Abstract
Background:
Tubular breasts are caused by connective tissue malformation and occur in puberty. The main clinical characteristics of the tubular breast are breast asymmetry, dense fibrous ring around the areola, hernia bulging of the areola, megaareola, and hypoplasia of quadrants of the breast. Pathology causes great psychological discomfort to patients.
Methods:
This study included 17 patients, aged 18 to 34 years, with tubular breast type II who had bilateral pathology and were treated from 2013 to 2016. They had surgical treatment by method of the clinic. Correction technique consisted of mobilization of the central part of the gland and formation of a glandular flap with vertical and horizontal scorings, which looks like a “chessboard,” that was sufficient to cover the lower pole of the implant. The flap was fixed to the submammary folds with stitches that prevented its reduction and accented a new submammary fold. To underscore the importance of the method and to study the structural features of the vascular bed of tubular breast tissue, a morphological study was conducted.
Results:
Mean follow-up time was 25 months (range between 13 and 37 mo). The proposed technique achieved good results. Complications (hematoma, circumareolar scarring, and “double-bubble” deformity) were identified in 4 patients.
Conclusions:
Our morphological study confirmed that tubular breast tissue has increased vascularity due to the vessels with characteristic minor malformation and due to the high restorative potential of the vascular bed. Therefore, an extended glandular flap could be freely mobilized without damaging its blood supply; thus, the flap in most cases covered the implant completely and good aesthetic results were achieved.
SURGICAL TREATMENT OF TUBULAR BREAST TYPE II
Tubular breasts are caused by connective tissue malformations and occur in puberty. Rees and Aston1 in 1976 described this pathology for the first time.
Clinical characteristics of the tubular breast include breast asymmetry, dense fibrous rings around the areola, hernia bulging of the areola, megaareola, hypoplasia of 2, 1, or all quadrants of the breast, narrowing of the breast base, and high location of submammary folds.2 Tubular deformity causes great psychological discomfort to patients and is most challenging for plastic surgeons to correct.
Several classifications of this pathology have been proposed. In 1996, von Heimburg et al3 classified this pathology into 4 types. The most common classification is that of Grolleau et al4, which includes 3 types of tubular breasts. In 2013, Costagliola et al5 modified the classification of Grolleau et al and included type О, which is characterized by isolated hernial protrusion of areola and normal breast base. Kolker and Collins6 classified deformities of tuberous breast and described treatment techniques for each individual.
According to Javier Orozco-Torres,7 patients with tubular breast type II underwent clinical correction more often (54.76%) than patients with type I or III tubular breasts.
Generally, treatment of a tubular breast type II includes releasing the constricted base; correcting ptosis, areola herniation, and preexisting asymmetry; and restoring a normal breast shape.
Surgical techniques that use implants and that do not use implants have been described, reflecting the reconstructive challenges associated with this deformity.8,9
The most popular method is the one suggested by Mandrekas et al.10 In this technique, after downward and upward prepectoral dissections, the constricting ring of the tubular breast is transected at the 6-o’clock semiaxis of the breast, thus creating 2 pillars in the inferior part of the breast. The pillars are then either just loosely reapproximated by using absorbable sutures or folded over each other to add volume to the inferior pole. In patients with small breasts, the use of implants should be considered.
Correcting tubular breast type II using only anatomical breast implants or Mandrekas method had several problems. High prepectoral dissection increased the risk of flap circulatory disorders, and mobilization only of the central part of the breast and its transection at 6-o’clock semiaxis did not always allow covering of the lower pole of the implant to the level of new submammary fold. Thus, there was a risk of development of contour irregularities in the lower pole of the breast due to reduction in the breast flap and risk of formation of double-bubble deformity in patients who initially had stiff submammary fold (5 cases in 31 of our operated patients). Moreover, unusually high level of vascularization of the mobilized breast flap was noticed.
Taking into consideration the high-level vascularization of the glandular flap, we hypothesized that it was safe to score the flap more widely in a vertical and horizontal manner, allowing the flap to extend considerably.
By comparing these facts and assumptions, we developed a technique to increase aesthetic outcomes and reduce the complication rate that we were experiencing in our clinical practice.
PATIENTS AND METHODS
Between 2013 and 2016, 17 patients were treated using our technique. Mean age of the patients was 26 years (range = 18 to 34 y). All were screened with mammography, ultrasound, and clinical examination. All patients had type II deformity based on Grolleau classification, bilateral deformities, and asymmetry.
Surgical Technique
Patients were marked preoperatively in the upright position. We marked the base of the breasts and the new inframammary folds. The area to be dissected was injected with dilute solution of epinephrine (1.5 mL of 1:1000 epinephrine added to 200 mL of normal saline). A circumareolar skin excision deepithelialization was performed to reduce the diameter of the mega-areola, according to preoperative planning. The inner part of the areola was incised, and the inferior pole of the breast was sharply undermined down to the pectoral fascia. Prepectoral dissection then was performed downward to the level of new inframammary fold. All attachments of surrounding tissues were released, creating a pocket for the lower pole of the implant. It was mandatory to leave an adequate thickness of the tissue to ensure its viability. The constricting fascial bands along the preexisting inframammary fold were incised by electrocautery. Then, dissection was performed upward. Breast parenchyma was dissected deep down to the pectoral fascia, leaving only the superior part of the gland attached to the pectoral wall. The dissection was also extended laterally and medially. After thorough hemostasis, the breast tissue was exteriorized through the periareolar opening, and its inner surface was scored consistently in a vertical and horizontal manner using electrocautery (Fig. 1).
Fig. 1.
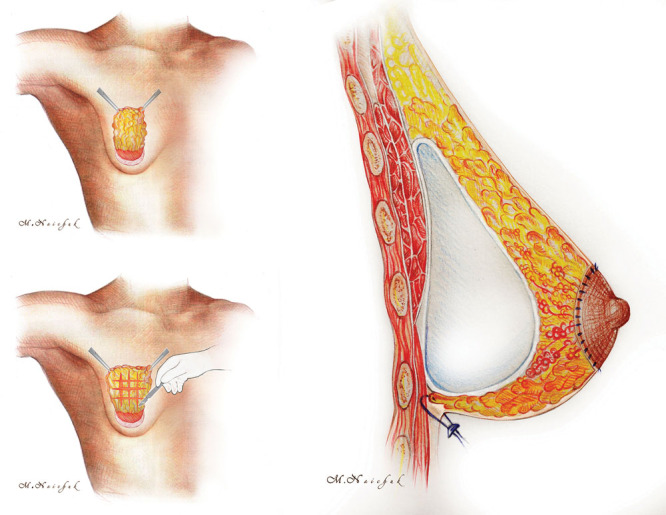
Schematic depiction of the tuberous breast correction.
The extended glandular flap so formed looked like a “chessboard” (Fig. 2A). Blood supply to the flap during surgery was controlled under direct vision. We formed a pocket for the implant in a subglandular plane to maximize the direct stretching effect of the implant on the dense tissues of the breast’s lower pole. In all cases, we used anatomical highly cohesive gel implants. After pocket irrigation with betadine, the implant was inserted. Then, the extended glandular flap was moved downward to cover the implant and reached the level of a new inframammary fold where it was fixed by a few transcutaneous stitches (Fig. 2B). To prevent dimpling, the external stitches were fixed using small cotton rolls. To control areola diameter, circumareolar mastopexy using interlocking suture was performed (Fig. 2C). A single drain was used for 3 to 5 days postoperatively.
Fig. 2.
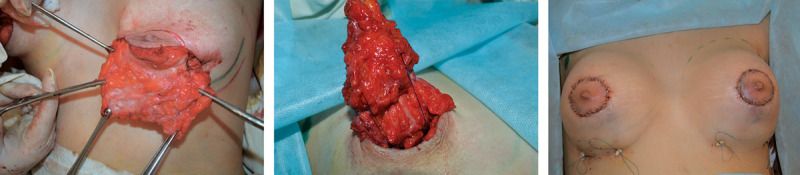
Surgical technique.
Morphological Studies
Samples of breast tissues taken from patients with different types of tubular breasts in our previous morphological studies were compared with similar tissue samples taken from 10 patients with primary breast augmentation. The areas selected for biopsies had similar characteristics and location. Samples were processed in a routine manner and stained with hematoxylin and eosin by van Gieson method. Histological studies were conducted using light-optical microscope “Olympus BX-43” (Olympus; Tokyo, Japan), with photodocumentation.
RESULTS
The average follow-up time was 25 months (range between 13 and 37 mo). The BREAST-Q postoperative satisfaction with breasts mean score was 82, psychosocial well-being mean score was 89, sexual well-being mean score was 86, and physical well-being mean score was 84. The aesthetic outcome was graded as good, fair, and poor based on criteria such as symmetry of the breasts, shape of lower pole, skin irregularities, diameter of new areola, and quality of circumareolar scars (Figs. 3,4).
Fig. 3.
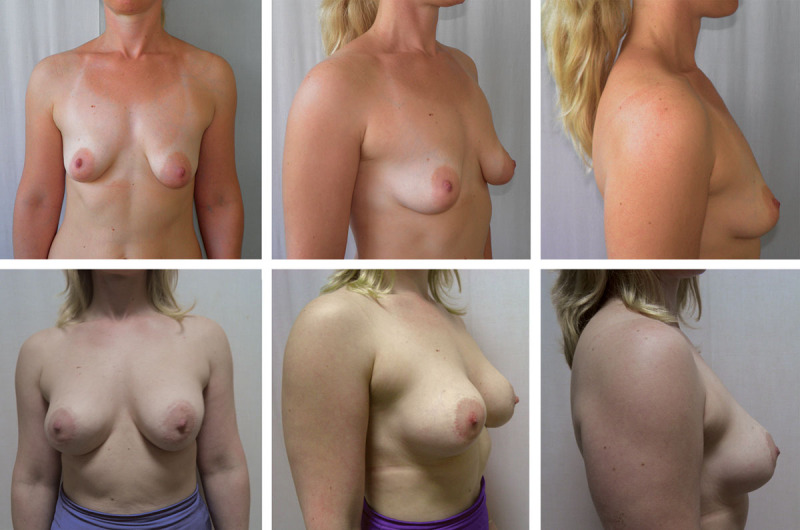
A 29-year-old patient with asymmetric tuberous breasts, type II. Above, Preoperative frontal, right oblique, and lateral views. Below, 24-month postoperative frontal, right oblique, and lateral views with subglandular placement of the high-profile anatomical cohesive gel silicone implants, 260 cm3.
Fig. 4.
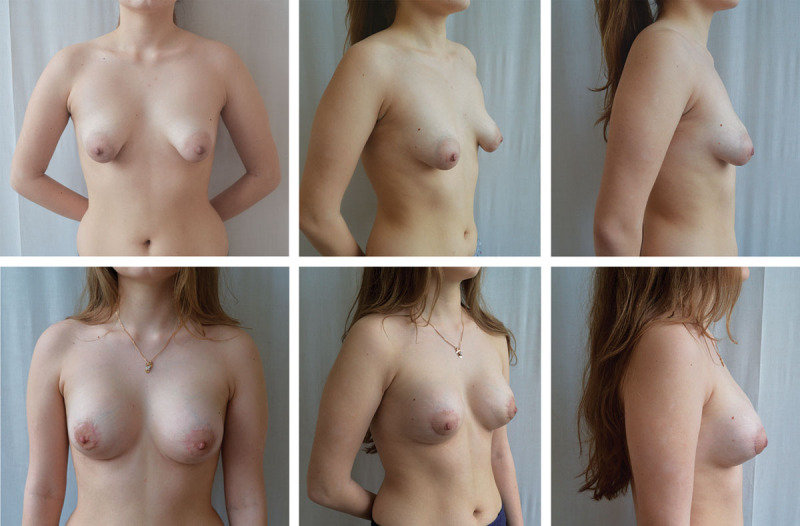
An 18-year-old patient with bilateral tuberous breasts, type II. Above, Preoperative frontal, oblique, and lateral views. Below, 12-month postoperative frontal, oblique, and lateral views with subglandular placement of the high-profile anatomical cohesive gel silicone implants, 200 cm3.
Complications were identified in 4 patients: hematoma in 1 patient, which was drained 4 hours after operation; circumareolar scarring in 1 patient; and mild “double-bubble” deformity in 2 patients, who initially had stiff submammary folds. But there was no evidence of capsular contracture, implant displacement, or palpation. These patients underwent surgery to correct minor aesthetic outcomes. Correction of circumareolar scarring using an interlocking suture was performed, and double-bubble deformity was corrected by fat grafting according to the technique of Tonnard et al.11 After correction, aesthetically pleasing results with good sensation were obtained in all 17 patients. Two patients, who delivered after surgery, had no feeding problems.
Morphological studies have shown that vascular bed of glandular flap of the tubular breast has a polymorphous structure and has capillaries, separate arterial and venous vessels of different diameters, and dysplastic vascular formations. Polymorphism of the vascular bed in the tissue of affected quadrants of the breast is expressed by sites of microangiomatosis, local areas of hemorrhagic leakage with a few sinusoidal and dysplastic vessels, and nonvascular areas without any capillaries; such areas with hemorrhagic leakage and individual vessels are clear signs of underdevelopment of the vascular bed, immaturity of capillaries, and congestive processes in those zones. Under surgical stress, these zones can be both the cause of bleeding and source of vascular bed restoration. There are also certain groups of vessels with normal ratio of arteries and veins (1:2), which can provide blood supply to individual lobules of the gland. But studies also revealed in these zones the absence of an important intermediate segment of vascular network, indicating the congenital nature of this pathology.
Locally, arteries and veins of small diameter were observed, which, based on their peculiar structure, ratio of diameters, state of endothelium, and signs of arteriovenous shunts, could be classified as minor malformation (Fig. 5). This indicates the adequate proliferative capacity of blood network to restore and enhance blood supply in areas of trauma, hypoxia, or ischemia. So, even when a large glandular flap is mobilized, the risk of its necrosis is less.
Fig. 5.
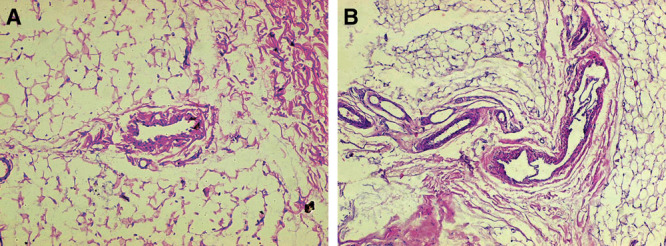
Gland of tubular breast, type II. A, Muscular artery in the loose connective tissue, whose structure looks like shunt, along with small capillaries. B, Vascular malformation. Clusters of arteries and veins of various diameters in connective tissue. The wall of the largest vessel has elements of venous and arterial structures with thin membrane valves. Hematoxylin and eosin stain, original magnification ×20.
DISCUSSION
Tubular breast represents a significant psychological problem in women. In general, the pathology becomes apparent in puberty and is characterized by narrow breast base, bulging of mega-areola, asymmetry, etc. Our morphological studies have found signs that confirm the congenital nature of this pathology, and our results are in agreement with those of the previous studies.6,12,13
Since the correction of type II deformity has a large number of problems, it cannot be classified as a reconstructive surgery. In the majority of cases, a satisfactory contouring of tubular breast could be obtained by widening its base, moving the inframammary fold down with creation of a lower pole using tissue or implant, and reshaping the areolar complex.
To create lower pole of the breast, some authors used anatomical implants for extending the base of the tubular breast.14 In our previous studies in patients with augmentation mammoplasty and patients with tubular breast type I, we noted the ability of the highly cohesive shaped implants to expand the skin of lower pole of the breast. So, it was logical to try them in patients with tubular breast type II as well. But it did not work in all cases, especially in patients with a stiff submammary fold. Textured shaped implants in patients with tubular breast type II were associated with increased rates of palpable and visible implant margins and double-bubble deformity. Using an implant is very important for achieving proper contour of the lower pole. Therefore, many methods are suggested to solve this problem. Hence, we used shaped implants in combination with mobilized and scored glandular flaps and achieved favorable results. Although we have used shaped implants in our study, the use of round implants should be considered, and their use needs to be studied further.
For patients who had sufficient volume of their own breast or did not want to increase their breasts’ volume, Ribeiro (1998) proposed to cut the breast horizontally into 2 flaps. The lower flap, bigger than the upper one, was folded down for creating the volume of lower pole.8 The technique provides good aesthetic results, but patients often wish to increase their breast volume.
In small-volume breasts, Puckett and Concannon9 proposed a high prepectoral dissection of the breast, cutting it in the posterior surface into 2 halves. On the one hand, it allows the formation of a glandular flap to cover the lower pole of the implants, but on the other hand, it increases the risk of circulatory disturbances in the flap, followed by its atrophy or scarring in the postoperative period. In such cases, contour irregularities in the lower pole of breast could be observed.
Kolker and Collins6 performed dissection in a perpendicular fashion through lower pole of the gland directly to the prepectoral fascia. Prefascial dissection was then carried out inferiorly to the limit of new inframammary fold, and radial scoring of the inferior dermoglandular flap was performed with electrocautery. From our point of view, this approach increases the risk for possible decrease in blood supply of the inferior dermoglandular flap, scarring, and contour irregularities of the lower pole.
Mandrekas et al10 dissected the breast parenchyma from the deep pectoral fascia, leaving only the superior part of the breast attached. Breast parenchyma was exteriorized through the periareolar opening and was transected with vertical incision along the middle, dividing fibrous ring and creating 2 breast pillars that allow breast parenchyma to redrape implant, but often the flap is too short to cover the lower pole of the implant. Therefore, in the postoperative period, it can be reduced, leading to contour irregularities of the lower pole of the breast.
Our method is different from other methods and has several advantages. It allows mobilization of the extended glandular flap from the central part of the breast by vertical and horizontal incisions to form the flap like a chessboard. This technique does not disturb blood supply to the flap. The entire breast is not damaged as in high prepectoral dissection, making it possible for the women to breastfeed after the surgery.
The method allows full coverage of the implant with the glandular flap without any tension and prevents reduction. Thanks to stitches, new submammary folds can be better accented.
We preferred textured, anatomical, highly cohesive gel implants. In all cases, we fixed them in the subglandular plane. This maneuver provided better aesthetic effects in postoperative periods because the dense skin of lower pole stretches better, and contour of the breast has a natural look.
One of the problems with tubular breast correction in follow-up, according to many authors, is the double-bubble deformity. This occurs because in some cases, the fibrous constricting ring and previous inframammary fold cannot be completely released.
Some authors use lipofilling to correct this complication.15,16 We also noted a beneficial effect of lipofilling in double-bubble correction. We obtained good aesthetic results using method of Tonnard et al.11
CONCLUSIONS
Correcting tubular breast type II is a challenge for plastic surgeons. To achieve aesthetically pleasing results, an adequate surgical approach is required. Our technique is simple and easy to perform.
Morphological studies have confirmed that tubular breast tissue has increased vascularity because of the vessels with a characteristic minor malformation and because of the high restorative potential of vascular bed. Therefore, an extended glandular flap could be freely mobilized without damaging its blood supply. In most cases, this allows covering of the implant completely, achieving good aesthetic results.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Rees TD, Aston SJ. The tuberous breast. Clin Plast Surg. 1976;3:339–347. [PubMed] [Google Scholar]
- 2.Shiffman MA. Breast augmentation: principles and practice. In: DeLuca-Pytell D, Piazza RC, Holding JC, et al., editors. In: The Incidence of Tuberous Breast Deformity in Asymmetric and Symmetric Mammoplasty Patients. Berlin, Heidelberg: Springer-Verlag; 2009. pp. 302–306. [Google Scholar]
- 3.von Heimburg D, Exner K, Kruft S, et al. The tuberous breast deformity: classification and treatment. Br J Plast Surg. 1996;49:339–345. doi: 10.1016/s0007-1226(96)90000-4. [DOI] [PubMed] [Google Scholar]
- 4.Grolleau JL, Lanfrey E, Lavigne B, et al. Breast base anomalies: treatment strategy for tuberous breasts, minor deformities, and asymmetry. Plast Reconstr Surg. 1999;104:2040–2048. doi: 10.1097/00006534-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Costagliola M, Atiyeh B, Rampillon F. Tuberous breast: revised classification and a new hypothesis for its development. Aesthetic Plast Surg. 2013;37:896–903. doi: 10.1007/s00266-013-0124-2. [DOI] [PubMed] [Google Scholar]
- 6.Kolker AR, Collins MS. Tuberous breast deformity: classification and treatment strategy for improving consistency in aesthetic correction. Plast Reconstr Surg. 2015;135:73–86. doi: 10.1097/PRS.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 7.Oroz-Torres J, Pelay-Ruata MJ, Escolán-Gonzalvo N, et al. Correction of tuberous breasts using the unfolded subareolar gland flap. Aesthetic Plast Surg. 2014;38:692–703. doi: 10.1007/s00266-014-0340-4. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro L, Canzi W, Buss A, Jr, et al. Tuberous breast: a new approach. Plast Reconstr Surg. 1998;101:42–50; discussion 51. doi: 10.1097/00006534-199801000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Puckett CL, Concannon MJ. Augmenting the narrow-based breast: the unfurling technique to prevent the double-bubble deformity. Aesthetic Plast Surg. 1990;14:15–19. doi: 10.1007/BF01578320. [DOI] [PubMed] [Google Scholar]
- 10.Mandrekas AD, Zambacos GJ, Anastasopoulos A, et al. Aesthetic reconstruction of the tuberous breast deformity. Plast Reconstr Surg. 2003;112:1099–1108; discussion 1109. doi: 10.1097/01.PRS.0000076502.37081.28. [DOI] [PubMed] [Google Scholar]
- 11.Tonnard P, Verpaele A, Peeters G, et al. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017–1026. doi: 10.1097/PRS.0b013e31829fe1b0. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman MA. Breast augmentation: principles and practice. In: Mandrekas AD, Zambacos GJ, editors. In: Aesthetic Reconstruction of the Tuberous Breast Deformity. Berlin, Heidelberg: Springer-Verlag; 2009. pp. 307–319. [Google Scholar]
- 13.Grotting JC, Neligan P. Plastic surgery: breast. In: Muti E, editor. In: Congenital Anomalies of the Breast. 3rd ed. Vol. 5. Elsevier Saunders; 2012. pp. 521–547. [Google Scholar]
- 14.Brown MH, Somogyi RB. Surgical strategies in the correction of the tuberous breast. Clin Plast Surg. 2015;42:531–549. doi: 10.1016/j.cps.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Delay E, Sinna R, Ho Quoc C. Tuberous breast correction by fat grafting. Aesthet Surg J. 2013;33:522–528. doi: 10.1177/1090820X13480641. [DOI] [PubMed] [Google Scholar]
- 16.Serra-Renom JM, Muñoz-Olmo J, Serra-Mestre JM. Treatment of grade 3 tuberous breasts with Puckett’s technique (modified) and fat grafting to correct the constricting ring. Aesthetic Plast Surg. 2011;35:773–781. doi: 10.1007/s00266-011-9686-z. [DOI] [PubMed] [Google Scholar]


