SUMMARY
Visceral leishmaniasis is a systemic and chronic disease and dogs are the main reservoir of the etiologic agent, Leishmania infantum (syn L. chagasi). A serological and molecular investigation of canine visceral leishmaniasis (CVL) was performed in the municipality of Alfenas, located in the southern region of Minas Gerais, where the disease is not endemic. Samples from 87 dogs were submitted to serological tests including the Dual Path Platform (DPP(r) ) CVL Bio-Manguinhos rapid test, an in-house enzyme-linked immunosorbent assay (ELISA) and an immunofluorescence antibody test (IFAT), as well as molecular techniques such as a conventional polymerase chain reaction (PCR) with the RV1/RV2 primers and a quantitative PCR (qPCR) with the LinJ31, Ldon and DNApol primers. Of the 87 serum samples, eight (9.2%) were positive for Leishmania using the DPP rapid test, but only four (4.6%) were confirmed by ELISA and two (2.3%) by IFAT. In these two serologically confirmed cases, spleen and liver samples were positive by all the employed molecular and parasitological procedures performed on spleen samples. When whole blood samples were used in the molecular assays, two samples (2.3%) were positive only by qPCR. DNA extracted and amplified from the spleens of seropositive dogs was sequenced, showing 100% of similarity with the Leishmania infantum (syn L. chagasi) sequence. Thus, the first cases of CVL have been confirmed in the Alfenas region, suggesting the importance of canine surveys in non-endemic municipalities for CVL to monitor disease progression and to prevent outbreaks.
KEYWORDS: Canine visceral leishmaniasis, Leishmania infantum, Serology, Polymerase chain reaction, Sequencing
INTRODUCTION
Leishmaniasis is caused by protozoa parasites from over 20 species of Leishmania, which are mainly transmitted by the bite of infected sand flies. Among the three main forms of leishmaniasis, i.e. cutaneous, mucocutaneous, and visceral, the latter is the most serious form of the disease 1 . The World Health Organization (WHO) estimates 200,000 to 400,000 new human cases of visceral leishmaniasis (VL) worldwide each year, and if not treated, VL may lead to death in a large number of cases 1 . In the Americas, VL is considered a zoonosis with a considerable impact on public health care 2 .
Canine visceral leishmaniasis (CVL) is an important parasitic disease because of its clinical characteristics, transmissibility and zoonotic potential 3 . Canine infection usually precedes the human one, and its presence in areas not previously considered endemic 4 has led to a revised national strategy that includes surveillance and preventive measures in areas considered at risk of infection 5 . In spite of all the control measures that have been taken in Brazil, the disease has spread to new areas 6 , 7 , 8 and it has now become a nationwide issue. In addition, human and canine cases have increased significantly in urban areas, even with the culling of VL seropositive dogs 6 .
The domestic dog (Canis familiaris) is considered the main urban reservoir of Leishmania infantum (syn L. chagasi), since it presents intense cutaneous parasitism and close contact with humans. In addition, the dog serves as a food source, attracting the vector, which is the phlebotomine sand fly species Lutzomyia longipalpis in the Americas, facilitating the transmission between animals and humans 9 . The clinical manifestations of VL in dogs are not specific, mimicking several other diseases. Ratings for different stages of the canine disease have been established based on clinical signs, serological diagnosis and laboratory profiles 10 .
Parasitological, serological and molecular methods can be used for the case definition of CVL 11 . The Brazilian Health Ministry recommends the DPP(r) (Dual Path Platform) CVL Bio-Manguinhos rapid test as a screening method and the enzyme-linked immunosorbent Assay (ELISA) Bio-Manguinhos (r) test as the confirmatory test in canine cases 12 .
In 1913, Migone recorded the first case of VL in Brazil, in the necropsy material of a patient from Mato Grosso State. Subsequently, new autochthonous human cases were described in the northern and northeastern regions of Brazil. L. longipalpis was described as the disease vector and the first cases of canine infection were discovered 6 .
VL as a zoonosis has a primarily rural character. However, the transmission of the disease has been described in several urban areas from different municipalities. The disease has undergone important changes in transmission patterns, having been described initially in rural and peri-urban environments, and more recently in urban areas such as Rio de Janeiro, Corumbá, Belo Horizonte, Araçatuba, Palmas, Três Lagoas, and Campo Grande, among other Brazilian cities. Currently, the disease has been registered in 19 out of 27 Brazilian States, with approximately 1,600 municipalities having autochthonous transmission 6 . Another important aspect is the frequent movement of individuals and their pets, contributing to the disease spread to non-endemic areas 13 .
The first cases of VL in Minas Gerais State were reported around 1940, in the northern region, especially in rural areas. The first description of a human patient occurred in 1953, in the city of Itanhomi 14 , located in the Valley of Rio Doce, in the northeastern region of the state. In 1989, the death of a two-year-old child from VL was reported in Belo Horizonte, the capital of the state 15 , and in the same study, the presence of infected dogs and of the vector L. longipalpis was verified in various districts of the city. Other cases were described in Governador Valadares 16 , Montes Claros 17 and Divinópolis 18 , municipalities in the northern region of the state, and in Juiz de Fora 19 and Lavras 20 , located in the southern region of the state. Currently, the region of Belo Horizonte, the state capital, has the highest incidence of VL and the higher risk of disease transmission 21 , 22 .
The control strategies for VL are to target the canine reservoir, using vector insecticides with a repellent effect (as well as euthanasia of infected dogs), and to improve diagnosis and appropriate treatment of reported human cases. However, these measures have not shown effectiveness in reducing the incidence of the disease. In this way, the standard methodology for surveillance should be based on a better definition of transmission in risk areas. The approach may involve the incorporation of surveillance activities in municipalities and states that are not endemic for this disease, aiming to avoid or to minimize the impact in transmission areas 6 .
Thus, the use of serological and molecular methods for the identification of VL-positive dogs seems to be relevant in the Alfenas region, where the present study was carried out. It is noteworthy that the study was conducted in a region considered not endemic for VL, located in the southern region of Minas Gerais State (21° 25'S, 45° 56'W). Since this region is characterized by the presence of a transient population composed of students and workers from various regions of Brazil, including some areas that are endemic for VL, this study may be relevant to obtain further data related to the spread of the disease to areas with no known cases of VL.
MATERIAL AND METHODS
Samples from 87 dogs were included in this study. The origin of the 64 animals provided by the municipal kennel in Alfenas (21° 26' S, 45° 57' W) was unknown. Among the 23 dogs provided by veterinary clinics located in Alfenas, only three were living in the same city; 20 were from other cities near Alfenas: one from Fama (21° 26 S, 45° 48 W), one from Poços de Caldas (21° 47' S, 46° 34' W), and 18 from Paraguaçu (21º 32' S, 45º 44' W). Serum samples were submitted to serological tests and whole blood samples to molecular techniques (conventional PCR and qPCR). Spleen and liver samples were collected from euthanized animals, which had positive serology and qPCR to leishmaniasis, and submitted to parasitological and molecular detection. The animals were evaluated regarding clinical signs such as the presence of onychogryphosis, skin alterations, eye lesions, lymphadenopathy, hepatomegaly and splenomegaly, as well as for chronic phase changes such as locomotor alterations mainly due to the deposition of immune complexes 23 , 10 .
The Ethics Committee on the Use of Animals (ECUA/UNIFAL-MG) under the registration number nº 507/2013 approved all the procedures performed with the animals.
Serological methods
DPP(r) CVL rapid test
The occurrence of canine visceral leishmaniasis (CVL) was investigated by means of the immunochromatographic Dual-Path Platform (DPP(r)) rapid test manufactured by Bio-Manguinhos, Rio de Janeiro, Brazil, following the protocol recommended by the manufacturer.
ELISA
Dogs were subjected to serological diagnosis using an in-house enzyme-linked immunosorbent assay (ELISA) containing a crude L. infantum antigen and anti-canine IgG conjugated to alkaline phosphatase, as described previously 24 . Each day, the absorbance values of eight negative sera from healthy dogs, living in non-endemic areas for CVL, were submitted to ELISA, and the average of their absorbance values plus two standard deviations was established as the cut-off. The samples with absorbance values lower or higher than the cut-off were considered to be negative or positive, respectively.
IFAT
For antibody detection by an immunofluorescence antibody test (IFAT), promastigotes of L. infantum were used as the antigen, and anti-dog IgG conjugated to fluorescein was used to reveal the antibodies, as described previously 25 . Animals were considered as positive when the serum presented a titer higher than 1:80.
Molecular detection
Conventional PCR
DNA extraction from whole blood samples was performed by the phenol-chloroform-isoamyl alcohol extraction protocol 26 with previously described changes 27 . The same protocol was used to extract DNA from spleen and liver samples collected of euthanized animals. DNA obtained from the promastigotes of L. infantum (MHOM/BR/72/LD/strain 46) was used as the positive control. The concentration of DNA samples was estimated using the Nanodrop 2000 (Thermo Scientific) and DNA concentrations were adjusted to 250 ng/µL when samples had higher concentrations.
Amplification
Conventional PCR was conducted using the primers RV1-RV2 (5'-CTT TTC TGG TCC CGC GGG TAG-G3' and 5'-CCA CCT GGC CTA TTT TAC ACC-A3'), a molecular marker used to amplify a 145 bp fragment from a variable region of the kDNA minicircle (LT1 region) specific for Leishmania donovani 27 , 28 , 29 . The PCR conditions were set as previously described 24 . To assess the success of DNA extraction and PCR inhibitors, canine samples were assayed using the GAPDH-4 primer set (5'-AGG CTG AGA ACG GGA AAC TT-3' and 5'-ATT AAG TTG GGG CAG GGA CT-3'), used to amplify a 911-bp fragment of the canine glyceraldehyde-3-phosphate dehydrogenase 30 . These reactions were performed using the same protocol described to the RV1-RV2 marker. All the positive PCR results for the canine GAPDH-4 confirmed the good quality of the dog DNA extractions. PCR products were electrophoresed in a 2% agarose gel and stained with ethidium bromide. The DNA fragments were visualized under UV lights. Fragment molecular sizes were estimated based on comparisons with a 100 bp ladder.
qPCR
Regarding qPCR, doubled labeled-TaqMan hydrolysis probes were used, with the primer sets LinJ31 24 , Ldon and DNAPol 31 , as described in Table 1. The reactions were performed on an ABI 7500 Real Time PCR System (Applied Biosystems), at a final volume of 20 µL per reaction. The volume contained 3 µL of DNA (samples or controls) along with 10 µL of 2x TaqMan Universal PCR Master Mix, 1 µL of a mix of the relevant forward and reverse primers at 18 µM, the TaqMan probe, labeled with FAM or HEX and NFQ as a quencher at 5 µM. Two negative controls and one positive control were added to the assay. The amplifications were performed as previously described 24 .
Table 1. Targets and sequences of primers used in the qPCR.
| Primer | Target | Sequence |
| LinJ31 | mRNA hypothetical protein | F: 5'CCG CGT GCC TGT CG3' R 5' CCC ACA CAA GCG GGA ACT3' FAM-5' CCT CCT TGG ACT TTG C 3' |
| Ldon | Actin region | F: 3'AAG TGC GAC ATT GAT GTG CGC'5; R: 3'AAG GTT GAG GAA CAT GGT CGA C'5 HEX-5'CC GGA CAG CAC GAT GTT CCC GTA C3' |
| DNApol | Polimerase DNA gene | F: 5'TGT CGCT TGC AGA CCA GATG-3'; R: 5'GCA TCG CAG GTG TGA GCAC 3' FAM-5'CAG CAA CAA CTT CGA GCC TGG CAC C 3' TAMRA |
Sequencing
Samples that were positive for L. infantum DNA were sequenced using the primer pair LGITSF2: 3'-GCA TGC CAT ATT CTC AGT GTC-5' and LGITSR2: 3'-GGC CAA CGC GAA GTT GAA TTC-5' was used for the reaction to identify the expected fragment of 418 bp related to the fragment of ITS2 (rRNA internal transcribed spacer 2) of the Leishmania genus 32 . BioEdit Sequence Alignment Editor version 8.1 (available from: http://bioedit.software.informer.com/) was used for the analysis and manual editing of the genetic sequences.
Parasitological detection
Tissue fragments were mounted on glass slides, dried and then stained with Instant Prov kit (Newprov, Pinhais, PR, Brazil), according to the manufacturer's instructions. Then, the slides were examined under an optical microscope at 100x magnification to search for amastigote forms of the parasite 33 .
Phlebotomine survey
A survey of phlebotomines was performed in the area of the municipal kennel and in the vicinity of the residences where VL-positive dogs were found in Paraguaçu. Catches were performed from 6:00 pm to 6:00 am, using modified CDC automatic light traps (CDC-M) 34 . Collected specimens were transported to the Microorganism Molecular Biology Laboratory of the Universidade Federal de Alfenas (UNIFAL) for preliminary screening.
RESULTS
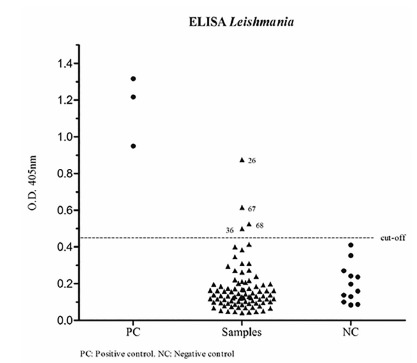
Only two of the studied animals, identified with numbers 67 and 68, showed clinical signs related to CVL. These dogs were treated at a veterinary clinic in Alfenas, but they were from Paraguaçu, Minas Gerais State, Brazil, a municipality close to Alfenas, the town where the study was conducted. The following clinical signs were observed in the affected dogs: onychogryphosis, skin flaking, conjunctivitis, hyperkeratinization, generalized lymphadenopathy, alopecia in the periocular region, tip of ears and tail, difficulty moving, polyarthritis and splenomegaly. Eight out of 87 (9.2%) studied serum samples were positive by the DPP(r) CVL rapid test (numbers 24, 26, 39, 44, 48, 56, 67 and 68). Nevertheless, 83 samples (95.4%) were negative, and only four (4.6%) were positive (numbers 26, 36, 67 and 68) by the ELISA test, as shown in Figure 1. Using the IFAT, two out of 87 studied samples (2.3%) were positive with titers of 1/640 (number 67) and 1/1,280 (number 68); 85 samples (97.7%) were negative.
Fig. 1. Distribution of the results of 87 serum samples obtained by ELISA Leishmania.
The conventional PCR for the detection of DNA from L. infantum was performed after DNA extraction from whole blood samples of 87 dogs. The RV1/RV2 primers were used for this assay, and the results were negative for all the samples. Nevertheless, as a control procedure, the results for the constitutively expressed gene GAPDH-4 were positive in all the 87 samples assessed by the conventional PCR. For the liver and spleen samples of the LV-positive dogs (numbers 67 and 68), the results of the RV1/RV2 conventional PCR were positive.
All the 64 samples from the municipal kennel dogs were negative for Leishmania spp. when the LinJ31 primers were used in the qPCR. Yet, out of 23 dogs from the veterinary clinics of Alfenas, two (numbers 67 and 68) showed positive qPCR results in blood, spleen and liver samples. In addition, blood samples from six seropositive dogs (numbers 24, 26, 36, 39, 44, 48 and 56) were submitted to qPCR, using the Ldon and DNApol primers, but results were negative. However, the blood, spleen and liver samples of dogs 67 and 68 were positive using the Ldon and DNApol primers.
Amplification products were submitted to DNA sequencing using the LGITSF2/LGITSR2 primers, which amplify an rRNA ITS2 fragment from Leishmania spp., to identify the species based on the molecular weight and the sequence composition of the analyzed fragment 32 . The nucleotide sequence was analyzed and edited using the BioEdit Sequence Alignment Editor, version 8.1. This sequence was compared with the one deposited in GenBank by means of BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and showed complete 100% of similarity with L. infantum (syn L. chagasi) (access AJ000304.1).
Results of the serological and molecular assays of nine dogs that had at least one positive result by the described methods are summarized in Table 2. In the parasitological assessments, the amastigote form was observed in spleen samples from dogs 67 and 68 (Fig. 2).
Table 2. Results of serological and molecular methods, according to the type of sample (serum, blood, liver, spleen) from nine dogs with at least one positive result.
| Dog number | Serological methods | Molecular methods | |||||||||
| Serum | Blood | Liver | Spleen | Blood | Liver | Spleen | |||||
| DPP(r) | ELISA | IFAT | Conventional PCR | qPCR* | |||||||
| 24 | + | + | - | - | ND | ND | - | ND | ND | ||
| 26 | + | - | - | - | ND | ND | - | ND | ND | ||
| 36 | - | + | - | - | ND | ND | - | ND | ND | ||
| 39 | + | - | - | - | ND | ND | - | ND | ND | ||
| 44 | + | - | - | - | ND | ND | - | ND | ND | ||
| 48 | + | - | - | - | ND | ND | - | ND | ND | ||
| 56 | + | - | - | - | ND | ND | - | ND | ND | ||
| 67 | + | + | + | - | + | + | + | + | + | ||
| 68 | + | + | + | - | + | + | + | + | + | ||
ND: No done; *To qPCR was used LinJ31, Ldon and DNApol primers.
Fig. 2. Amastigotes forms observed after Instant Prov kit staining of the imprinting of spleen.

A phlebotomine survey was performed in the area of the Alfenas municipal kennel and in the city of Paraguaçu, where the CVL-positive dogs were found. However, no Lutzomyia species were identified among the captured insects during the studied period.
DISCUSSION
Several studies have shown that dogs with subclinical infections by L. infantum have a low parasitic load in tissues, mainly in the skin. With the disease progression, the parasite can be found in other organs, such as the spleen, where it induces cellular activation soon after infection, considered the key factor responsible for the parasite multiplication 35 . This immune response profile varies according to the individual's immune system 35 . In this way, the antibody production may depend on the phase of infection and the degree of immune response, while the molecular techniques and their detection limits will depend on the type of sample used for DNA analysis 35 . This may have occurred with the samples 26, 67 and 68 in the present study. These samples presented positive results by serology but they were negative by the conventional PCR. Regarding the sample 26, a second attempt was made to collect material in order to clarify the case. However, this animal died as a result of a dog fight. Currently, the Brazilian Ministry of Health program for visceral leishmaniasis control recommends that serological surveys of canine cases are performed by screening using the DPP(r) CVL Bio-Manguinhos rapid test and an ELISA for confirmation 6 . When a dog is positive by these serological tests, euthanasia is indicated as a control measure to prevent the disease spread to humans and to other animals 6 . In Brazil, VL shows singular geographic, climate and social aspects because of its wide geographical distribution, involving the northern, northeastern, midwest and southeastern regions. In the 1990s, about 90% of VL notified cases occurred in the northeastern region. Nevertheless, in the past 10 years, epidemiological data have demonstrated the urbanization of VL 6 , and despite the control measures that were put in place, this disease has expanded to the whole country.
This disease has spread to several areas previously considered non-endemic, for example the cases reported in Rio de Janeiro State 36 and CVL outbreaks in the countryside of São Paulo State 37 . These facts justify performing surveillance surveys to prevent the spread of the disease to other animals and humans. These measures are already being recommended by the Ministry of Health 6 .
In the present study, it was possible to detect L. infantum infection by conventional PCR only in the spleen and liver samples from dogs 67 and 68. Nevertheless, by qPCR, the results were positive for all the tested samples of both dogs. These results can be attributed to the higher sensitivity of qPCR when compared with the conventional PCR; the former is able to detect as few as 0.001 of a parasite per reaction, corresponding to about 0.2 parasites/ mL of the sample 38 .
The detection of anti-Leishmania antibodies by serological tests and the confirmation by conventional PCR and qPCR, matched with identification by sequencing showing full compatibility with L. infantum, point to the first two cases of CVL in the Alfenas region, in dogs from Paraguaçu. According to owner information, one of the mentioned dogs was never in a CVL endemic area, which confirms this dog as the first autochthonous CVL case in this municipality. The second dog was collected from the street, hampering the identification of the appropriate origin of this case. However, the two dogs lived together for a long time. This finding of VL positive dogs in the veterinary clinics of Alfenas suggest a flow of VL positive animals into the municipality as the result the movement of people, who are often accompanied by their pets.
The first case of CVL was found in 1999 in another city in the southern region of Minas Gerais State, Bom Sucesso (21°02' S, 44°45'W, about 150 km from Alfenas). In the following years, serological surveys for CVL were performed, and positive results were detected in the dogs of this city as well as dogs from other cities. A phlebotominic survey was performed, but Lutzomyia-carrying insects were not found in this municipality 39 , 40 . In the city of Lavras (21°14'S, 44°59'W, about 140 km from Alfenas), in an area of raising chicks 20 , specimens of L. longipalpis were found as well as dogs diagnosed with CVL. Cases of CVL have also been reported in another study carried out in Juiz de Fora 19 (21°14' S, 42°14'W, about 380 km from Alfenas and 240 km from Lavras), another city of the southern region of Minas Gerais, close to Rio de Janeiro State.
The southern region of Minas Gerais State has a great number of coffee plantations in rural as well as peri-urban areas, often close to an inhabited environment. A study in the town of Machado (21°39'S, 45°55'W, about 30 km from Alfenas) performed a phlebotominic survey of a number of coffee plantations and found specimens related to the transmission of American tegumentary leishmaniasis (ATL) 41 . In this way, the presence of phlebotomines in this kind of plantation, which are abundant in the area, increases the risk of Leishmania transmission to humans and animals. Studies on the phlebotominic fauna in the town of Conceição Aparecida (21°06'S, 46°13'W, about 50 km from Alfenas) have shown vector species for ATL transmission and specimens of L. longipalpis 42 . However, Leishmania infected humans or dogs were not found in Conceição Aparecida. A sandfly survey was conducted in Espírito Santo do Pinhal (22º06'S, 46º26'W, about 170 km from Alfenas), a city located in the state of São Paulo close by the cities of Southern Minas Gerais State. In this study, several specimens of CVL and ATL vectors were found in urban and rural areas. It sounded an alert to the public health services in relation to the risk of transmission to humans, since L. infantum positive dogs were found in this city 43 .
In the present study, a positivity rate of approximately 10% was observed by CVL serology (nine positive samples from a total of 87 dogs); however, detecting the two positive cases was only possible through the utilization of different methodologies, especially molecular methods and sequencing. Thus, when analyzing the findings of studies performed in nearby towns, it is possible to assess the spread of the disease to the southern region of Minas Gerais State, a region that has been considered non-endemic, with no verified cases of VL. In addition, the present results demonstrate the contribution of molecular techniques as a more efficient diagnostic tool compared to serological methods, as recommended for the diagnosis of canine cases in VL control programs.
This study confirmed the growth of CVL endemic areas in the southern and southeastern regions of Minas Gerais, with the presence of infected dogs and vectors for CVL transmission. These areas are associated with the CVL endemic areas of the neighboring states of São Paulo and Rio de Janeiro. This fact may facilitate the flow of CVL vectors and reservoirs. The species L. longipalpis is considered to be the main vector for CVL transmission, and is prevalent in endemic areas. This species is highly adaptable to the environmental conditions of inhabited urban areas 44 . In this study, specimens of L. longipalpis or other phlebotomine species were not found; thus, the mechanism of CVL transmission remains unclear. Further surveys must be carried out to confirm the absence of phlebotomines in the studied areas, since the collection period may have been short for this observation. Moreover, the participation of ticks in the infectious cycle of CVL should also be considered 45 .
CONCLUSION
This study demonstrated that, during the studied period, dogs from Paraguaçu that were treated at veterinary clinics in Alfenas were found to be positive for CVL by serological, molecular and parasitological methods, as well as by sequencing for L. infantum. Nevertheless, phlebotomines were not found in the studied area. LV has undergone considerable expansion in recent years, which justifies initiating epidemiological surveillance using serological and molecular tests in non-endemic areas as way of tracking disease spread and controlling CVL outbreaks.
ACKNOWLEDGMENTS
Fellowship Capes number 1418863, AUXPE PNPD number 2391/2011, PVNS 1277021, technical support municipal Kennel and Department of Epidemiological Surveillance and Health of the Municipality of Alfenas/MG, Brazil.
REFERENCES
- 1.World Health Organization . Leishmaniasis. 2015. who.int/mediacentre/factsheets/fs375/en [Google Scholar]
- 2.Dantas-Torres F, Brandão-Filho SP. Visceral leishmaniasis in Brazil revisiting paradigms of epidemiology and control. Rev Inst Med Trop Sao Paulo. 2006;48:151–156. doi: 10.1590/s0036-46652006000300007. [DOI] [PubMed] [Google Scholar]
- 3.Gavgani AS, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children a matched-cluster randomised trial. Lancet. 2002;360:374–379. doi: 10.1016/s0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- 4.Barão SC, de Fonseca Camargo-Neves VL, Resende MR, da Silva LJ. Human asymptomatic infection in visceral leishmaniasis a seroprevalence study in an urban area of low endemicity. Preliminary results. Am J Trop Med Hyg. 2007;77:1051–1053. [PubMed] [Google Scholar]
- 5.Steindel M, Menin A, Evangelista T, Stoco PH, Marlow MA, Fleith RC. Outbreak of autochthonous canine visceral leishmaniasis in Santa Catarina, Brazil. Pesq Vet Bras. 2013;33:490–496. [Google Scholar]
- 6.Brasil. Ministério da Saúde . Manual de vigilância e controle da leishmaniose visceral. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 7.Oliveira CD, Assunção RM, Reis IA, Proietti FA. Spatial distribution of human and canine visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brasil, 1994-1997. Cad Saude Publica. 2001;17:1231–1239. doi: 10.1590/s0102-311x2001000500023. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira CD, Morais MH, Machado-Coelho GL. Visceral leishmaniasis in large Brazilian cities challenges for control. Cad Saude Publica. 2008;24:2953–2958. doi: 10.1590/s0102-311x2008001200026. [DOI] [PubMed] [Google Scholar]
- 9.Camargo-Neves VL. Manual de vigilância e controle da leishmaniose visceral americana do Estado de São Paulo. São Paulo: Secretaria da Saúde; 2006. [Google Scholar]
- 10.Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86–86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melo MN. Leishmaniose visceral no Brasil desafios e perspectivas. Rev Bras Parasitol Vet. 2004;23(1):41–45. [Google Scholar]
- 12.Ministério da Saúde . Secretaria de Vigilância em Saúde. Esclarecimentos sobre substituição do protocolo diagnóstico da leihsmaniose visceral canina (LVC) Brasilia: Ministério da Saúde; 2011. [Google Scholar]
- 13.Forattini OP. Ecologia, epidemiologia e sociedade. São Paulo: EDUSP; 1992. [Google Scholar]
- 14.Magalhães PA, Mayrink W, Costa CA, Melo MN, Dias M, Batista SM. Calazar na zona do Rio Doce - Minas Gerais resultados de medidas profiláticas. Rev Inst Med Trop Sao Paulo. 1980;22:197–202. [PubMed] [Google Scholar]
- 15.Genaro O, da Costa CA, Williams P, Silva JE, Rocha NM, Lima SL. Ocorrência de calazar em área urbana da grande Belo Horizonte, MG. Rev Soc Bras Med Trop. 1990;23:121–121. doi: 10.1590/s0037-86821990000200011. [DOI] [PubMed] [Google Scholar]
- 16.Barata RA, Peixoto JC, Tanure A, Gomes ME, Apolinário EC, Bodevan EC. Epidemiology of visceral leishmaniasis in a reemerging focus of intense transmission in Minas Gerais State, Brazil. Biomed Res Int. 2013;2013:405083–405083. doi: 10.1155/2013/405083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prado PF, Rocha MF, Sousa JF, Caldeira DI, Paz GF, Dias ES. Epidemiological aspects of human and canine visceral leishmaniasis in Montes Claros, State of Minas Gerais, Brazil, between 2007 and 2009. Rev Soc Bras Med Trop. 2011;44:561–566. doi: 10.1590/s0037-86822011000500006. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira-Neto RG, da Silva ES, Nascimento RA, Belo VS, Cd de Oliveira, Pinheiro LC. Canine visceral leishmaniasis in an urban setting of Southeastern Brazil an ecological study involving spatial analysis. Parasit Vectors. 2014;7:485–485. doi: 10.1186/s13071-014-0485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castro-Júnior JG, Freire ML, Campos SP, Scopel KK, Porrozzi R, Da Silva ED. Evidence of Leishmania (Leishmania) infantum infection in dogs from Juiz de Fora, Minas Gerais State, Brazil, based on immunochromatographic dual-path platform (DPP(r)) and PCR assays. Rev Inst Med Trop Sao Paulo. 2014;56:225–229. doi: 10.1590/S0036-46652014000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barçante TA, Botelho MC, Freitas HF, Soares GD, Barçante JM. Note First report of the main vector of visceral leishmaniasis in America, Lutzomyia longipalpis (Lutz Neiva, 1912) (Diptera Psychodidae : Phlebotominae), in southern Minas Gerais State, Brazil. J Vector Ecol. 2015;40:412–414. doi: 10.1111/jvec.12182. [DOI] [PubMed] [Google Scholar]
- 21.Lara-Silva FO, Michalsky EM, Fortes-Dias CL, Fuiza VO, Pessanha JE, Regina-Silva S. Epidemiological aspects of vector, parasite, and domestic reservoir in areas of recent transmission and no reported human cases of visceral leishmaniasis in Brazil. Acta Trop. 2015;148:128–136. doi: 10.1016/j.actatropica.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 22.de Araújo VE, Pinheiro LC, Almeida MC, de Menezes FC, Morais MH, Reis IA. Relative risk of visceral leishmaniasis in Brazil a spatial analysis in urban area. PLoS Negl Trop Dis. 2013;7:75. doi: 10.1371/journal.pntd.0002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paltrinieri S, Solano-Gallego L, Fondati A, Lubas G, Gradoni L, Castagnaro M. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J Amer Vet Med Assoc. 2010;236:1184–1191. doi: 10.2460/javma.236.11.1184. [DOI] [PubMed] [Google Scholar]
- 24.Colombo FA, Odorizzi RM, Laurenti MD, Galati EA, Canavez F, Pereira-Chioccola VL. Detection of Leishmania (Leishmania) infantum RNA in fleas and ticks collected from naturally infected dogs. Parasitol Res. 2011;109:267–274. doi: 10.1007/s00436-010-2247-6. [DOI] [PubMed] [Google Scholar]
- 25.De Nardo CD, Rossi CN, Laurenti MD, Marcondes M. Detecção de anticorpos anti-Leishmania infantum syn chagasi em cães de São José do Rio Preto, São Paulo. Braz J Vet Res Anim Sci. 2011;48:425–428. [Google Scholar]
- 26.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. Plainview: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Gomes AH, Ferreira IM, Lima ML, Cunha EA, Garcia AS, Araújo MF. PCR identification of Leishmania in diagnosis and control of canine Leishmaniasis. Vet Parasitol. 2007;144:234–241. doi: 10.1016/j.vetpar.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Le Fichoux Y, Quaranta JF, Aufeuvre JP, Lelievre A, Marty P, Suffia I. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in southern France. J Clin Microbiol. 1999;37:1953–1957. doi: 10.1128/jcm.37.6.1953-1957.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravel S, Cuny G, Reynes J, Veas F. A highly sensitive and rapid procedure for direct PCR detection of Leishmania infantum within human peripheral blood mononuclear cells. Acta Trop. 1995;59:187–196. doi: 10.1016/0001-706x(95)00079-t. [DOI] [PubMed] [Google Scholar]
- 30.Kullberg M, Nilsson MA, Arnason U, Harley EH, Janke A. Housekeeping genes for phylogenetic analysis of eutherian relationships. Mol Biol Evol. 2006;23:1493–1503. doi: 10.1093/molbev/msl027. [DOI] [PubMed] [Google Scholar]
- 31.Bretagne S, Durand R, Olivi M, Garin J, Sulahian A, Rivollet D. Real-time PCR as a new tool for quantifying Leishmania infantum in liver in infected mice. Clin Diag Lab Immunol. 2001;8:828–831. doi: 10.1128/CDLI.8.4.828-831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Almeida ME, Steurer FJ, Koru O, Herwaldt BL, Pieniazek NJ, da Silva AJ. Identification of Leishmania spp by molecular amplification and DNA sequencing analysis of a fragment of rRNA internal transcribed spacer 2. J Clin Microbiol. 2011;49:3143–3149. doi: 10.1128/JCM.01177-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurr E. The rational use of dyes in biology, and general staining methods. London: Leonard Hill; 1965. [Google Scholar]
- 34.Natal D, Marucci D, Reis IM, Galati EA. Modificação da armadilha CDC com testes para coletas de flebotomíneos (Diptera) Rev Bras Entomol. 1991;35:697–700. [Google Scholar]
- 35.Reis LE, Coura-Vital W, Roatt BM, Bouillet LE, Ker HG, Fortes de Brito RC. Molecular diagnosis of canine visceral leishmaniasis a comparative study of three methods using skin and spleen from dogs with natural Leishmania infantum infection. Vet Parasitol. 2013;197:498–503. doi: 10.1016/j.vetpar.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 36.de Campos MP, da Silva DA, Madeira MF, Velho AA, Jr, Figueiredo FB. First autochthonous case of canine visceral leishmaniasis in Volta Redonda, Rio de Janeiro, Brazil. Rev Bras Parasitol Vet. 2013;22:424–426. doi: 10.1590/S1984-29612013000300018. [DOI] [PubMed] [Google Scholar]
- 37.von Zuben AP, Angerami RN, Castagna C, Baldini MB, Donalisio MR. The first canine visceral leishmaniasis outbreak in Campinas, State of São Paulo Southeastern Brazil. Rev Soc Bras Med Trop. 2014;47:385–388. doi: 10.1590/0037-8682-0126-2013. [DOI] [PubMed] [Google Scholar]
- 38.Francino O, Altet L, Sánchez-Robert E, Rodriguez A, Solano-Gallego L, Alberola J. Advantages of real-time PCR assay for diagnosis and monitoring of canine leishmaniosis. Vet Parasitol. 2006;137:214–221. doi: 10.1016/j.vetpar.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Silva MR, Marques MJ, Romanha AJ, Santa-Rosa IC, Carneiro CM, Reis AB. Autochthonous canine visceral leishmaniasis in a non-endemic area Bom Sucesso, Minas Gerais State, Brazil. Cad Saude Publica. 2008;24:281–286. doi: 10.1590/s0102-311x2008000200006. [DOI] [PubMed] [Google Scholar]
- 40.Silva MR, Santa Rosa IC. Levantamento de leishmaniose visceral canina em Bom Sucesso, Minas Gerais. Acta Sci Vet. 2005;33:69–74. [Google Scholar]
- 41.Alexander B, Oliveria EB, Haigh E, Almeida LL. Transmission of Leishmania in coffee plantations of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2002;97:627–630. doi: 10.1590/s0074-02762002000500005. [DOI] [PubMed] [Google Scholar]
- 42.Loiola CF, Silva DA, Galati EA. Phlebotomine fauna (Diptera Psychodidae) and species abundance in an endemic area of American cutaneous leishmaniasis in southeastern Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2007;102:581–585. doi: 10.1590/s0074-02762007005000050. [DOI] [PubMed] [Google Scholar]
- 43.Colla-Jacques FE, Casanova C, Prado AP. Study of sand fly fauna in an endemic area of American cutaneous leishmaniasis and canine visceral leishmaniasis in the municipality of Espírito Santo do Pinhal, São Paulo, Brazil. Mem Inst Oswaldo Cruz. 2010;105:208–215. doi: 10.1590/s0074-02762010000200017. [DOI] [PubMed] [Google Scholar]
- 44.Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil a review. Mem Inst Oswaldo Cruz. 2005;100:811–827. doi: 10.1590/s0074-02762005000800001. [DOI] [PubMed] [Google Scholar]
- 45.Campos JH, Costa FA. Participation of ticks in the infectious cycle of canine visceral leishmaniasis, in Teresina, Piauí, Brazil. Rev Inst Med Trop Sao Paulo. 2014;56:297–300. doi: 10.1590/S0036-46652014000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]


