SUMMARY
The aim of this retrospective study was to review all the notified cases of multidrug-resistant tuberculosis (MDR-TB) in São Paulo State (Brazil), as well as to describe and discuss the clinical, microbiological and radiologic aspects in a single reference center, within the same state, from 2000 to 2012. There were 1,097 notifications of MDR-TB in São Paulo State over this period, 70% affecting men aged on average 38 years (10-77). There was a significant fall in the MDR-TB mortality rate from 30% to 8% (2000-2003 versus 2009-2012). The same trend was observed in the cases studied at the reference center. The number of notified cases increased and death rate reduced from 37.5% (2000-2005) to 3.4% (2006-2012). Among the 48 drug-resistant TB cases, 17 non-tuberculous Mycobacteria were isolated in the sputum culture of nine patients, without any clinical significance. TB and fungus co-infection was diagnosed in 15% (7/48) of these cases: three with confirmed chronic pulmonary aspergillosis and four with positive serological markers for paracoccidioidomycosis. Overall, the reports show that MDR-TB diagnosis and cure rates have increased, while the mortality rate has decreased significantly in São Paulo State including in the studied reference center.
KEYWORDS: Multidrug resistant tuberculosis, Treatment outcome, Non-tuberculous, Mycobacteria, Lung Diseases, Fungal
INTRODUCTION
Tuberculosis (TB) continues to be an important worldwide public health problem, affecting millions of persons per year. Although it can be treated effectively, it is still the major cause of deaths due to infectious disease in adults 1 . Brazil is one of the 22 countries that concentrate 80% of the total number of TB cases, although the incidence and mortality of TB have been slowly and progressively decreasing over the last decades. However, new challenges regarding the management of the disease are increasingly important, especially the TB and human immunodeficiency virus (HIV) co-infection, which affects 12% of the TB cases around the world and 9.8% of cases in Brazil 1 , 2 . More recently, there has been an increase in the percentage of multidrug-resistant TB (MDR-TB) notified in the world 3 .
Increased bacillary resistance is also observed in Brazil according to the data of national surveys of resistance to anti-tuberculous drugs, the first conducted between 1995 and 1997 and the second between 2007 and 2008. An increase of almost 40% in primary resistance to isoniazid (from 4.4% to 6.0%) and of 30% in resistance to combined isoniazid and rifampicin (from 1.1% to 1.4%) were observed 4 , 5 . The number of multidrug-resistant tuberculosis (MDR-TB) cases in 2014 was 702, compared with 334 in 2001. There has been a consistent increase in the number of MDR-TB cases in Brazil in the past 15 years. This increase was particularly high between 2004-2005 and 2009-2010, with 22.6% and 47.3% increments from one year to another, respectively. It is important to consider that in the latter period, the National Tuberculosis Program began to prioritize culture and sensitivity testing for all retreatment cases and in the most vulnerable populations 1 , 6 .
The first report of extensively drug-resistant TB (XDR-TB) case was published in 2006 and it was characterized by resistance to rifampicin, isoniazid, fluoroquinolones and to a second-line injectable drug 7 . Over the last 10 years, XDR-TB cases have been reported in many countries, including Brazil. It is estimated that all the XDR-TB cases reported in 2012 corresponded to 9.6% of MDR-TB cases, but this is a condition still highly under diagnosed. Only 23% of the patients with a diagnosis of MDR-TB had samples tested for second-line drugs in 2011 8 - 10 , and the occurrence of XDR-TB is an indirect indicator of failure in the management of MDR-TB cases 1 .
There is a lack of data about the situation of TB resistance currently available in Brazil, with widely diverse reports varying according to the region where the cases were detected 11 - 15 . The aim of the present study was to review the notifications of all cases of MDR-TB in the state of São Paulo, as well as to describe and discuss the clinical and microbiological aspects of all notified cases at a reference center for the northeast and northwest regions of the state from 2000 to 2012.
MATERIAL AND METHODS
This is a retrospective study of MDR-TB cases notified in the state of São Paulo from 2000 to 2012, with a detailed description of the patients with drug-resistant TB seen at the reference center for MDR-TB of the University Hospital, School of Medicine of Ribeirão Preto (HCRP), University of São Paulo.
Information for the state of São Paulo
Inclusion criteria
We included data of TB patients infected by Mycobacterium tuberculosis resistant to at least rifampicin and isoniazid. These cases had been notified in the state of São Paulo, and the data were available in the system of information on special treatment of TB (SITE-TB - http://sitetb.org/), from 2000 to 2012. The following data were collected: age, sex, place and date of notification, and the patient outcome (death, cure, failure and abandonment of treatment). During the study period, some patients were notified more than once (failure, abandonment and/or retreatment), but only patients with a notification interval greater than 18 months, were considered for analysis in this study, as this is the shortest time for full treatment of the MDR-TB cases, whereas other cases would represent recurrence of the disease.
Exclusion criteria
Patients with TB whose bacillus did not show resistance to rifampicin and isoniazid were excluded.
Data source
The SITE-TB website (http://sitetb.org/) was the basic source of information.
Drug-resistant of TB cases notified at HCRP reference center
Inclusion criteria
All TB cases with diagnosis of drug resistance seen and followed up at the outpatient clinic of HCRP during the study period were included, as long as they had at least 36 months of follow-up after the diagnosis of resistant TB. The medical records of all patients were reviewed with special attention to clinical, imaging and microbiology recorded information.
The diagnosis of resistance was confirmed by culture, identification and drug-susceptibility testing for all the patients.
Sample collection and processing
The clinical specimens collected on the occasion of the diagnostic investigation of TB were processed according to the routine established by the Mycobacteria Laboratory of HCRP for these cases: direct examination after Ziehl-Neelsen staining and incubation in liquid culture medium by means of the automated system MGIT 960(r) . When the result was positive the sample was submitted again to bacilloscopy in order to confirm the AFB and detect possible false-positivity of the system and samples were cultured on solid Lowenstein-Jensen medium. The identification of M. tuberculosis complex was performed by polymerase chain reaction (PCR) that amplifies a 123 pair fragment of Mycobacterium tuberculosis 16 . The M. tuberculosis isolates suspected of being resistant (previously treated TB patients, patients with treatment failure, health professionals and prisoners) were sent to the Central Reference Laboratory for Mycobacteria to perform the drug-susceptibility testing, as described by Palacci et al. 17 .
Non-tuberculous mycobacteria were also sent to the Central Reference Lab for species identification by a phenotypic technique and molecular typing (PCR coupled to the Restriction Fragment Length Polymorphism - RFLP, and the analysis of a 441 bp fragment from the hsp65 gene (PRA-hsp65) 18 .
Paracoccidioides brasiliensis and Aspergillus infection were diagnosed based on mycological investigation (direct observation and culture), histological and serologic methods. A counter immunoelectrophoresis test was used to detect antibodies raised to both fungi.
Exclusion criteria
Patients with M. tuberculosis detected, but showing drug susceptibility or a mono-resistance profile to the first-line drugs were excluded from this study.
Data source
Information was obtained from the medical records of the patients and from the notification and follow-up forms of the epidemiological surveillance of HCRP. For the analysis of the patient evolution and outcome according to the case notification, patients were divided into three periods: Period 1 (P1): 2000-2003, Period 2 (P2): 2004-2008 and Period 3 (P3): 2009-2012, for both situations: 1) those cases notified in the state of São Paulo and 2) the subgroup of patients seen at the reference center of HCRP.
Statistical analysis
A comparative analysis of the death risk and the chance of cure during different time periods (P1, P2, P3) was performed using the Pearson chi-square test for the state of São Paulo, and the two-tailed Fisher test for the patients seen at the reference center of HCRP. Based on these analyses it was possible to observe a trend regarding mortality, cure and other outcomes for both groups, São Paulo State and Ribeirão Preto reference center.
Ethical aspects
The study was approved by the Ethics Committee of HCRP according to the process number 8595/2011.
RESULTS
MDR-TB in the state of São Paulo
During the study period, 958 patients were notified as having MDR-TB in the state of São Paulo. For this group of patients there were 1,097 notifications, as some of them were notified more than once with an interval of more than 18 months, over a period of 13 years.
The mean age of patients was 38.3 years (range: 10-77 years) and 70% were males. Most cases were notified at outpatient clinics in the capital city of São Paulo (54.2%); 26.1% at outpatient clinics in the countryside areas; 17.7% at reference hospitals for drug-resistant TB and 2% at prison hospitals of São Paulo State.
Analysis of the course and outcome of the cases notified in the state of São Paulo revealed a significant increase in the number of cases notified within a little more than a decade, 266 during the period from 2000 to 2003; 362 from 2004 to 2008 and 469 from 2009 to 2012. The outcomes recorded in the notifications revealed an important fall in mortality due to MDR-TB, of 30%, 12% and, 8% respectively, when the three periods were compared. There was also a significant increase in the proportion of completed treatments and the cure of cases: 53% (2000 to 2003); 56% (2004 to 2008) and 68% (2009 to 2012) (Table 1).
Table 1. Outcomes of MDR-TB cases notified in the state of São Paulo comparing three time periods .
| MDR-TB outcome SP State | P1: 2000-2003 | P2: 2004-2008 | P3: 2009-2012 | |||
| n | % | n | % | n | % | |
| Death due to MDR-TB* | 80 | 30% | 44 | 12% | 36 | 8% |
| Death due to other causes | 5 | 2% | 11 | 3% | 14 | 3% |
| Cure# | 140 | 53% | 238 | 66% | 318 | 68% |
| Treatment failure | 12 | 4% | 27 | 8% | 33 | 7% |
| Abandonment of treatment | 23 | 9% | 41 | 11% | 64 | 13% |
| Others | 6 | 2% | 1 | 0 | 4 | 1% |
| Total | 266 | 100% | 362 | 100% | 469 | 100% |
*Relative risk of death due to MDR-TB: (P2 x P1) RR=0.40 (0.29-0.56); (P3 x P1) RR=0.25 (0.18-0.37) p < 0.001. # Chance of cure (P2 x P1) RR=1.25 (1.09-1.42); (P3 x P1) RR=1.29 (1.14-1.45) p < 0.001. Pearson's chi-square test.
There was a significant reduction in mortality due to MDR-TB in the state of São Paulo over the three periods analyzed when comparing Period 2 (2004-2008) with Period 1 (2000-2003), with a relative risk (RR) of 0.40 (0.29-0.56) (p < 0.001), and Period 3 (2009-2012) to Period 1 with RR of 0.25 (0.18-0.37) (p < 0.001). The same was observed regarding the chance of cure during the three periods. When Period 2 (2004-2008) was compared to Period 1 (2000-2003) RR was 1.25 (1.09-1.42) (p = 0.001) and when Period 3 (2009-2012) was compared to Period 1, RR was 1.29 (1.14-1.45) (p < 0.001).
Resistant TB in the reference outpatient clinic of HCRP
Until 2012, the reference outpatient clinic of HCRP was the first one regarding the number of cases in the countryside of the state, and the third one in number of MDR-TB notified in the state of São Paulo during this period. There were 43 notifications of MDR-TB and two cases of XDR-TB. In this analysis, three cases of patients with disease caused by poly-resistant Mycobacterium tuberculosis, including resistance to rifampicin or isoniazid plus an additional first-line drug were also included. All cases were reviewed and their characteristics are described in the present study.
At the reference center of HCRP the risk of death and the chance of cure showed a behavior similar to that observed for the state of São Paulo. There was an increase, of 8 (2000 to 2003), to 15 (2004 and 2008), and to 25 (2009 to 2012) in the number of notified cases (Table 2). Regarding the outcomes, there was a mortality fall due to drug-resistant TB and an increase in the number of treatment completion and cure over these three periods: 25% (2000-2003); 66.7% (2004-2008) and 86.4% (2009-2012) (Pearson's chi-square test).
Table 2. Outcomes of drug resistant TB cases at MDR-TB service of HCRP comparing three time periods.
| MDR-TB outcome HCRP | P1: 2000-2003 | P2: 2004-2008 | P3: 2009-2012 | |||
| n | % | n | % | N | % | |
| Death due to MDR-TB | 4 | 50.0% | 2 | 13.3% | 2 | 8.0% |
| Cure | 2 | 25.0% | 10 | 66.7% | 22 | 88.0% |
| Abandonment of treatment | 1 | 12.5% | 2 | 13.3% | 0 | 0.0% |
| Others | 1 | 12.5% | 1 | 6.7% | 1 | 4.0% |
| Total | 8 | 100% | 15 | 100% | 25 | 100% |
* Relative risk of death due to MDR-TB: (P2 x P1) RR=0.27 (0.66-1.07); (P3 x P1) RR=0.18 (0.05-0.72), p < 0.05. # Chance of cure (P2 x P1) RR=2.67 (0.95-7.48) p = 0.089 (not significant); (P3 x P1) RR=3.45 (1.61-7.40) p = 0.003 (two-tailed Fisher test).
There was a significant reduction over time in the drug-resistant TB mortality among the patients seen at the reference service of HCRP. Comparison between Period 2 (2004-2008) and Period 1 (2000-2003) showed RR = 0.27 (0.66-1.07), p = 0.131 and comparison between Period 3 (2009-2012) and Period 1 showed RR = 0.18 (0.05-0.72), p = 0.023. The same behavior was observed regarding the chance of cure when Period 3 (2009-2012) was compared to Period 1 (2000-2003), with RR = 3.45 (1.61-7.40), p = 0.003 (two-tailed Fisher test). The chance of cure was not statistically significant when Period 2 (2004-2008) was compared to Period 1 (2000-2003), with RR 2.67 (0.95-7.48), p = 0.089.
Clinical, radiologic and microbiological findings in drug-resistant TB cases at HCRP
Half of the patients (24/48) seen at the HCRP center lived in the Ribeirão Preto region, with only four of them residing in the city of Ribeirão Preto. Twenty-three of the remaining 24 patients were from the northeastern region of the state of São Paulo, and one was from the state of Mato Grosso (Brazilian Midwest region). Two patients with XDR-TB were from the same region (northwestern part of São Paulo State), but they had no history of previous contact. The absolute majority of patients (81.2% N = 39) had a history of previous TB treatment (a minimum of one and a maximum of five previous treatments). Only three patients had confirmed HIV infection with lymphocyte T CD4+ counts lower than 100 cells/mm 3 . All the other TB diagnosed patients were tested and had a negative HIV serology.
Nine patients had no previous history of completed TB treatment and only three of them fulfilled the criterion for primary resistance of M. tuberculosis, less than 30 days of treatment when culture and drug susceptibility tests were performed. The other six patients were under regular TB treatment for four to five months when the sputum cultures were positive (Table 3). Until the end of 2009, the regular TB treatment in Brazil included rifampicin, isoniazid and pyrazinamide. After 2010, ethambutol was added to the scheme 2 .
Table 3. Demographic, clinical and radiological characterization of the 48 cases of drug resistant TB cases at the reference service of HCRP.

All patients had pulmonary disease and the chest X-ray or chest tomography revealed an apical opacification in 79.2% (38), bilateral opacification in 66.7% (32) and cavity images in 62.5% (30) of the notified cases in Ribeirão Preto.
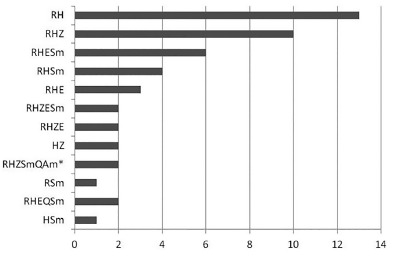
Figure 1 shows the susceptibility profile of M. tuberculosis isolated from patients seen at HCRP. There was a high prevalence of resistance to rifampicin plus isoniazid, but various types of combined resistance to two and three drugs was also observed. We included in the analysis four cases of polyresistance, i.e., resistance to rifampicin or isoniazid plus at least an additional first-line drug of the anti-TB scheme.
Fig. 1. Profile of resistance to tuberculostatic drugs in the 48 cases studied at HCRP. R: rifampicin; H: isoniazide; Z: pyrazinamide; E: etambutol; Sm: streptomycin; Q: quinolone; Am: amikacin. * Two cases confirmed as TBXDR.
During this 13-year period, the predominant MDR-TB treatment scheme included a five-drug regimen with an injectable aminoglycoside (streptomycin), quinolones (ofloxacin or levofloxacin), ethambutol, pyrazinamide and terizidone. The most common side-effects observed with the use of the therapeutic schemes for MDR-TB were otoxicity and vertigo induced by the aminoglycosides, arthralgia with the use of quinolones and pyrazinamide, diarrhea with the use of quinolones, optic neuritis with the use of etambutol, and depression associated with terizidone.
After the beginning of treatment, the time needed for M. tuberculosis culture conversion was less than six months in 35 patients (72.9%), seven to 12 months for 10 (20.8%) patients, and more than 12 months for three (6.3%) cases. In two patients there were no culture conversion, and complementary investigation confirmed XDR-TB. One of these patients died and the other was transferred for treatment to a hospital specialized in TB in the state, with a good evolution.
Another interesting finding observed during the follow-up of these patients was the growth of 14 non-tuberculous Mycobacteria isolated from cultures of 10 patients who were notified from 2000 to 2012, at HCRP. The NTM species isolated by automated culture were: M. avium (1), M. kansasii (2), M. fortuitum (2), M. chelonae (1), M. gordonae (1), and seven other species were not identified.
In addition, there were seven cases with TB and fungus co-infection. Three cases with pulmonary chronic aspergillosis had been confirmed by Aspergillus fumigatus culture and the detection of specific antibodies. There were also four patients with resistant TB who had high titers of antibodies against Paracoccidioides brasiliensis detected by counter immunoelectrophoresis.
DISCUSSION
Over the last two decades, the worldwide incidence of TB reached a peak in 2004, showing a slow decline thereafter in various regions of the globe. The goal established by WHO and followed by Brazil was to reduce the incidence of TB by 50% between 1995 and 2015 and has been consistently reached in terms of the reduction of number of cases of the disease 1 , 2 , 3 . However, the results for TB associated with HIV, MDR-TB and XDR-TB are going in the opposite direction 1 , 3 , 6 . The proportion of resistant cases in Brazil is still lower than what is observed in many regions of the world, with a similar epidemiological and social situation. However, due to a greater access to test drug susceptibility, an increase in the number of notified drug-resistant TB in the last decade6,7 has been observed worldwide. Our findings go in the same direction, showing that the number of MDR-TB notified in the state of São Paulo also increased in the past decade. Along with the increase in the drug-resistance, another reasonable explanation for these findings is the increased capacity to detect drug-resistant TB cases in the state 17 . The demographic characteristics of the cases in São Paulo State and for the reference center of HCRP were similar to those reported in other Brazilian studies regarding age and sex 11 , 12 , 14 .
Despite the increase in the notified cases, the analysis of the outcomes of MDR-TB in the state of São Paulo revealed an expressive reduction of mortality and a significant increase in the cure rate and completed treatments among the MDR-TB cases. On the other extreme, the chance of cure was greater in the last two periods compared to the first one. Many factors may be involved in this improvement, but the creation of specialized services and training of multiprofessional teams for the diagnosis, follow-up and treatment are thought to be related to the better results observed along these 13 years. Porter and Teisberg described a positive correlation between the concentration of difficult and less common cases at few super-specialized services and the experience of the teams, resulting in better outcomes 19 .
Despite the increase in the cure rate and the reduction of deaths, it is interesting to note that the rate of abandonment of treatment has remained practically stable over the last 40 years. The mean rate of abandonment of TB treatment was 12% in the early 1980's, 14% in the 1990 decade, and even higher in the Brazilian capital cities 20 , 21 . In this study, the abandonment of treatment for MDR-TB cases in the state of São Paulo, from 2000-2012, revealed a mean rate of 11.6%.
In an analysis of the fist Brazilian surveillance from 1995 to 1998 data of the main MDR-TB service in the state of São Paulo, Fiuza de Melo et al. observed that about 50% of the patients with TB and post-primary resistance had a history of previous treatment or abandonment of treatment 21 . In Ribeirão Preto, 39/48 (81.2%) of the notified patients had been submitted to a previous treatment for TB. Several studies have supported the relationship between the previous use of anti-TB drugs, especially when taken in an irregular manner, and the development of resistance 11 - 15 .
A more detailed analysis of the 48 cases notified at the reference service of HCRP revealed the occurrence of bilateral opacification and the presence of apical caverns in chest imaging exams as the most frequent findings in this series, similar to the findings of Siqueira et al. who reported the presence of caverns in 36 (72%) of their 50 cases, in addition to bilateral involvement in 38 (76%) of the 50 cases studied in Rio de Janeiro 14 .
Most of the patients studied at HCRP (27.1%) were found to be resistant only to rifampicin and isoniazid (13/48), in disagreement with the cases of drug-resistant TB notified in Rio de Janeiro where resistance to rifampicin, isoniazid and two more first-line drugs was the most frequent drug susceptibility profile 14 .
Detected resistance to isoniazid is reported to be predictive to streptomycin resistance. In this study, 18 (38.3%) isolates out of 47, which were resistant to isoniazid showed a concomitant resistance to streptomycin. This association may occur in up to 50% of the times. However, as the clinical reliability of this test is not very high 22 , even if the drug susceptibility test indicates a susceptible isolate, amikacin, capreomycin and kanamycin should be considered as the injectable drugs to be used in the MDR-TB scheme. In Brazil, streptomycin is the first injectable drug of choice, except if the patient has been treated with this drug before 10 . All patients with history of previous use of streptomycin, in the Ribeirão Preto reference center, were treated with amikacin.
In the HCRP, there were two (9.1%) out of 44 patients with confirmed XDR-TB. One died and the other evolved to cure after a long treatment in a specialized hospital for drug-resistant TB in the State. Although it is still a rare occurrence, 77 countries worldwide had reported at least one case by the end of 2011. Information from countries with reliable data suggests that almost 10% of MDR‐TB cases worldwide are also XDR‐TB 3 , 22 , 23 .
For the majority of patients (73%), negative culture results were observed six months after the beginning of the drug-resistant treatment. In contrast, negative culture results were observed between 15 and 60 days after the beginning of the standard treatment 2 for patients with susceptible TB. The culture continued to be positive for up to 12 months among 27% (13/48) of cases, showing the need to extend the use of the aminoglycoside or the total treatment time (from 18 to 24 months). In two of these cases negativity of cultures did not take place since they were XDR-TB cases.
The concomitant presence of positive cultures for non-tuberculous Mycobacteria in patients under drug-resistant TB treatment was not considered as a clinically relevant finding. These patients were not treated and it had no impact in the patients' outcomes. Pulmonary TB sequelae and residual fibrosis may be an appropriate environment for non-tuberculous Mycobacteria colonization 24 .
The occurrence of active Aspergillus infection associated with active or sequelae TB residual cavern is a well described condition 25 , even though cases of aspergillosis and drug-resistant TB 26 , 27 are scarce. TB and paracoccidioidomycosis (PCM) co-infection is very common in South America, especially in the northeastern region of the São Paulo State, which is an endemic area. Around 8.3% of PCM cases used to be diagnosed associated with tuberculosis in high endemic areas28, 29. However, there is only one published report of concomitant PCM and drug-resistant TB in a patient with acquired immunodeficiency syndrome and hepatitis C, from Peru 30 . These fungi and drug-resistant TB association needs to be considered in endemic areas, whenever TB shows microbiological improvement (AFB sputum smear and negative culture) but there are persistent pulmonary symptoms.
Despite compiling important data about drug-resistant TB, this retrospective study has limitations because it deals with secondary data from a national surveillance databank as well as with patients' records. Along the 13-year study period, many significant changes happened in the country, in the population and in the health services (offer/demand and access) and in the drug-resistant TB treatment, which probably interfered in the observed outcomes.
CONCLUSION
In the studied period there was a clear trend towards the increase of drug-resistant TB diagnosis and cure rates, while a significant decrease in mortality in São Paulo State was observed.
ACKNOWLEDGMENTS
We are grateful to Prof. José Fernando de Castro Figueiredo (in memoriam), the founder of the MDR-TB outpatient clinic in Ribeirão Preto. We would like to thank Daisy Nakamura Sato (PhD) for reviewing the manuscript, and Vera Maria Neder Galesi (M.D.), Coordinator of the São Paulo State Division for TB Control.
REFERENCES
- 1.World Health Organization . Global tuberculosis report 2015. 20. Geneva: WHO; 2015. [Google Scholar]
- 2.Conde MB, Melo FA, Marques AM, Cardoso NC, Pinheiro VG, Dalcin PT. III Brazilian Thoracic Association Guidelines on Tuberculosis. J Bras Pneumol. 2009;35:1018–1048. doi: 10.1590/s1806-37132009001000011. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . End TB strategy. Geneva: WHO; 2015. [Google Scholar]
- 4.Braga JU, Barreto AM, Hijjar MA. Inquérito epidemiológico da resistência às drogas usadas no tratamento da tuberculose no Brasil 1995-97, IERDTB Parte III: Principais resultados. Bol Pneumol Sanit. 2003;11:76–81. [Google Scholar]
- 5.Kritski AL. Multidrug-resistant tuberculosis emergence a renewed challenge. J Bras Pneumol. 2010;36:157–158. doi: 10.1590/s1806-37132010000200001. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira GP, Torrens AW, Bartholomay P, Barreira D. Tuberculosis in Brazil last ten years analysis 2001-2010. Braz J Infect Dis. 2013;17:218–233. doi: 10.1016/j.bjid.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs - worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. [PubMed] [Google Scholar]
- 8.Parida SK, Axelsson-Robertson R, Rao MV, Singh N, Master I, Lutckii A. Totally drug-resistant tuberculosis and adjunct therapies. J Intern Med. 2015;277:388–405. doi: 10.1111/joim.12264. [DOI] [PubMed] [Google Scholar]
- 9.Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K. Surveillance of anti-tuberculosis drug resistance in the world an updated analysis, 2007-2010. Bull World Health Organ. 2012;90:111–119D. doi: 10.2471/BLT.11.092585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Centro de Referência Professor Hélio Fraga. Projeto MSH. Tuberculose multirresistente: guia de vigilância epidemiológica Rio de Janeiro: Ministério da Saúde; 2007. [Google Scholar]
- 11.Seiscento M, Fiúza de Melo FA, Ide J, Neto, Noronha AM, Afiune JB, Inomata T. Tuberculose multirresistente (TBMR) aspectos clínico-laboratoriais, epidemiológicos e terapêuticos. J Pneumol. 1997;23:237–244. [Google Scholar]
- 12.Souza MB, Antunes CM, Garcia GF. Multidrug-resistant Mycobacterium tuberculosis at a referral center for infectious diseases in the state of Minas Gerais, Brazil sensitivity profile and related risk factors. J Bras Pneumol. 2006;32:430–437. doi: 10.1590/s1806-37132006000500010. [DOI] [PubMed] [Google Scholar]
- 13.Telles MA, Ferrazoli L, Waldman EA, Giampaglia CM, Martins MC, Ueki SY. A population-based study of drug resistance and transmission of tuberculosis in an urban community. Int J Tuberc Lung Dis. 2005;9:970–976. [PubMed] [Google Scholar]
- 14.Siqueira HR, Freitas FA, Oliveira DN, Barreto AM, Dalcolmo MP, Albano RM. Clinical evolution of a group of patients with multidrug-resistant TB treated at a referral center in the city of Rio de Janeiro, Brazil. J Bras Pneumol. 2009;35:54–62. doi: 10.1590/s1806-37132009000100008. [DOI] [PubMed] [Google Scholar]
- 15.Marques M, Cunha EA, Ruffino-Netto A, Andrade SM. Drug resistance profile of Mycobacterium tuberculosis in the state of Mato Grosso do Sul, Brazil, 2000-2006. J Bras Pneumol. 2010;36:224–231. doi: 10.1590/s1806-37132010000200011. [DOI] [PubMed] [Google Scholar]
- 16.Bollela VR, Sato DN, Fonseca BA. Problemas na padronização da reação em cadeia de polimerase para o diagnóstico de tuberculose pulmonar. Rev Saude Publica. 1999;33:281–286. doi: 10.1590/s0034-89101999000300009. [DOI] [PubMed] [Google Scholar]
- 17.Palacci M, Ueki SY, Sato DN, Telles MA, Curcio M, Silva EA. Evaluation of mycobacteria growth indicator tube for recovery and drug susceptibility testing of Mycobacterium tuberculosis isolates from respiratory specimens. J Clin Microbiol. 1996;34:762–764. doi: 10.1128/jcm.34.3.762-764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zamarioli LA, Coelho AG, Pereira CM, Nascimento AC, Ueki SY, Chimara E. Descriptive study of the frequency of nontuberculous mycobacteria in the Baixada Santista region of the state of São Paulo, Brazil. J Bras Pneumol. 2008;34:590–594. doi: 10.1590/s1806-37132008000800008. [DOI] [PubMed] [Google Scholar]
- 19.Porter ME, Teisberg EO. Redefining health care: creating value-based competition on results. Boston: Harvard Business School Press; 2006. [Google Scholar]
- 20.Diniz LS, Gerhardt G, Miranda JA, Manceau JN. Efetividade do tratamento da tuberculose em oito municípios de capitais brasileiras. Bol Pneumol Sanit. 1995;3:6–19. [Google Scholar]
- 21.de Melo FA, Afiune JB, Ide J, Neto, de Almeida EA, Spada DT, Antelmo AN. Aspectos epidemiológicos da tuberculose multirresistente em serviço de referência na cidade de São Paulo. Rev Soc Bras Med Trop. 2003;36:27–34. [PubMed] [Google Scholar]
- 22.Caminero JA, Sotgiu G, Zumla A, Miglioi GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet. 2010;10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 23.Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–387. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenai S, Rodrigues C, Mehta A. Time to identify and define non-tuberculous mycobacteria in a tuberculosis-endemic region. Int J Tuberc Lung Dis. 2010;14:1001–1008. [PubMed] [Google Scholar]
- 25.Ohba H, Miwa S, Shirai M, Kanai M, Eifuku T, Suda T. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med. 2012;106:724–729. doi: 10.1016/j.rmed.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Musella RM, Castagnino JP, Maiolo E, Negroni R, Arechavala A, Santiso G. Problemas clínicos en micología médica problema nº 24. Rev Iberoam Micol. 2007;24:75–78. doi: 10.1016/s1130-1406(07)70018-9. [DOI] [PubMed] [Google Scholar]
- 27.Kumar AA, Shantha GP, Jeyachandran V, Rajkumar K, Natesan S, Srinivasan D. Multidrug resistant tuberculosis co-existing with aspergilloma and invasive aspergillosis in a 50 year old diabetic woman a case report. Cases J. 2008;1:303–303. doi: 10.1186/1757-1626-1-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellissimo-Rodrigues F, Machado AA, Martinez R. Paracoccidioidomycosis epidemiological features of a 1,000 cases series from a hyperendemic area on the southeast of Brazil. Am J Trop Med Hyg. 2011;85:546–550. doi: 10.4269/ajtmh.2011.11-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellissimo-Rodrigues F, Bollela VR, da Fonseca BA, Martinez R. Endemic paracoccidioidomycosis relationship between clinical presentation and patients' demographic features. Med Mycol. 2013;51:313–318. doi: 10.3109/13693786.2012.714529. [DOI] [PubMed] [Google Scholar]
- 30.Nunura RJ, Salazar MD, Vásquez LT, Endo GS, Rodríguez FA, Zerpa LR. Paracoccidioidomicosis y TBC-MR en portador de VIH/VHC. Rev Chilena Infectol. 2010;27:551–555. [PubMed] [Google Scholar]


