Abstract
Background:
The prevailing theory for capsular contracture after breast augmentation is a subclinical capsular infection. A capsulectomy, site change, and implant replacement are recommended. An open capsulotomy leaves the capsule in the patient. Theoretically, such a procedure would be ineffective because it does not remove the infected tissue. Recurrences occurred frequently in women treated in the 1970s when leaky silicone gel implants were in use. Open capsulotomy has not been studied in women implanted with third-generation devices.
Methods:
Seventy-five consecutive women with Baker III/IV capsular contractures after breast augmentation treated with open capsulotomies between 1996 and 2016 were retrospectively evaluated. The original implants were usually saline-filled (72.2%). Replacements were all smooth and round, and 92.6% were also saline-filled.
Results:
Seventeen women (22.7%) developed a recurrent capsular contracture. Two patients (2.7%) experienced a second recurrence. Patients with ruptured silicone gel implants (n = 13) had a significantly greater risk of recurrence (P = 0.01). There was no significant difference in recurrence rates comparing patients whose intact implants were reinserted (12.5%) with women whose intact implants were replaced (18.2%). Povidone–iodine irrigation did not affect the recurrence rate. Capsular contracture was corrected with 1 procedure in 77.3% of patients and 2 procedures in 97.3% of patients.
Conclusions:
Open capsulotomy is a safe and effective treatment that avoids the additional morbidity and cost of a capsulectomy. The findings challenge the infected biofilm theory of capsular contracture. Open capsulotomy deserves reconsideration by plastic surgeons.
The prevailing explanation for capsular contracture is chronic inflammation caused by a bacterial biofilm.1–9 The “gold standard” treatment calls for a capsulectomy, along with a site change and implant exchange.2,7,9 However, Wan and Rohrich,7 in a recent systematic review, found little supportive evidence for this recommendation. Recurrence rates after surgical treatment are sparsely reported.5
Capsulectomies require greater dissection than open capsulotomies, increasing the level of difficulty, operating time, and patient discomfort.5,8 There is additional bleeding and an increased risk of pneumothorax after total capsulectomy.1,5,8 To avoid morbidity, a lesser procedure is preferred. However, open capsulotomies are typically regarded as inadequate, and more disposed to recurrence of a capsular contracture.7 This opinion is based on studies published in the 1970s and 1980s10–15 evaluating women treated with leaky second-generation silicone gel implants. Although open capsulotomies were commonly performed in the 1980s,16 this procedure was largely replaced by capsulectomy17 after investigators implicated bacterial biofilms as a cause of capsular contractures.18,19
Clinical experience with earlier generation implants should not be relied upon when evaluating third- generation devices.6,20 This study was undertaken to determine the efficacy of open capsulotomy alone as a treatment for capsular contracture in breast augmentation patients treated with modern, third-generation breast implants, including saline implants, that were not evaluated in early studies.10–15 This is an important clinical question because saline implants were used almost exclusively in the United States from 1992 to 2006 during the silicone gel moratorium, and continue to be a popular choice among patients,5 representing approximately 30% of breast augmentation patients in the United States today (Mentor Worldwide LLC, Personal communication, May 2016).
PATIENTS AND METHODS
A retrospective chart review was conducted from 1996 to 2016. All women who underwent an open capsulotomy by the author were included. All patients had Baker III/IV contractures. Only cosmetic breast augmentation and augmentation/mastopexy patients were included. Three patients with extensive capsular calcification, treated with capsulectomies, were excluded. Breast reconstruction patients were also excluded. This study was determined to be exempt from institutional review board oversight by Chesapeake Institutional Review Board Services.
Surgery
All patients underwent outpatient surgery using total intravenous anesthesia (Fig. 1). Patients received cefazolin 1 g IV before surgery and 3 doses of cephalexin 500 mg orally twice daily postoperatively. An inframammary approach, usually along the original scar, was used exclusively. This approach optimizes exposure. The existing capsule was circumferentially incised either partially or totally (Fig. 2). In patients with existing subpectoral pockets, the original (expanded) pockets were reused, with no attempt to dissect a new tissue plane. When the original implants were subglandular, a new submuscular pocket was developed whenever possible,5,7 with no attempt to remove or suture the original capsule. New subpectoral pockets were created using sharp dissection to release the inferior pectoralis origin, and blunt dissection of the pocket.
Fig. 1.
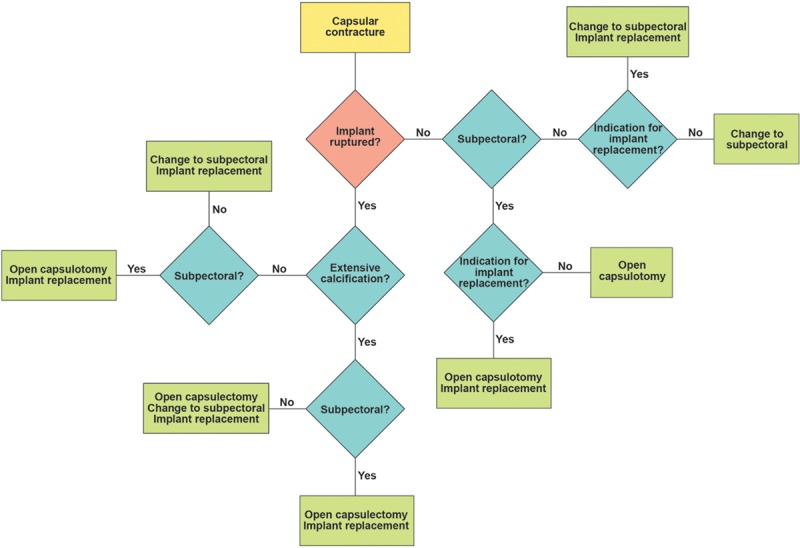
Treatment algorithm. Indications for implant replacement, apart from rupture, are subjective and include old implants (usually >10 y), size change, preference for a saline-filled or smooth implant, a deflation on the contralateral side, and warranty renewal. Implant manufacturers now provide free replacement implants for variable periods (3–10 y) after implantation.
Fig. 2.
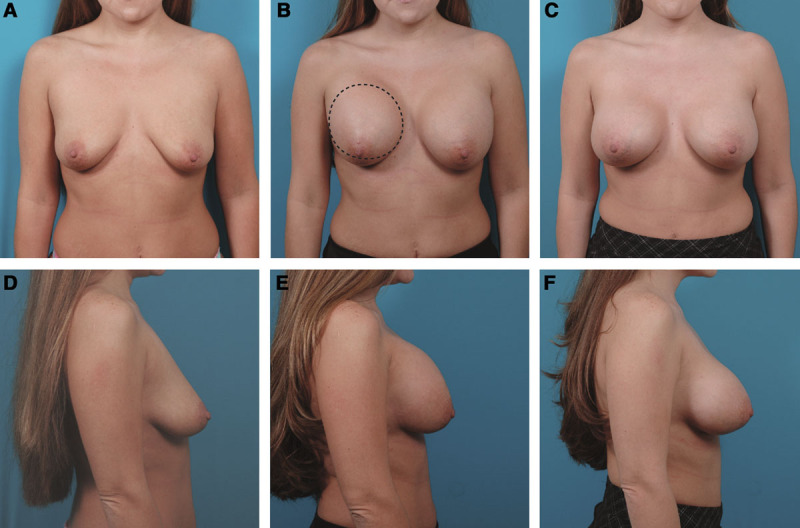
This 29-year-old woman developed a Baker III capsular contracture after her breast augmentation using Mentor smooth, round, Moderate Plus profile saline-filled implants inflated to 420 cc. She is seen before (A, D) and 1 month after her breast augmentation (B, E). Two months after her breast augmentation, she underwent a right open capsulotomy with reinsertion of the same implant in the same subpectoral pocket. The hatched line indicates the capsulotomy incision. She is seen 1 month after the capsulotomy (C, F). She had no recurrence.
Dilute povidone–iodine solution was used before 2000. Only saline solution was used for irrigation subsequently because of (probably unwarranted21) concerns regarding the effect of antibiotics on implants. The beginning of the study coincided with the author’s transition from textured to exclusively smooth, round implants, based on a questionable clinical benefit and possibly more leaks and wrinkles using textured devices.22,23 Drains, nipple shields, implant funnels, and acellular dermal matrix were not used. Patients were instructed to wear a bra day and night for at least 2 weeks, not to massage their breasts, and to avoid vigorous physical activity for 1 month.
Statistical Analysis
A chi-square test was used to compare categorical data. The cumulative incidence of capsular contractures per patient was estimated using Kaplan–Meier analysis, calculated from the date of implant insertion to the date of the reported complication. A value of P < 0.05 was considered significant.
RESULTS
Seventy-five women with Baker III/IV contractures (Fig. 3) underwent open capsulotomies (Table 1). In 68 patients (90.7%), the existing implants were subpectoral. Among the 7 patients with subglandular implants, 6 were changed to a subpectoral pocket. The mean time lapse between implantation and diagnosis of a capsular contracture was 4.0 years. The mean interval between the first open capsulotomy and diagnosis of a recurrent contracture was 8.4 months.
Fig. 3.
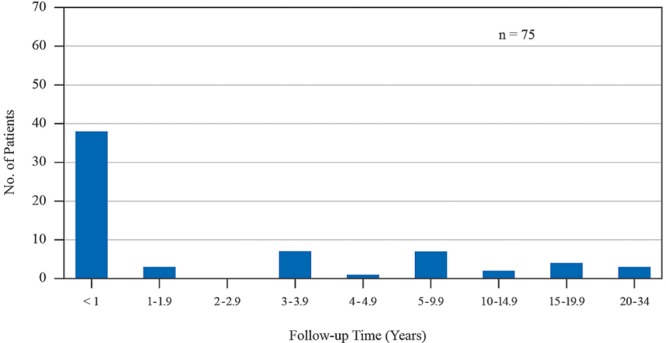
Time interval between the original breast augmentation and diagnosis of capsular contracture in 75 consecutive women treated with open capsulotomy during 1996 to 2016.
Table 1.
Patient Data for 75 Patients Treated with Open Capsulotomy
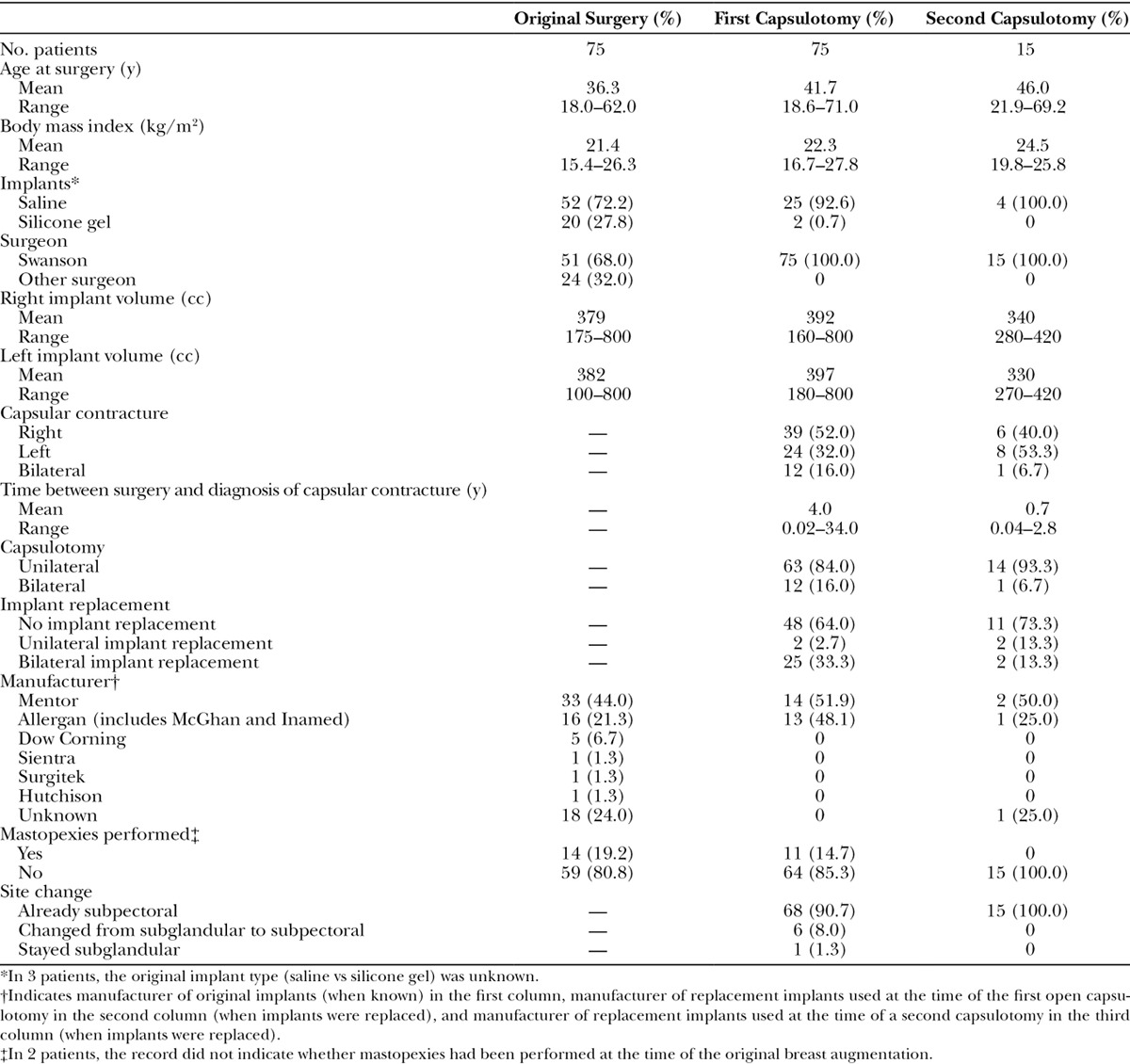
Seventeen patients (22.7%) returned with a recurrence (Fig. 4). Fifteen women were re-treated; 2 women elected not to have additional surgery. Two patients (2.7%) returned with a third capsular contracture; neither patient elected to have it treated. In 12 women (including 3 bilateral contractures, only 1 of which recurred on both sides), the recurrence was on the same side, and in 5 patients the recurrence was on the contralateral side. Thirteen patients were found to have ruptured silicone gel implants or silicone bleed at surgery; 9 of these patients had recurrences (69.2%). Replacement of a leaking silicone gel implant significantly increased the risk of a recurrent contracture (P = 0.01) compared with intact implant replacement. There was no significant difference comparing recurrence rates in patients with implants that were reinserted (6/48 patients, 12.5%) versus intact implants that were replaced (2/11 patients, 18.2%).
Fig. 4.
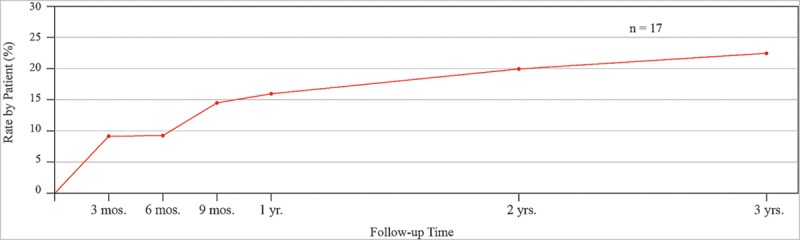
Kaplan–Meier analysis: Cumulative incidence of recurrent capsular contracture in 17 consecutive women after open capsulotomy.
Indications for implant replacement (frequently more than 1) included: old implants, usually >10 years (n = 15), larger size (n = 13), preference for saline (n = 8), a deflation on the contralateral side (n = 4), suspected implant rupture (n = 2), and smaller size (n = 1).
Twenty-six women who received povidone–iodine irrigation during surgery (before 2000) had a similar (P > 0.05) recurrence rate (19.2%) to patients treated subsequently with saline irrigation (17.2%).
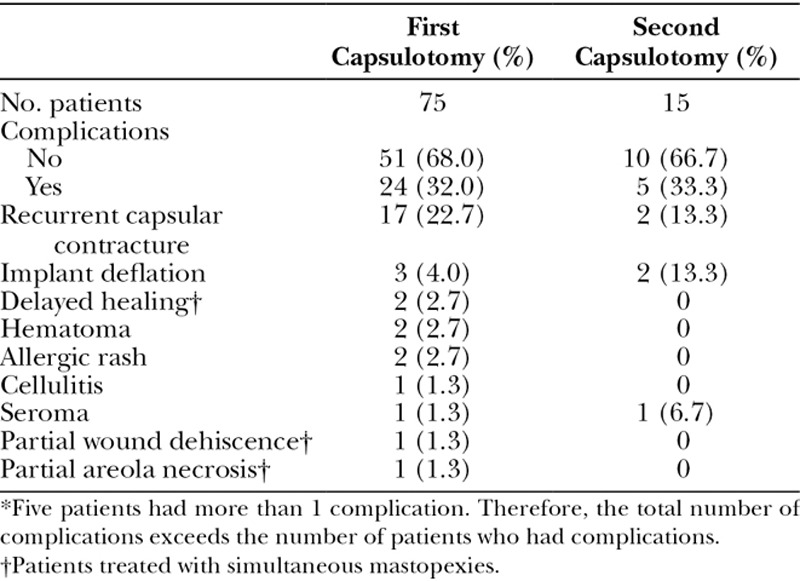
Complications are listed in Table 2. The most common complication was a recurrent capsular contracture (22.7%). Although not part of the study group, the 3 women treated with capsulectomies (heavily calcified capsules) during the study period did not experience recurrences.
Table 2.
Complications*
Implants manufactured by Mentor Corporation (Santa Barbara, Calif.) were used predominantly by the author from 1996 to 2009. Allergan (Irvine, Calif.) implants were inserted primarily from 2010 to 2016. Consequently, most women received Mentor implants (Table 1). The group sizes were insufficient for comparison of capsular contracture rates by manufacturer.
DISCUSSION
Existing Studies Comparing Open Capsulotomy and Capsulectomy
A recent systematic review7 compared the rate of recurrent capsular contracture after open capsulotomies (0%–54%) with capsulectomies (0%–53%), finding them similar. All except one study of open capsulotomies meeting the authors’7 inclusion criteria were published between 1972 and 1987.10–15 Moufarrege et al15 reported a 54% failure rate after open capsulotomies. Little and Baker12 reported 6 recurrences among 18 open capsulotomies (33%). Hipps et al11 reported a 34% capsular contracture rate, and 46% of contractures were bilateral. In all 3 studies, second-generation devices had been implanted between 1973 and 1978 and many of the women had undergone closed capsulotomies. By contrast, Freeman’s10 1972 study reported only 2 recurrences after 35 capsulotomies (5.7%) in patients treated with more durable first-generation devices. The most recent study of open capsulotomy, published in 1999, also evaluated patients treated predominantly (70%) with second-generation devices; no recurrence rates were provided.24
In the only head-to-head comparison in women treated with the same (albeit, second generation) implants, Hipps et al11 reported a 34% recurrence rate after capsulectomy (n = 29) and a very similar 31% recurrence rate after open capsulotomy (n = 26). Other studies of capsulectomy, all published after 2000,3,17,25,26 evaluated women with third-generation implants. Costagliola et al25 reported no recurrences after capsulectomies, but these patients were treated with steroids. Caffee26 reported a recurrence rate of 30.7% after capsulectomy and subpectoral placement of new textured saline implants. Collis and Sharpe17 reported an 11% recurrence rate after total capsulectomy and a 50% recurrence rate after a partial “anterior disc” capsulectomy, although the follow-up time was shorter for patients treated with total capsulectomy. In 2012, Hester et al3 reported a 53.4% recurrence rate after traditional capsulectomy.
From 1963 to 1992, over 95% of all breast implants were silicone gel implants; only 5% were saline-filled.27 First-generation implants had a thick gel and a thick elastomeric shell.4,27 Second-generation implants, implanted in the 1970s, had much thinner envelopes.4,27 Peters et al27 reported a 95% failure rate for second-generation implants 12 years after implantation. Silicone leakage into the tissues is known to increase the inflammatory response and increase the risk of contracture.28,29 In response to this problem, in the early 1980s breast implant companies started manufacturing third-generation implants that contained an additional barrier layer.4,27
Improved implant design compromises any comparison of open capsulotomies performed decades ago with capsulectomies performed more recently,7 hence the need for evaluation of open capsulotomy in women treated with third-generation devices. A prospective cohort study comparing capsulotomy and capsulectomy would be ideal, but impractical in view of the requirement for equipoise.
Etiology of Capsular Contracture
Infection as a factor predisposing to capsular contracture is supported by numerous microbiological studies that have cultured organisms from the capsule.18,19,30,31 Many investigators advocate an infectious etiology2,4,6,19,32–36 and recommend numerous steps to optimize sterility at the time of implant insertion. Reaching beyond a correlation, some researchers now claim that bacterial biofilm infections cause capsular contracture.34–36 Tamboto et al34 investigated capsular contracture using a porcine model, injecting Staphylococcus epidermidis around miniature silicone gel implants. Capsular contractures developed in 28 of 36 inoculated pockets (78%), but also occurred in 7 of 15 uninoculated pockets (47%).
There are problems with the theory of a purely infectious etiology. Positive and negative bacterial cultures from implants and capsules may be obtained from women with and without capsular contractures.30,31,37–40 Jacombs et al33 detected 20-fold (72-fold in vitro) more bacteria attached to textured implants than smooth implants in their porcine model, and more growth of biofilm, despite similar capsular contracture rates. Capsular contractures can occur years after implantation and the cumulative risk increases over time (Fig. 3),1,2,23 which would not be expected if the cause were a bacterial infection acquired at surgery. Capsular contractures occur more frequently after breast reconstruction using implants.1,2,23,41 The source of bacteria in postmastectomy patients is unclear. Curiously, a histological study42 found that mast cells, the predominant inflammatory cell in hypertrophic scars, disappear as the capsular contracture becomes more severe. Poppler et al,43 using specialized cultures and scanning electron microscopy in women undergoing expander/implant exchange, were unable to find a correlation between biofilm formation and capsular contracture. These investigators43 theorize that stressful stimuli might lead independently to inflammation and a biofilm. Paradoxically, antimicrobial therapy may even induce biofilm formation to confer resistance.44,45
The limitations of an infectious etiology are evident in the clinical findings of this study. An open capsulotomy leaves all of the capsule (and biofilm) in the patient, virtually guaranteeing treatment failure. Yet, this simple maneuver is 77.3% effective after 1 release and 97.3% effective after 2 capsulotomies. The success rate is even higher in patients with intact implants, in whom free silicone gel is not a factor; 86.4% had no recurrence. Moreover, there was no change in the recurrence rate after povidone–iodine irrigation was replaced with saline irrigation.
Another theory for capsular contracture is based on the mechanical action of myofibroblasts1,7,9,43,46,47 and abnormal collagen deposition.9,42,43 A capsulotomy breaks this mechanical force.7 It is possible, although unproven, that nonpathogenic bacteria may be protective against pathogens38 (similar to the flora of the digestive tract) and attempts to alter the microbial environment with antibiotics may be counterproductive. Triple antibiotic irrigation is recommended.6,48 However, recent studies have found no benefit in capsular contracture rates comparing triple antibiotic with saline irrigation.49,50 Other studies challenge the efficacy of perioperative systemic antibiotics.51,52
For the period 2002 to 2012, the author’s capsular contracture rate after primary breast augmentation was 6%,53 similar to other series,3,54 and to manufacturer core study data (8.1%–18.9%),20,41,55–57 which are regarded as most robust.5,7 It is not clear that conforming to numerous technical recommendations4,35 (apart from the usual sterile technique) makes a difference in the capsular contracture rate.3
Site Change
Some investigators recommend a subglandular/subpectoral site change, or a neopocket.58–60 In their comparison of capsulectomy and site change versus open capsulotomy and no site change, Wan and Rohrich7 find evidence to support a site change. However, as with the comparison of capsulectomy and capsulotomy, the difference in implant shell integrity is a confounding factor. An open capsulotomy in women treated in the 1970s with second-generation implants11,12,15 is more likely to fail in the presence of ruptured implants and silicone bleed.
An open capsulotomy limits the wound area and theoretically minimizes inflammation and fibroblast activity by preserving the existing capsule. The subpectoral location seems to be at a lower risk for capsular contracture,1,5,6,16,56 a finding often attributed to more separation from nonsterile breast tissue,1,6,18,19 although there is still plenty of contact. The number of patients undergoing a site change in this study (n = 6) was too small to make any conclusions regarding effect on capsular contracture rate. Regardless of capsular contracture risk, replacing a subglandular implant in the submuscular plane provides additional tissue cover and optimizes upper pole aesthetics.5,7
Implant Replacement
In this study, the subgroup of patients whose ruptured or leaking silicone gel implants were replaced had a significantly (P = 0.01) higher risk of recurrence than patients whose intact implants were replaced, underscoring the increased risk associated with silicone gel leakage.
Perhaps counterintuitively, patients whose implants were not replaced were at no greater risk of recurrence than women whose intact implants were replaced. This finding contrasts with a recent core study of Sientra (Santa Barbara, Calif.) breast implants, which found a reduced recurrence risk in women whose implants were replaced.57 This finding was based on the analysis of a subgroup of 68 patients (of 150) whose contractures responded to treatment.57 The investigators noted that 3 study sites had a much higher rate of capsular contracture than the others.57 Confounding variables must be considered.
Although implant exchange is generally recommended,2,4,6,7,9,57 the recipient site is not sterile.1,6,38,61 A new implant is likely to be quickly colonized by the bacteria already present in adjacent breast tissue.38 Nevertheless, other indications for replacement include the following: a size change, warranty renewal, or to replace textured implants with smooth devices. Implant manufacturers have recently started providing free replacement implants for patients developing capsular contractures within 3 to 10 years of implantation.62–64
Smooth versus Textured Implants
Textured implants were designed to minimize the rate of capsular contracture, but there may be no advantage over smooth implants when the implant is placed submuscularly.4,65,66 Shaped implants have not been shown to be superior to round implants in breast augmentation.5,8 Textured implants are at greater risk for rippling and deflation than smooth implants.7,23 Recently, textured implants have been implicated in late seromas, double capsules,67 and anaplastic large-cell lymphoma.68 Smooth implants are now favored by the majority of surveyed plastic surgeons.8 Smooth implants may reduce the recurrence rate when used as replacement implants.7 A recent core study with a 10-year follow-up identified the lowest capsular contracture rate in patients treated with smooth subpectoral implants inserted through an inframammary incision.56
Capsulectomy versus Capsulotomy
A capsulectomy involves much more dissection than a capsulotomy.69 There is less remaining tissue to provide implant cover and a greater potential for nerve injury.70 A drain may be needed.5 It can be technically difficult, and dangerous, to remove capsular tissue from the axilla and the chest wall.1,60,69 Accordingly, an anterior capsulectomy may be recommended,69 although this recommendation begs the question, is a capsulectomy really necessary if part of the capsule is left in the patient?
A unilateral open capsulotomy typically requires 20 to 30 minutes. Capsulectomy adds about 1 hour of operating time.69 Because there is minimal submuscular dissection (unless a new subpectoral pocket is created), there is little discomfort. Patients return to their usual activities within a few days. Even in the event of a recurrence, the patient has experienced minimal cost and morbidity. The recurrence rate of 22.7% overall after open capsulotomy (and 13.6% for patients with intact implants) compares favorably with recurrence rates reported after capsulectomy (25%–53.4%).3,57
In the case of thin, noncalcified capsules, there seems to be no harm in leaving the capsule in the patient.71 The capsule around saline implants is usually absorbed.72 The low seroma rate (1.3%) indicates that leaving the capsule in situ rarely leads to fluid accumulation. A capsulectomy, either partial or full, may be reserved for thick, calcified capsules.7 Removal of all calcification is unnecessary.71 Capsular calcifications are not usually a source of confusion for radiologists.69 Acellular dermal matrix is thought to reduce capsular contracture by serving as a barrier and reducing inflammation and scarring.73 This objective is also accomplished with a capsulotomy.
Treatment Recommendations
Recent recommendations to avoid recurrence include capsulectomy, site change, new implants, bloodless dissection, antibiotic irrigation, glove change, covering the incision site with an adhesive barrier, form-stable implants, a sleeve or funnel, nipple shields, and acellular dermal matrix.2,4–6,35 In 1981, Brody and Latts74 lamented the lack of controlled studies leading to a “shotgun approach using every means ever reported,” commenting that “the enthusiastic espousal of circumstantial evidence becomes dogma.” In the years since, investigators have frequently noted the lack of scientific data7,75,76 and the shortcomings of treatment dictated by clinical impressions alone.75
In 2012, Hester et al3 commented that breast-pocket irrigation, site changes, and submuscular or dual plane implant placement had minimal identifiable effect on the rate of capsular contracture. Despite capsulectomy, site change when appropriate, and implant replacement, these experienced surgeons reported a recurrence rate of 53.4%, prompting them to start incorporating acellular dermal matrix.
A recent survey8 and review articles4,5,8 do not include open capsulotomy as a treatment option. Twenty percent of plastic surgeons use silicone gel implants exclusively.8 Although silicone gel implants are thought to have a more ideal feel characteristic,5 this difference may be negligible, particularly when the implant is placed submuscularly. The appearance of saline-filled implants is the same.5 Implant deflation is easier to detect, and treatment of a capsular contracture is likely to be uncomplicated and successful. Saline implants are less expensive than silicone gel implants and patient satisfaction is extremely high with both devices.16,77,78
CONCLUSIONS
Capsular contracture after a breast augmentation may be safely and effectively treated with an open capsulotomy, reducing patient morbidity and cost associated with alternative treatments.
ACKNOWLEDGMENTS
The author thanks Christina Engel, R.T., for data collection and Gwendolyn Godfrey for illustrations.
Footnotes
Disclosure: The author has no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the author.
REFERENCES
- 1.Araco A, Caruso R, Araco F, et al. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124:1808–1819. doi: 10.1097/PRS.0b013e3181bf7f26. [DOI] [PubMed] [Google Scholar]
- 2.Adams WP., Jr Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clin Plast Surg. 2009;36:119–26, vii. doi: 10.1016/j.cps.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Hester TR, Jr, Ghazi BH, Moyer HR, et al. Use of dermal matrix to prevent capsular contracture in aesthetic breast surgery. Plast Reconstr Surg. 2012;130(5 Suppl 2):126S–136S. doi: 10.1097/PRS.0b013e3182605d18. [DOI] [PubMed] [Google Scholar]
- 4.Adams WP, Jr, Mallucci P. Breast augmentation. Plast Reconstr Surg. 2012;130:597e–611e. doi: 10.1097/PRS.0b013e318262f607. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014;133:567e–583e. doi: 10.1097/PRS.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 6.Chong SJ, Deva AK. Understanding the etiology and prevention of capsular contracture: translating science into practice. Clin Plast Surg. 2015;42:427–436. doi: 10.1016/j.cps.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Wan D, Rohrich RJ. Revisiting the management of capsular contracture in breast augmentation: a systematic review. Plast Reconstr Surg. 2016;137:826–841. doi: 10.1097/01.prs.0000480095.23356.ae. [DOI] [PubMed] [Google Scholar]
- 8.Hidalgo DA, Sinno S. Current trends and controversies in breast augmentation. Plast Reconstr Surg. 2016;137:1142–1150. doi: 10.1097/01.prs.0000481110.31939.e4. [DOI] [PubMed] [Google Scholar]
- 9.Ajdic D, Zoghbi Y, Gerth D, et al. The relationship of bacterial biofilms and capsular contracture in breast implants. Aesthet Surg J. 2016;36:297–309. doi: 10.1093/asj/sjv177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman BS. Successful treatment of some fibrous envelope contractures around breast implants. Plast Reconstr Surg. 1972;50:107–113. doi: 10.1097/00006534-197208000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hipps CJ, Raju R, Straith RE. Influence of some operative and postoperative factors on capsular contracture around breast prostheses. Plast Reconstr Surg. 1978;61:384–389. doi: 10.1097/00006534-197803000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Little G, Baker JL., Jr Results of closed compression capsulotomy for treatment of contracted breast implant capsules. Plast Reconstr Surg. 1980;65:30–33. doi: 10.1097/00006534-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Sugimoto T. Open capsulotomy for capsular contracture: a new procedure for the prevention of recurrence. Aesthetic Plast Surg. 1982;6:225–230. doi: 10.1007/BF01570652. [DOI] [PubMed] [Google Scholar]
- 14.Herman S. The Même implant. Plast Reconstr Surg. 1984;73:411–414. doi: 10.1097/00006534-198403000-00012. [DOI] [PubMed] [Google Scholar]
- 15.Moufarrege R, Beauregard G, Bosse JP, et al. Outcome of mammary capsulotomies. Ann Plast Surg. 1987;19:62–64. doi: 10.1097/00000637-198707000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Gutowski KA, Mesna GT, Cunningham BL. Saline-filled breast implants: a Plastic Surgery Educational Foundation multicenter outcomes study. Plast Reconstr Surg. 1997;100:1019–1027. doi: 10.1097/00006534-199709001-00028. [DOI] [PubMed] [Google Scholar]
- 17.Collis N, Sharpe DT. Recurrence of subglandular breast implant capsular contracture: anterior versus total capsulectomy. Plast Reconstr Surg. 2000;106:792–797. doi: 10.1097/00006534-200009040-00006. [DOI] [PubMed] [Google Scholar]
- 18.Deva AK, Chang IC. Bacterial biofilms: a cause for accelerated capsular contracture? Aesthet Surg J. 1999;19:130–133. [Google Scholar]
- 19.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. doi: 10.1097/01.PRS.0000054768.14922.44. [DOI] [PubMed] [Google Scholar]
- 20.Cunningham B. The Mentor Core Study on silicone MemoryGel breast implants. Plast Reconstr Surg. 2007;120(Suppl 1):19S–29S; discussion 30S–32S. doi: 10.1097/01.prs.0000286574.88752.04. [DOI] [PubMed] [Google Scholar]
- 21.Adams WP, Jr, Conner WC, Barton FE, Jr, et al. Optimizing breast-pocket irrigation: the post-betadine era. Plast Reconstr Surg. 2001;107:1596–1601. doi: 10.1097/00006534-200105000-00049. [DOI] [PubMed] [Google Scholar]
- 22.Alpert BS, Lalonde DH. MOC-PS(SM) CME article: breast augmentation. Plast Reconstr Surg. 2008;121(4 Suppl):1–7. doi: 10.1097/01.prs.0000305933.31540.5d. [DOI] [PubMed] [Google Scholar]
- 23.Handel N, Cordray T, Gutierrez J, et al. A long-term study of outcomes, complications, and patient satisfaction with breast implants. Plast Reconstr Surg. 2006;117:757–767; discussion 768. doi: 10.1097/01.prs.0000201457.00772.1d. [DOI] [PubMed] [Google Scholar]
- 24.Embrey M, Adams EE, Cunningham B, et al. Factors associated with breast implant rupture: pilot of a retrospective analysis. Aesthetic Plast Surg. 1999;23:207–212. doi: 10.1007/s002669900269. [DOI] [PubMed] [Google Scholar]
- 25.Costagliola M, Atiyeh BS, Rampillon F. An innovative procedure for the treatment of primary and recurrent capsular contracture (CC) following breast augmentation. Aesthet Surg J. 2013;33:1008–1017. doi: 10.1177/1090820X13502035. [DOI] [PubMed] [Google Scholar]
- 26.Caffee HH. Capsule injection for the prevention of contracture. Plast Reconstr Surg. 2002;110:1325–1328. doi: 10.1097/01.PRS.0000025628.86133.EE. [DOI] [PubMed] [Google Scholar]
- 27.Peters W, Smith D, Lugowski S. Failure properties of 352 explanted silicone-gel breast implants. Can J Plast Surg. 1996;4:55–58. [Google Scholar]
- 28.Caffee HH. The influence of silicone bleed on capsule contracture. Ann Plast Surg. 1986;17:284–287. doi: 10.1097/00000637-198610000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Moyer HR, Ghazi BH, Losken A. The effect of silicone gel bleed on capsular contracture: a generational study. Plast Reconstr Surg. 2012;130:793–800. doi: 10.1097/PRS.0b013e318262f174. [DOI] [PubMed] [Google Scholar]
- 30.Burkhardt BR, Fried M, Schnur PL, et al. Capsules, infection, and intraluminal antibiotics. Plast Reconstr Surg. 1981;68:43–49. doi: 10.1097/00006534-198107000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Netscher DT, Weizer G, Wigoda P, et al. Clinical relevance of positive breast periprosthetic cultures without overt infection. Plast Reconstr Surg. 1995;96:1125–1129. doi: 10.1097/00006534-199510000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Adams WP., Jr Discussion: subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:843–844. doi: 10.1097/PRS.0b013e3181e605e9. [DOI] [PubMed] [Google Scholar]
- 33.Jacombs A, Tahir S, Hu H, et al. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plast Reconstr Surg. 2014;133:471e–480e. doi: 10.1097/PRS.0000000000000020. [DOI] [PubMed] [Google Scholar]
- 34.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:835–842. doi: 10.1097/PRS.0b013e3181e3b456. [DOI] [PubMed] [Google Scholar]
- 35.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328. doi: 10.1097/PRS.0b013e3182a3c105. [DOI] [PubMed] [Google Scholar]
- 36.Hu H, Johani K, Almatroudi A, et al. Bacterial biofilm infection detected in breast implant-associated anaplastic large-cell lymphoma. Plast Reconstr Surg. 2016;137:1659–1669. doi: 10.1097/PRS.0000000000002010. [DOI] [PubMed] [Google Scholar]
- 37.Virden CP, Dobke MK, Stein P, et al. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plast Surg. 1992;16:173–179. doi: 10.1007/BF00450610. [DOI] [PubMed] [Google Scholar]
- 38.Thornton JW, Argenta LC, McClatchey KD, et al. Studies on the endogenous flora of the human breast. Ann Plast Surg. 1988;20:39–42. doi: 10.1097/00000637-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 39.Schreml S, Heine N, Eisenmann-Klein M, et al. Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammaplasty. Ann Plast Surg. 2007;59:126–130. doi: 10.1097/01.sap.0000252714.72161.4a. [DOI] [PubMed] [Google Scholar]
- 40.Peters W, Smith D, Fornasier V, et al. An outcome analysis of 100 women after explantation of silicone gel breast implants. Ann Plast Surg. 1997;39:9–19. doi: 10.1097/00000637-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham B, McCue J. Safety and effectiveness of Mentor’s MemoryGel implants at 6 years. Aesthetic Plast Surg. 2009;33:440–444. doi: 10.1007/s00266-009-9364-6. [DOI] [PubMed] [Google Scholar]
- 42.Moyer KE, Ehrlich HP. Capsular contracture after breast reconstruction: collagen fiber orientation and organization. Plast Reconstr Surg. 2013;131:680–685. doi: 10.1097/PRS.0b013e31828189d0. [DOI] [PubMed] [Google Scholar]
- 43.Poppler L, Cohen J, Dolen UC, et al. Histologic, molecular, and clinical evaluation of explanted breast prostheses, capsules, and acellular dermal matrices for bacteria. Aesthet Surg J. 2015;35:653–668. doi: 10.1093/asj/sjv017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffman LR, D’Argenio DA, MacCoss MJ, et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;34:737–751. doi: 10.5301/ijao.5000027. [DOI] [PubMed] [Google Scholar]
- 46.Baker JL, Jr, Chandler ML, LeVier RR. Occurrence and activity of myofibroblasts in human capsular tissue surrounding mammary implants. Plast Reconstr Surg. 1981;68:905–912. doi: 10.1097/00006534-198112000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Hwang K, Sim HB, Huan F, et al. Myofibroblasts and capsular tissue tension in breast capsular contracture. Aesthetic Plast Surg. 2010;34:716–721. doi: 10.1007/s00266-010-9532-8. [DOI] [PubMed] [Google Scholar]
- 48.Adams WP, Jr, Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plast Reconstr Surg. 2006;117:30–36. [PubMed] [Google Scholar]
- 49.Drinane JJ, Bergman RS, Folkers BL, et al. Revisiting triple antibiotic irrigation of breast implant pockets: a placebo-controlled single practice cohort study. Plast Reconstr Surg Glob Open. 2013;1:e55. doi: 10.1097/GOX.0b013e3182aa8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfeiffer P, Jørgensen S, Kristiansen TB, et al. Protective effect of topical antibiotics in breast augmentation. Plast Reconstr Surg. 2009;124:629–634. doi: 10.1097/PRS.0b013e3181addc68. [DOI] [PubMed] [Google Scholar]
- 51.Gylbert L, Asplund O, Berggren A, et al. Preoperative antibiotics and capsular contracture in augmentation mammaplasty. Plast Reconstr Surg. 1990;86:260–267; discussion 268. [PubMed] [Google Scholar]
- 52.Mirzabeigi MN, Mericli AF, Ortlip T, et al. Evaluating the role of postoperative prophylactic antibiotics in primary and secondary breast augmentation: a retrospective review. Aesthet Surg J. 2012;32:61–68. doi: 10.1177/1090820X11430830. [DOI] [PubMed] [Google Scholar]
- 53.Swanson E. Prospective comparative clinical evaluation of 784 consecutive cases of breast augmentation and vertical mammaplasty, performed individually and in combination. Plast Reconstr Surg. 2013;132:30e–45e; discussion 46e–47e. doi: 10.1097/PRS.0b013e3182910b2e. [DOI] [PubMed] [Google Scholar]
- 54.Codner MA, Mejia JD, Locke MB, et al. A 15-year experience with primary breast augmentation. Plast Reconstr Surg. 2011;127:1300–1310. doi: 10.1097/PRS.0b013e318205f41b. [DOI] [PubMed] [Google Scholar]
- 55.Maxwell GP, Van Natta BW, Murphy DK, et al. Natrelle style 410 form-stable silicone breast implants: core study results at 6 years. Aesthet Surg J. 2012;32:709–717. doi: 10.1177/1090820X12452423. [DOI] [PubMed] [Google Scholar]
- 56.Spear SL, Murphy DK Allergan Silicone Breast Implant U.S. Core Clinical Study Group. Natrelle round silicone breast implants: core study results at 10 years. Plast Reconstr Surg. 2014;133:1354–1361. doi: 10.1097/PRS.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stevens WG, Calobrace MB, Harrington J, et al. Nine-year core study data for Sientra’s FDA-approved round and shaped implants with high-strength cohesive silicone gel. Aesthet Surg J. 2016;36:404–416. doi: 10.1093/asj/sjw015. [DOI] [PubMed] [Google Scholar]
- 58.Spear SL, Carter ME, Ganz JC. The correction of capsular contracture by conversion to “dual-plane” positioning: technique and outcomes. Plast Reconstr Surg. 2006;118(7 Suppl):103S–113S; discussion 114S. [PubMed] [Google Scholar]
- 59.Maxwell GP, Gabriel A. The neopectoral pocket in revisionary breast surgery. Aesthet Surg J. 2008;28:463–467. doi: 10.1016/j.asj.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 60.Lee HK, Jin US, Lee YH. Subpectoral and precapsular implant repositioning technique: correction of capsular contracture and implant malposition. Aesthetic Plast Surg. 2011;35:1126–1132. doi: 10.1007/s00266-011-9714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bartsich S, Ascherman JA, Whittier S, et al. The breast: a clean-contaminated surgical site. Aesthet Surg J. 2011;31:802–806. doi: 10.1177/1090820X11417428. [DOI] [PubMed] [Google Scholar]
- 62. Mentor Warranty and Enhanced Warranty for MemoryGel Breast Implants and MemoryShape breast implants. www.mentorwwllc.com/Documents/01012010RevBMGWarranty.pdf. Accessed May 4, 2016.
- 63. Natrelle Warranty. https://www.natrelle.com/warranty. Accessed May 4, 2016.
- 64. Sientra CapCon Program Information Card. http://feelgood.sientra.com/augmentation-reconstruction-resources. Accessed May 5, 2016.
- 65.Barnsley GP, Sigurdson LJ, Barnsley SE. Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2006;117:2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 66.Hammond D, Handel N, Canady J, et al. Impact of surgical approach, together with placement and breast implant texturing on capsular contracture: an analysis of 10-year prospective multicenter data. Plast Reconstr Surg. 2014;134(4S-1):90–91. [Google Scholar]
- 67.Hall-Findlay EJ. Breast implant complication review: double capsules and late seromas. Plast Reconstr Surg. 2011;127:56–66. doi: 10.1097/PRS.0b013e3181fad34d. [DOI] [PubMed] [Google Scholar]
- 68.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. doi: 10.1097/PRS.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 69.Young VL. Guidelines and indications for breast implant capsulectomy. Plast Reconstr Surg. 1998;102:884–891; discussion 892–894. [PubMed] [Google Scholar]
- 70.Sanger JR. Guidelines and indications for breast implant capsulectomy (Discussion). Plast Reconstr Surg. 1998;102:892. [PubMed] [Google Scholar]
- 71.Baran CN, Peker F, Ortak T, et al. A different strategy in the surgical treatment of capsular contracture: leave capsule intact. Aesthetic Plast Surg. 2001;25:427–431. doi: 10.1007/s00266-001-0003-0. [DOI] [PubMed] [Google Scholar]
- 72.Friedman HI, Friedman AC, Carson K. The fate of the fibrous capsule after saline implant removal. Ann Plast Surg. 2001;46:215–221. doi: 10.1097/00000637-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Mofid MM. Acellular dermal matrix in cosmetic breast procedures and capsular contracture. Aesthet Surg J. 2011;31(7 Suppl):77S–84S. doi: 10.1177/1090820X11418201. [DOI] [PubMed] [Google Scholar]
- 74.Brody GS, Latts JR. Capsules, infection, and intraluminal antibiotics (Discussion). Plast Reconstr Surg. 1981;68:48–49. doi: 10.1097/00006534-198107000-00010. [DOI] [PubMed] [Google Scholar]
- 75.Cooper MA. A plea for more science regarding articles on breast implant capsular contracture. Plast Reconstr Surg. 2000;105:809–810. doi: 10.1097/00006534-200002000-00072. [DOI] [PubMed] [Google Scholar]
- 76.Schaub TA, Ahmad J, Rohrich RJ. Capsular contracture with breast implants in the cosmetic patient: saline versus silicone–a systematic review of the literature. Plast Reconstr Surg. 2010;126:2140–2149. doi: 10.1097/PRS.0b013e3181f2b5a2. [DOI] [PubMed] [Google Scholar]
- 77.Rohrich RJ, Reece EM. Breast augmentation today: saline versus silicone–what are the facts? Plast Reconstr Surg. 2008;121:669–672. doi: 10.1097/01.prs.0000298115.96337.72. [DOI] [PubMed] [Google Scholar]
- 78.Swanson E. Prospective outcome study of 225 cases of breast augmentation. Plast Reconstr Surg. 2013;131:1158–1166; discussion 1167–1168. doi: 10.1097/PRS.0b013e318287a0e1. [DOI] [PubMed] [Google Scholar]


