Abstract
Objective
Survival of immune and non-immune cells relies on Axl, a receptor tyrosine kinase, which is implicated in hypertension. Activated T lymphocytes are involved in regulation of high blood pressure. The goal of the study was to investigate the role of Axl in T lymphocyte functions and its contribution to salt-dependent hypertension.
Approach and Results
For the first time we report increased apoptosis in peripheral blood from Axl−/− mice due to lower numbers of white blood cells mostly lymphocytes. In vitro studies showed modest reduction in interferon gamma production in Axl−/− Th1 cells. Axl did not affect basic proliferation capacity or production of interleukin 4 in Axl−/− Th2 cells. However, competitive repopulation of Axl−/− bone marrow or adoptive transfer of Axl−/− CD4+ T cells to Rag1−/− mice showed robust effect of Axl on T lymphocytes expansion in vivo. Adoptive transfer of Axl−/− CD4+ T cells was protective in a later phase of deoxycorticosterone-acetate and salt hypertension. Reduced numbers of CD4+ T cells in circulation and in perivascular adventitia decreased vascular remodeling and increased vascular apoptosis in the late phase of hypertension.
Conclusions
These findings suggest that Axl is critical for survival of T lymphocytes especially during vascular remodeling in hypertension.
Keywords: Axl, lymphocyte, hypertension, apoptosis, vascular remodeling
Introduction
Tyro3, Axl and Mertk (TAM) family of receptor tyrosine kinases has been implicated in regulation of multiple organ systems1. Growth arrest-specific protein 6 (Gas6) and Protein S are potent anticoagulants and ligands for the TAM receptors2, 3. However, mice with genetic deletion of all three TAM receptors showed normal hemostasis1. It was later reported that Gas6 and TAM receptors are involved in regulation of thrombus stabilization4. More severe lymphoproliferative disorder was described in adult TAM triple knockout mice5. The observed autoimmune phenotypes was linked to TAM functions in innate immune cells, e.g. macrophages (Mϕs), dendritic cells (DCs), and Natural Killer (NK) cells6. Initial data in humans showed that Axl is restricted to innate immune cells and upregulation of Axl is predominant in myeloid leukemias7. However, recent reports suggested substantial roles for Axl and Mertk in T and B cell leukemias8-10. Thus, the role of TAM family and Axl in particular on lymphocyte functions in homeostasis and under pathological conditions remain unclear.
A recent human study (3,679 untreated hypertensive individuals; The Framingham Heart Study) showed that apoptosis, T cell activation, and T cell differentiation are enriched in a gene-gene interactions network for a mutation in SH2B adaptor protein 3 (SH2B3) that cause increased blood pressure (BP)11. Not surprisingly, one of the sub-networks of the whole blood transcriptome included the Gas6/Axl pathway in relation to high BP genetic susceptibility11. Previous genetic studies in a Sabra rat model of salt-sensitive hypertension identified Axl as a candidate gene12. Experiments in global knockout mice confirmed pathophysiological role for Gas6 and Axl in development of deoxycorticosterone-acetate (DOCA)-salt hypertension13, 14. Specifically, our group showed that Axl-dependent signals control vascular smooth muscle cells survival and promote vascular remodeling after 6 weeks of DOCA-salt13. We recently reported that Axl expression in bone marrow (BM)-derived cells is responsible for early and late phases of DOCA-salt hypertension15. Adaptive immune cells such as T lymphocytes contribute to vascular dysfunction and DOCA-salt hypertension16. Recent study showed that both CD4+ and CD8+ T cells producing interferon gamma (IFN-γ) are involved in elevation of blood pressure and kidney damage after repeated hypertensive stimuli17. Interestingly, the Th1 response in the retina was also shown to be regulated through Axl/Mertk in mice18. The primary focus of this study was on the role of Axl in T lymphocyte functions and its contribution to salt-dependent hypertension.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Increased apoptosis of white blood cells in peripheral blood from Axl−/− mice
Others and we showed that Axl plays a key role in survival of various cell types19. We double-stained peripheral leukocytes from Axl littermates with AnnexinV and PI (Fig. 1). The lower-right quadrant of each flow chart shows early apoptotic leukocytes, while the upper-right quadrant necrotic (late apoptotic) cells in Axl mice (Fig. 1A). Quantification of flow cytometry demonstrated that Axl−/− had significantly higher apoptotic leukocytes compared to Axl+/+ mice (Fig. 1B). Of note, the AnnexinV+PI+ cell numbers didn’t reach statistical significance between Axl genotypes (Fig. 1B). We found that the levels of apoptosis were the same in BM from Axl littermates (not shown). Evaluation of splenocytes from adult Axl littermates showed similar numbers of CD3+ T or NK1.1+ cells. The frequencies of naive CD62Lhigh/CD44low and CD62Llow/CD44high memory T cells were similar between Axl−/− and Axl+/+ mice. We also found no differences in B and T lymphocytes in spleens and lymph nodes between Axl genotypes (not shown). However, Axl−/− mice had significantly lower (~40%) total white blood cells and lymphocytes count in peripheral blood compared to their Axl+/+ littermates. In addition, Axl deletion resulted in higher CD3+AnnexinV+ and CD3−AnnexinV+ lymphocytes in blood (Fig. I). Hemoglobin levels were slightly reduced in Axl−/− mice. Taken together, our data suggest that Axl controls survival of T lymphocytes in peripheral blood.
Figure 1. Critical role of Axl in leukocytes survival.
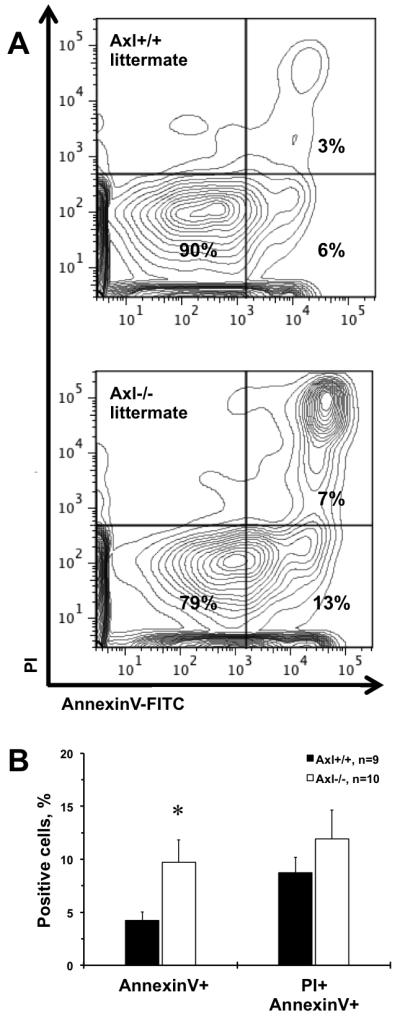
Representative flow data for: Axl+/+ littermate; Axl−/− littermate (A). The lower-right quadrant represents early apoptotic leukocytes (AnnexinV+), while the upper-right quadrant – necrotic cells (PI+AnnexinV+) from Axl mice peripheral blood. B. Quantitation of apoptotic, necrotic and live cells between Axl genotypes. Black bars represent Axl+/+ mice. Open bars represent Axl−/− mice. Values are mean±SEM. *, p<0.05 vs. Axl+/+. n, Number of mice.
Competitive repopulation of bone marrow cells showed less Axl−/− T cells
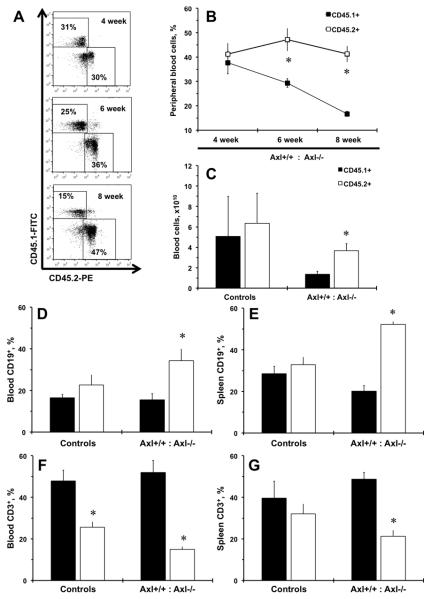
Original report on deletion of three TAM receptors resulted in a severe lymphoproliferative disorder in mice5. In order to explore the role of Axl on T cells in vivo we evaluated homeostatic expansion of Axl+/+ (CD45.1+) vs. Axl−/− (CD45.2+) bone marrow cells in competitive repopulation experiment (Fig. 2). We were able to define Axl genotype origin by dual flow cytometry with anti-CD45.1 and anti-CD45.2 antibodies of white blood cells from peripheral blood from chimeras over 8 weeks time-course (Fig. 2A). Significantly more CD45.2+ than CD45.1+ cells were found at 6 and 8 weeks in chimeras after bone marrow transplant (BMT) suggesting the role of Axl on immune homeostasis (Fig. 2B). At the end of experiment (8 week) we collected peripheral blood and confirmed higher numbers of CD45.2+ cells from Axl−/− mice (Fig. 2C). As shown by original report in triple TAM knockout5, the immune population of the chimeras was shifted towards increase in Axl−/− B lymphocytes (CD45.2+CD19+) both in blood and spleen (Fig. 2D-E). There was dramatic decrease in Axl−/− T cells (CD45.2+CD3+) in blood and spleen from chimeric mice after competitive repopulation (Fig. 2F-G). Lymphocyte frequencies in spleen were similar between Axl genotype controls as we observed in naïve vs. memory T cells (Fig. 2E,G). Analyses of innate immune cells suggested the role for Axl in repopulation of blood monocyte/Mϕ (CD11b+) but did not affect DCs (CD11c+) or NK (NK1.1+) cells (Fig. II). There were no differences in innate immune subsets in spleens from Axl genotype controls or repopulated chimeras (Fig. II). These findings confirmed our hematological data and suggest that Axl is required for peripheral T lymphocyte expansion from BM.
Figure 2. Competitive repopulation of Axl bone marrow cells after bone marrow transplant.
A. Representative flow charts of double stained CD45.1 (Axl+/+) and CD45.2 (Axl−/−) peripheral leukocytes from Axl chimeric mice. B. A time-course of competitive repopulation (CD45.1+ and CD45.2+; 50% and 50%) after 8 weeks of bone marrow transplant (BMT). C. Total numbers of CD45.1+ and CD45.2+ cells in Axl chimeras at 8 weeks after BMT. D. Percentages of CD19+ cells in blood. E. Percentages of CD19+ cells in spleen. F. Percentages of CD3+ cells in blood. G. Percentages of CD3+ cells in spleen. Black bars represent Axl+/+ (CD45.1+) cells. Open bars – Axl−/− (CD45.2+) cells. Values are mean±SEM. *, p<0.05 vs. Axl+/+. Controls, n=4. Axl chimeras, n=10.
Axl regulates CD4+ T cell repopulation in vivo
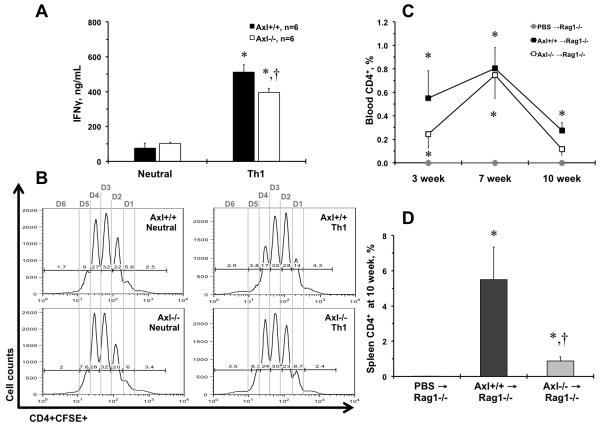
It is possible that the Axl−/− lymphocytes decline could be due to alteration in innate immune cells under competitive repopulation of BM, as was reported in double and triple TAM knockouts before5, 18. However, previous studies suggested that dual deletion of Mertk and Axl affected Th1 polarization in vitro18. In our experiments Axl−/− T cells showed modest but significant decrease in IFN-γ under Th1 polarizing conditions (Fig. 3A). The basic capacity to proliferate in response to T cell receptor stimulation was unaffected in cultured CD4+ T cell from Axl−/− vs. Axl−/− mice, with similar cell number yields (Fig. 3B). Although, there was a slight delay in the initial two cell divisions that was compensated in division 4 in Axl−/− vs. Axl−/− Th1 cells (Fig. 3B; Fig. III). We also found that production of interleukin 4 (IL-4) was similar between Axl genotypes under Th2 polarization (Fig. IV). Thus, Axl showed a modest effect on Th1 polarization of CD4+ T lymphocytes in vitro.
Figure 3. Axl regulates T cell functions.
A. Secretion of interferon gamma (IFN-γ) under Th1 conditions. Black bar represent Axl+/+ cells. Open bar represent Axl−/− cells. *, p<0.05 vs. Neurtal conditions. †, p<0.05 vs. Axl+/+ Th1 cells. n, Number of replicates. B. Representative flow charts of CFSE proliferation of Axl CD4+ T cell in 6 cell divisions (Division 1 – Division 6). n=5. C. A time-course of CD4+ T cell repopulation after adoptive transfers to Rag1−/− mice in blood. Grey circle and grey line show Rag1−/− mice injects with PBS (n=4). Black squares and black line – Axl+/+ CD4+ → Rag1−/− mice (n=6). Open squares and black line – Axl−/− CD4+ → Rag1−/− mice (n=6). D. Percentages of CD4+ T cells in spleen after 10 weeks after adoptive transfer to Rag1−/− mice. Open bar shows PBS → Rag1−/− mice. Dark grey bar – Axl+/+ CD4+ → Rag1−/− mice. Light grey bar – Axl+/+ CD4+ → Rag1−/− mice. Values are mean±SEM. *, p<0.05 vs. PBS → Rag1−/− mice. †, p<0.05 vs. Axl+/+ CD4+ → Rag1−/− mice.
To avoid potential effects of Axl depletion in innate immune compartment, we adoptively transferred Axl−/− or Axl+/+ CD4+ T cells to recombination activating gene 1 knockout (Rag1−/−) mice that lack adaptive immunity but contain Axl-sufficient innate immunity (Fig. 3C-D). In our experiments frequencies of CD4+ T cell were under detection level in peripheral blood from Rag1−/− mice injected with phosphate-buffered saline (PBS; Fig. 3C-D). Adoptive transfer of Axl+/+ T cells resulted in significant increase in percentage of CD4+ cells over 10 weeks of repopulation that peaked at the 7 week (Fig. 3C). Axl−/− CD4+ T cell showed slightly slower repopulation after 3 weeks and non-significant increase at 10 weeks after adoptive transfer to Rag1−/− mice (Fig. 3C). Decreased repopulation of Axl−/− CD4+ T cells in blood was confirmed in spleens from adoptively transferred Rag1−/− mice (Fig. 3D). Taken together, our findings suggest that Axl is important for CD4+ T lymphocyte expansion and/or survival in vivo in the presence of Axl in innate immune compartment.
Axl is important for CD4+ T cell survival and the late phase of DOCA-salt hypertension
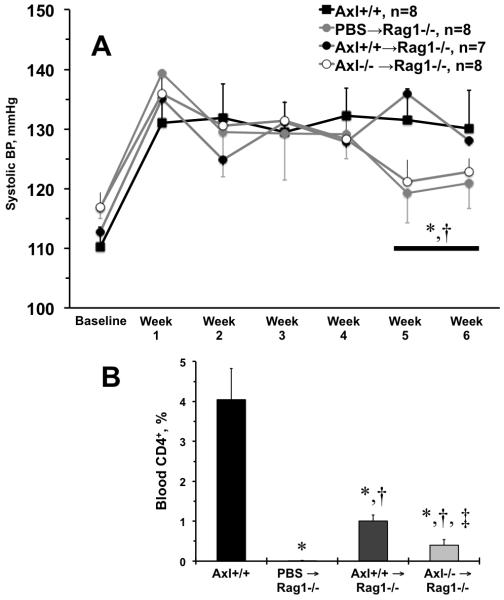
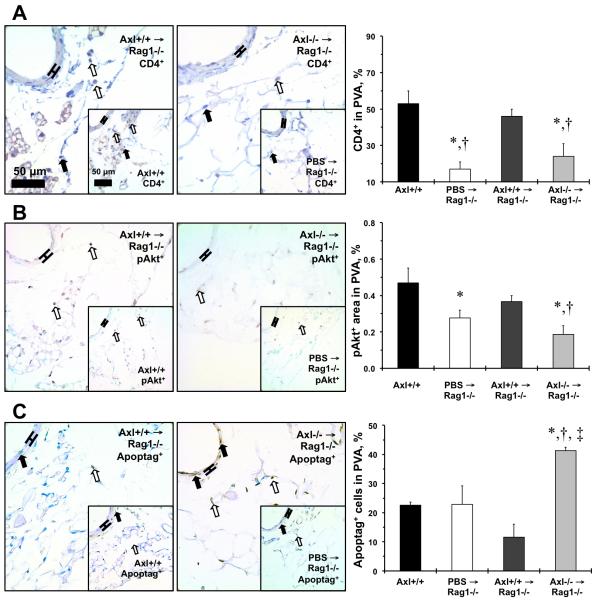
Production of IFN-γ by CD4+ and CD8+ T cells was recently shown to contribute to BP increase and kidney damage after repeated hypertensive stimuli17. Similar to our report in Axl chimeras15, we found reduction in arterial expression of IFN-γ (and Th1-dependent pathways) in Axl−/− mice after 6 weeks of DOCA-salt (not shown). We performed adoptive transfers of CD4+ T cells from Axl+/+ or Axl−/− to Rag1−/− mice and compared BP changes after DOCA-salt to that in Rag1−/− injected with PBS (PBS→Rag1−/−) or Axl+/+ mice (Fig. 4). As expected in this model16, Axl+/+ mice showed significant increase in systolic BP, while PBS→Rag1−/− mice were protected from hypertension after 5-6 weeks of DOCA-salt (Fig. 4A). Adoptive transfer of Axl−/− CD4+ T cells (Axl−/−→Rag1−/−) was also protective for BP increases as compared to Axl+/+→Rag1−/− or Axl+/+ mice after 5-6 weeks of DOCA-salt (Fig. 4A). CD4+ T cells were detected in blood in Axl−/−→Rag1−/− but were significantly reduced compared to Axl+/+→Rag1−/− or Axl+/+ mice after 5 weeks of DOCA-salt (Fig. 4B). Levels of BP reflected reduced medial thickening of mesenteric arteries in PBS→Rag1−/− and Axl−/−→Rag1−/− vs. Axl+/+→Rag1−/− or Axl+/+ mice after 6 weeks of DOCA-salt. We also noted significantly reduced adventitial compartment in arteries from Rag1−/− controls and Axl−/−→Rag1−/− mice. Previous report suggested that majority of T lymphocytes reside within perivascular adventitia (PVA) in hypertension16. As we observed by flow cytometry in peripheral blood, immunohistochemistry mirrored presence of CD4+ in PVA from Axl+/+→Rag1−/− or Axl+/+ mice after 6 weeks of DOCA-salt (Figs. 4B, 5A). In contrast, PBS→Rag1−/− and Axl−/−→Rag1−/− mice showed significantly less CD4+ immunoreactivity in PVA (Fig. 5A). Detected CD4+ staining in PBS→Rag1−/− mice could be due to cross-reactivity of anti-CD4 antibody to endothelial cells in PVA. One of the major pro-survival signals that is controlled by Axl in vasculature is phosphorylated protein kinase B (pAkt) after DOCA-salt13. In PVA we observed significant decrease in pAkt+ in PBS→Rag1−/− compared to Axl+/+, while Axl−/−→Rag1−/− mice had less pAkt relative to Axl+/+→Rag1−/− or Axl+/+ mice after 6 weeks of DOCA-salt (Fig. 5B). Percentage of apoptotic cells in PVA was elevated in Axl−/−→Rag1−/− mice only (Fig. 5C). Thus, Axl is required for presence of CD4+ T cells not only in circulation but also important for vascular inflammatory response, survival, and remodeling in hypertension.
Figure 4. Axl controls circulating CD4+ T cells during late phase of salt-dependent hypertension.
A. Changes in systolic blood pressure (BP) after deoxycorticosterone acetate (DOCA) and salt in mice after adoptive transfer of CD4+ T cells to Rag1−/− mice time-course of. Black squares and black line show Axl+/+ mice. Grey circles and grey line – PBS → Rag1−/− mice. Black squares and grey line – Axl+/+ CD4+ → Rag1−/− mice. Open circles and grey line – Axl−/− CD4+ → Rag1−/− mice. B. Percentage of CD4+ cells in peripheral blood from experimental mice. Black bar shows Axl+/+. Open bar – PBS → Rag1−/− mice. Dark grey bar – Axl+/+ CD4+ → Rag1−/− mice. Light grey bar – Axl−/− CD4+ → Rag1−/− mice. Values are mean±SEM. *, p<0.05 vs. Axl+/+. †, p<0.05 vs. PBS → Rag1−/− mice. ‡, p<0.05 vs. Axl+/+ CD4+ → Rag1−/− mice. n, Number of mice in each group.
Figure 5. Immunohistochemical evaluation of the arteries from mice after CD4+ T cell repopulation and DOCA- salt hypertension.
A. CD4+ cells and quantification in the perivascular adventitia (PVA) of mesenteric artery. B. p-Akt+ cells and quantification in the PVA of mesenteric artery. C. Apoptag+ cells and quantification in the PVA of mesenteric artery. Black bars show Axl+/+. Open bars – PBS → Rag1−/− mice. Dark grey bars – Axl+/+ CD4+ → Rag1−/− mice. Light grey bars – Axl−/− CD4+ → Rag1−/− mice. Values are mean±SEM. *, p<0.05 vs. Axl+/+. †, p<0.05 vs. PBS → Rag1−/− mice. ‡, p<0.05 vs. Axl+/+ CD4+ → Rag1−/− mice. n=3 per each group.
Discussion
For the first time we report that Axl is required for lymphocyte survival in peripheral blood in a mouse. Single deletion of Axl resulted in modest effect on CD4+ T cells production of IFN-γ. A defective expansion of T lymphocytes was evident in competitive repopulation of Axl−/− BM, which was also resulted in an increase in B cells with decline in circulating myeloid cells. Late repopulation of CD4+ T cells was dependent on Axl after adoptive transfer to Rag1−/− mice. Finally, presence of CD4+ T lymphocytes in blood and PVA could explain Axl-dependent effects on vascular remodeling in the late phase of DOCA-salt hypertension.
The TAM family of receptor tyrosine kinases (particularly Mertk and Axl) are shown in tempering the immune response in murine Mϕs, DCs and NK cells6, 20. Genetic defect in the TAM receptors is shown to lead to various autoimmune disorders, including arthritis, experimental autoimmune orchitis, and lupus in mice following a pathological insult5, 21, 22. Studies using single or double gene-knockout mice demonstrated that Mertk or Axl suppresses innate immune responses via inhibiting expression of pro-inflammatory cytokines through transcriptional upregulation of the suppressor of cytokine signaling 1 (SOCS1) and SOCS3 proteins23. In contrast, we have shown that Axl could inhibit SOCS1 and promote signal transducer and activator of transcription 1 (STAT1) signaling leading to immune modulation and activation of smooth muscle cells in vein grafts or after vascular injury24, 25. We also reported that Axl is important for vascular and kidney dysfunction by regulating immune cells in DOCA-salt hypertension13, 15. Collectively, our findings indicate distinct roles for the TAM family (e.g., Axl vs. Mertk) on immune modulation, and Mertk likely regulates immunosuppression observed in single or double TAM knockouts. Mertk is the primary TAM receptor that is involved in the engulfment and efficient clearance of apoptotic cells26, and in an effective resolution of acute inflammation27. Our new data suggest that Axl is required for homeostasis of white blood cells (lymphocytes) in peripheral blood.
Initial findings implied that Axl expression is restricted to normal myeloid cells with preference in myeloid (~60%) vs. lymphoid (2%) leukemias7. Triple TAM knockout mice exhibited enlarged spleens and lymph nodes with a predominance of CD4+ over CD8+ T cells or B cells after 1-2 months of age5. It was concluded that over-activated Mϕs and DCs are responsible for lymphoproliferation and autoimmunity in TAM knockout mice. However, deletion of Mertk exhibited larger spleens compared to lack of Axl or Tyro3 among single or double knockouts5. In fact, clinical studies showed that constitutively active Axl expressed in B cells and promotes survival and proliferation in a chronic B lymphocytic leukemia8. In addition, Mertk is reported to be ectopically express on B and T cells and might be involved in the progression of a variety of human cancers, including T cell acute lymphoblastic leukemia9. Most recent results showed that Mertk and Protein S are expressed in human T cells and upon activation facilitate an autocrine proliferation10. Furthermore, lack of Mertk and Axl in mice affected retinal CD4+ T cell polarization towards Th1, but the underlying mechanism is not clear18. In here we described a novel haematological phenotype in a single Axl−/− suggesting a significant role for Axl for T lymphocyte survival that might affect production of IFN-γ. Our in vivo repopulation studies in chimeras or Rag1−/− mice strongly support the role of Axl in T lymphocyte expansion. The likely mechanism is related to decrease in Axl-dependent activation of Akt, which is one of the key pro-survival signals in Th1 cells in sepsis28. Recent studies argue that a higher proliferation and metabolic activity of T cell29, and its differentiation to Th1 cells is mediated by the phosphoinositide 3-kinase (PI3K)/Akt signaling30. Therefore, Axl-dependent signals could be critical for T cell fitness and might be involved in late phases of vascular pathologies. Slight changes in Axl−/− cell divisions under Th1 polarization in vitro could explain more robust effects of Axl deletion in vivo. A premature mitosis in antigen-specific Th1 cells was shown to be regulated by the balance between c-fos and nuclear protein kinase Wee131. Similarly, recent pharmacological experiments in several cancer cell lines suggested a synergism between Axl inhibition and blocking cell cycle kinases that control mitotic cell entry32. Thus, Axl might regulate long-term activation of T lymphocytes by altering cell division and survival signals.
Increased attention to adaptive immunity revealed a key role for T lymphocytes in regulation of vascular dysfunction and experimental hypertension after Angiotensin II (AngII) or DOCA-salt16. As in a typical immune response, hypertensive stimuli increase isoketals in DCs that promote immune activation of T cells and progression of hypertension33. Further activated DCs enhance T cell proliferation and promote their polarization towards Th1 contributing to high BP in patients with preeclampsia34. There are growing experimental evidence on the primary role for CD8+ T cells in the development of hypertension35. However, a recent report17 showed that both CD4+ and CD8+ T cells-producing IFN-γ increased BP and promoted kidney damage in a repeated hypertensive stimuli model. Likewise, patients with resistant arterial hypertension reported to have increased circulating levels of Th1 cells36. High BP is regulated by multiple genomic loci, which was recently shown in untreated hypertensive individuals11. The authors identified a causal mutation (rs31184504 C/T) within SH2B3 gene. Gene expression signatures from a whole blood-derived RNA in these individuals lead to generation of co-expression networks and the one of the key drivers was HS2B3. Pathway analyses suggested that apoptosis, T cell activation, and T cell differentiation were the most significant in predisposition to hypertension11. The SH2B3 is also known as lymphocyte adaptor protein (LNK) regulates hematopoiesis and lymphocyte differentiation, and implicated in myeloproliferative and inflammatory disorders37. Over-production of IFN-γ was restricted to CD8+ and CD4+ T cells in Lnk−/− mice in experimental hypertension38. The SH2B3-constructed co-expression network of human peripheral blood also included the Gas6/Axl pathway in relation to high BP genetic susceptibility11. We previously showed that deletion of Axl in hematopoietic cells dramatically reduced Mϕs and DCs, and increased accumulation of B cells in the kidney and prevented initiation (1 week) of DOCA-salt hypertension15. The protective effects are due to down-regulation of kidney expression of IFN-γ and Th1-dependent pathways in Axl chimeras15. However, expression of Axl in BM-derived cells was also contributed to BP and vascular remodeling in the late phase (6 week) of DOCA-salt hypertension15. In the current study we showed that Axl is critical for the late survival of CD4+ T cells in peripheral blood and locally via significant increase in pAkt expression and decrease in percentage of apoptotic cells in the PVA. Observed increases in medial apoptosis in Rag1−/− adoptively transferred with Axl−/− CD4+ T cells suggest an important regulatory effect between lymphocytes and smooth muscle cells in late hypertension. On the other hand, an initial vascular response to injury is mostly driven by the immune activation of Axl in smooth muscle cells with modest effect from innate or adaptive immune cells24, 25. Collectively, our findings uncover distinct roles for Axl between vascular and immune cells in cardiovascular diseases (vascular injury vs. hypertension).
Measurements of the hemodynamic parameters using tail-cuff plethysmograhy have some limitations associated with stress. However, many laboratories including ours have validated tail-cuff BP measurements by radiotelemetry method. Our recent study in inbred mouse strains showed significant correlation between our tail-cuff training protocol and radiotelemetry measurements of systolic BP39. We are confident in our results collected by tail-cuff method because of BP levels reflected degree of arterial remodeling obtained by histology across mice after DOCA-salt. It is possible that Axl-dependent signals in innate immune cells and B lymphocytes are also involved in pathogenesis of DOCA-salt hypertension. A recent report on genetic or pharmacological depletion of B cells attenuated Ang II-induced hypertension and vessel remodeling40. Investigation of the TAM family on B lymphocyte function in hypertension warrant additional studies.
In summary, we conclude that Axl is critical for survival of CD4+ T cells not only in circulation but also in vascular inflammatory response and vascular remodeling in hypertension. Our study offers a new therapeutic avenue that might target pro-hypertensive T lymphocytes in peripheral blood and ameliorate T cells at the vascular site.
Supplementary Material
Highlights.
Axl, a receptor tyrosine kinase, is required for peripheral T lymphocyte survival
Axl-mediated CD4+ T cell survival is critical for elevated blood pressure and vascular remodeling in the late phase of hypertension
Acknowledgments
We would like to thank Dr. Jixiang Xia, Janice Gerloff, Kathy Donlon, and Sara Ture for help with animal handling, hematological, and histological evaluation.
Sources of funding
This study was supported in part by NIH grant HL105623 to V.A.K. and by NIAID A1072690 to D.J.F.
Abbreviations
- AngII
Angiotensin II
- BM
Bone marrow
- BMT
Bone marrow transplant
- BP
Blood pressure
- DC
Dendritic cell
- Gas6
Growth arrest-specific protein 6
- NK cell
Natural Killer cell
- TAM
Tyro3, Axl and Mertk family of receptor tyrosine kinases
- Mϕ
Macrophage
- SH2B3
SH2B adaptor protein 3
- DOCA
Deoxycorticosterone-acetate
- IFN-γ
Interferon gamma
- IL-4
Interleukin 4
- LNK
Lymphocyte adaptor protein
- Th1
Type 1 T helper cell
- Th2
Type 2 T helper cell
- pAkt
Phosphorylated protein kinase B
- PBS
Phosphate-buffered saline
- PI3K
Phosphoinositide 3-kinase
- PVA
Perivascular adventitia
- Rag1
Recombination activating gene 1
- SOCS1
Suppressor of cytokine signaling 1
- SOCS3
Suppressor of cytokine signaling 3
- STAT1
Signal transducer and activator of transcription 1
Footnotes
Conflict of Interest Disclosure
None
References
- 1.Lu Q, Gore M, Zhang Q, Camenisch T, Boast S, Casagranda F, Lai C, Skinner MK, Klein R, Matsushima GK, Earp HS, Goff SP, Lemke G. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 2.Stitt TN, Conn G, Gore M, Lai C, Bruno J, Radziejewski C, Mattsson K, Fisher J, Gies DR, Jones PF, et al. The anticoagulation factor protein s and its relative, gas6, are ligands for the tyro 3/axl family of receptor tyrosine kinases. Cell. 1995;80:661–670. doi: 10.1016/0092-8674(95)90520-0. [DOI] [PubMed] [Google Scholar]
- 3.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, Collen D, Dahlback B, Carmeliet P. Deficiency or inhibition of gas6 causes platelet dysfunction and protects mice against thrombosis. Nature medicine. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 4.Angelillo-Scherrer A, Burnier L, Flores N, et al. Role of gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. The Journal of clinical investigation. 2005;115:237–246. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 6.Lemke G, Rothlin CV. Immunobiology of the tam receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neubauer A, Fiebeler A, Graham DK, et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84:1931–1941. [PubMed] [Google Scholar]
- 8.Ghosh AK, Secreto C, Boysen J, Sassoon T, Shanafelt TD, Mukhopadhyay D, Kay NE. The novel receptor tyrosine kinase axl is constitutively active in b-cell chronic lymphocytic leukemia and acts as a docking site of nonreceptor kinases: Implications for therapy. Blood. 2011;117:1928–1937. doi: 10.1182/blood-2010-09-305649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandao LN, Winges A, Christoph S, Sather S, Migdall-Wilson J, Schlegel J, McGranahan A, Gao D, Liang X, Deryckere D, Graham DK. Inhibition of mertk increases chemosensitivity and decreases oncogenic potential in t-cell acute lymphoblastic leukemia. Blood cancer journal. 2013;3:e101. doi: 10.1038/bcj.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabezon R, Carrera-Silva EA, Florez-Grau G, Errasti AE, Calderon-Gomez E, Lozano JJ, Espana C, Ricart E, Panes J, Rothlin CV, Benitez-Ribas D. Mertk as negative regulator of human t cell activation. Journal of leukocyte biology. 2015;97:751–760. doi: 10.1189/jlb.3A0714-334R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huan T, Meng Q, Saleh MA, et al. Integrative network analysis reveals molecular mechanisms of blood pressure regulation. Molecular systems biology. 2015;11:799. doi: 10.15252/msb.20145399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yagil C, Hubner N, Monti J, Schulz H, Sapojnikov M, Luft FC, Ganten D, Yagil Y. Identification of hypertension-related genes through an integrated genomic-transcriptomic approach. Circulation research. 2005;96:617–625. doi: 10.1161/01.RES.0000160556.52369.61. [DOI] [PubMed] [Google Scholar]
- 13.Korshunov VA, Daul M, Massett MP, Berk BC. Axl mediates vascular remodeling induced by deoxycorticosterone acetate salt hypertension. Hypertension. 2007;50:1057–1062. doi: 10.1161/HYPERTENSIONAHA.107.096289. [DOI] [PubMed] [Google Scholar]
- 14.Park JK, Theuer S, Kirsch T, Lindschau C, Klinge U, Heuser A, Plehm R, Todiras M, Carmeliet P, Haller H, Luft FC, Muller DN, Fiebeler A. Growth arrest specific protein 6 participates in doca-induced target-organ damage. Hypertension. 2009;54:359–364. doi: 10.1161/HYPERTENSIONAHA.109.129460. [DOI] [PubMed] [Google Scholar]
- 15.Batchu SN, Hughson A, Gerloff J, Fowell DJ, Korshunov VA. Role of axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension. 2013;62:302–309. doi: 10.1161/HYPERTENSIONAHA.113.01382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. The Journal of experimental medicine. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itani HA, Xiao L, Saleh MA, et al. Cd70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circulation research. 2016;118:1233–1243. doi: 10.1161/CIRCRESAHA.115.308111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye F, Han L, Lu Q, Dong W, Chen Z, Shao H, Kaplan HJ, Li Q. Retinal self-antigen induces a predominantly th1 effector response in axl and mertk double-knockout mice. J Immunol. 2011;187:4178–4186. doi: 10.4049/jimmunol.1101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korshunov VA. Axl-dependent signalling: A clinical update. Clin Sci (Lond) 2012;122:361–368. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothlin CV, Lemke G. Tam receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng T, Zhang Y, Chen Q, Yan K, Han D. Toll-like receptor-mediated inhibition of gas6 and pros expression facilitates inflammatory cytokine production in mouse macrophages. Immunology. 2012;135:40–50. doi: 10.1111/j.1365-2567.2011.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van den Brand BT, Abdollahi-Roodsaz S, Vermeij EA, Bennink MB, Arntz OJ, Rothlin CV, van den Berg WB, van de Loo FA. Therapeutic efficacy of tyro3, axl, and mer tyrosine kinase agonists in collagen-induced arthritis. Arthritis and rheumatism. 2013;65:671–680. doi: 10.1002/art.37786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng S, Hedl M, Abraham C. Tam receptor-dependent regulation of socs3 and mapks contributes to proinflammatory cytokine downregulation following chronic nod2 stimulation of human macrophages. J Immunol. 2015;194:1928–1937. doi: 10.4049/jimmunol.1401933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerloff J, Korshunov VA. Immune modulation of vascular resident cells by axl orchestrates carotid intima-media thickening. The American journal of pathology. 2012;180:2134–2143. doi: 10.1016/j.ajpath.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batchu SN, Xia J, Ko KA, Doyley MM, Abe J, Morrell CN, Korshunov VA. Axl modulates immune activation of smooth muscle cells in vein graft remodeling. American journal of physiology. Heart and circulatory physiology. 2015;309:H1048–1058. doi: 10.1152/ajpheart.00495.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. Phagocytosis and clearance of apoptotic cells is mediated by mer. Nature. 2001;411:207–211. doi: 10.1038/35075603. [DOI] [PubMed] [Google Scholar]
- 27.Wan E, Yeap XY, Dehn S, et al. Enhanced efferocytosis of apoptotic cardiomyocytes through myeloid-epithelial-reproductive tyrosine kinase links acute inflammation resolution to cardiac repair after infarction. Circulation research. 2013;113:1004–1012. doi: 10.1161/CIRCRESAHA.113.301198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bommhardt U, Chang KC, Swanson PE, Wagner TH, Tinsley KW, Karl IE, Hotchkiss RS. Akt decreases lymphocyte apoptosis and improves survival in sepsis. J Immunol. 2004;172:7583–7591. doi: 10.4049/jimmunol.172.12.7583. [DOI] [PubMed] [Google Scholar]
- 29.Ray JP, Staron MM, Shyer JA, Ho PC, Marshall HD, Gray SM, Laidlaw BJ, Araki K, Ahmed R, Kaech SM, Craft J. The interleukin-2-mtorc1 kinase axis defines the signaling, differentiation, and metabolism of t helper 1 and follicular b helper t cells. Immunity. 2015;43:690–702. doi: 10.1016/j.immuni.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arimura Y, Shiroki F, Kuwahara S, Kato H, Dianzani U, Uchiyama T, Yagi J. Akt is a neutral amplifier for th cell differentiation. The Journal of biological chemistry. 2004;279:11408–11416. doi: 10.1074/jbc.M309063200. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki H, Komai K, Ouyang Z, Murata M, Hikasa M, Ohgiri M, Shiozawa S. C-fos/activator protein-1 transactivates wee1 kinase at g(1)/s to inhibit premature mitosis in antigen-specific th1 cells. The EMBO journal. 2001;20:4618–4627. doi: 10.1093/emboj/20.16.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson C, Ye X, Pham T, Lin E, Chan S, McNamara E, Neve RM, Belmont L, Koeppen H, Yauch RL, Ashkenazi A, Settleman J. Axl inhibition sensitizes mesenchymal cancer cells to antimitotic drugs. Cancer research. 2014;74:5878–5890. doi: 10.1158/0008-5472.CAN-14-1009. [DOI] [PubMed] [Google Scholar]
- 33.Kirabo A, Fontana V, de Faria AP, et al. Dc isoketal-modified proteins activate t cells and promote hypertension. The Journal of clinical investigation. 2014;124:4642–4656. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Tao YM, Cheng XY, Zhu TF, Chen ZF, Yao H, Su LX. Dendritic cells derived from preeclampsia patients influence th1/th17 cell differentiation in vitro. International journal of clinical and experimental medicine. 2014;7:5303–5309. [PMC free article] [PubMed] [Google Scholar]
- 35.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG. Oligoclonal cd8+ t cells play a critical role in the development of hypertension. Hypertension. 2014;64:1108–1115. doi: 10.1161/HYPERTENSIONAHA.114.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magen E, Feldman A, Cohen Z, Alon DB, Minz E, Chernyavsky A, Linov L, Mishal J, Schlezinger M, Sthoeger Z. Circulating endothelial progenitor cells, th1/th2/th17-related cytokines, and endothelial dysfunction in resistant hypertension. The American journal of the medical sciences. 2010;339:117–122. doi: 10.1097/MAJ.0b013e3181c6a968. [DOI] [PubMed] [Google Scholar]
- 37.Devalliere J, Charreau B. The adaptor lnk (sh2b3): An emerging regulator in vascular cells and a link between immune and inflammatory signaling. Biochemical pharmacology. 2011;82:1391–1402. doi: 10.1016/j.bcp.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 38.Saleh MA, McMaster WG, Wu J, et al. Lymphocyte adaptor protein lnk deficiency exacerbates hypertension and end-organ inflammation. The Journal of clinical investigation. 2015;125:1189–1202. doi: 10.1172/JCI76327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batchu SN, Smolock EM, Dyachenko IA, Murashev AN, Korshunov VA. Autonomic dysfunction determines stress-induced cardiovascular and immune complications in mice. J Am Heart Assoc. 2015;4(5):e001952. doi: 10.1161/JAHA.115.001952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan CT, Sobey CG, Lieu M, et al. Obligatory role for b cells in the development of angiotensin ii-dependent hypertension. Hypertension. 2015;66:1023–1033. doi: 10.1161/HYPERTENSIONAHA.115.05779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.