SUMMARY
Human T-cell lymphotropic virus (HTLV) may affect the clinical course of human immunodeficiency virus 1 (HIV1). Both infections are common in endemic areas because these viruses share similar routes of transmission. The aim of this study was to estimate the seroprevalence of HTLV1/2 in a population of HIV1-infected patients in the state of Goiás, Midwestern Brazil. Of the 505 studied patients, four (0.79%) were positive for anti-HTLV1/2 by enzyme-linked immunosorbent assay (ELISA), with HTLV1 infection confirmed by line immunoassay (LIA) and polymerase chain reaction (PCR) in all of the ELISA-positive samples. No cases of HTLV2 infection were observed. The prevalence of HTLV1/HIV1 coinfection was 0.79% (4/505; 95% CI: 0.25-2.16). All the coinfected patients reported sexual risk behaviors and only one reported intravenous drug use. Sequencing of the viral long terminal repeat (LTR) region and phylogenetic analysis revealed that the four HTLV1 isolates belonged to the Transcontinental a subgroup of the Cosmopolitan (1a) subtype, the most frequent subgroup detected in Brazil. This study shows a low prevalence of HTLV1/2 in HIV1-infected patients in Midwestern Brazil.
KEYWORDS: HTLV, HIV-1, Coinfection, Prevalence, Subtype
INTRODUCTION
Human T-cell lymphotropic virus type 1 (HTLV1), the causative agent of adult T-cell leukemia/lymphoma (ATLL) and tropical spastic paraparesis/ HTLV1-associated myelopathy (TSP/HAM), is also related to uveitis and other inflammatory diseases 1 . Worldwide, it is estimated that 5-10 million people are infected with HTLV1. This virus is endemic in Japan, sub-Saharan Africa, the Caribbean and some parts of South America 2 , 3 . Based on genetic analyses of the viral long terminal repeat (LTR) region, HTLV1 has been classified into seven subtypes (1a-g). The 1a or Cosmopolitan subtype Transcontinental subgroup is the most widespread 1 , 2 . Human T-cell lymphotropic virus type 2 (HTLV2) has also been associated with myelopathy and other neurological disorders 4 . HTLV2 is largely present in indigenous populations in the Americas, as well as in intravenous drug users. This virus is subdivided into four subtypes (a-d). HTLV2c is the most prevalent subtype in Brazil 3 .
Coinfection between HTLV1/2 and human immunodeficiency virus 1 (HIV1) is common in endemic areas, because these viruses share similar routes of transmission such as sexual contact, breastfeeding, blood transfusion and intravenous drug use (IDU). Although HTLV and HIV1 coinfection remains poorly understood, higher rates of myelopathy and other neurological disorders have been observed 5 .
In Brazil, a South American country, few data have been reported on the prevalence of HTLV infection among HIV1-positive patients in recent years 6 - 10 , and no studies regarding HTLV/HIV coinfection in Midwestern Brazil has been published so far. Therefore, the aim of this study was to estimate the seroprevalence of HTLV1/2 in a population of HIV1-infected patients in the state of Goiás.
MATERIAL AND METHODS
A cross-sectional study was conducted in a population of HIV1-positive patients at the Hospital de Doenças Tropicais (HDT) Dr. Anuar Auad. This hospital is located in the state of Goiás (6,610,681 inhabitants), the most populous state in Midwestern Brazil. The HDT is the largest public referral hospital for infectious diseases in the region, receiving an average of 600 new HIV1 cases per year. From April 2009 to March 2010, all the patients were invited to participate in the study during regular medical visits at the outpatient unit of the HDT. Individuals were eligible if they were adults (18 years old or older), infected with HIV1, antiretroviral drug therapy-naïve, and agreed to answer a structured questionnaire and have a blood sample collected. After the signature of the informed consent, they were interviewed so as to record their demographic and behavioral characteristics. Clinical data, T CD4+ lymphocyte counts, and HIV1 viral load measurements were obtained from medical records using the most recent data available at the time of the interview.
Blood was collected (10 mL) from all the participants. HTLV infection was detected by enzyme-linked immunosorbent assay (ELISA) (anti-HTLV, Murex HTLV-I+II, Murex Biotech, Dartford, UK) and confirmed by immunoassay (INNO-LIA HTLV I/II, Innogenetics Biotechnology for Healthcare, Ghent, Belgium).
Whole blood samples of anti-HTLV seropositive patients were subjected to DNA extraction using the QIAamp DNA Blood Mini kit (QIAGEN Inc., Hilden, Germany) according to the manufacturer's instructions. Polymerase chain reactions (PCR) targeting the tax and LTR regions of HTLV1 and HTLV2 were performed 11 - 13 . PCR products were purified using the QIAamp PCR purification kit (QIAGEN Inc., Hilden, Germany) and were submitted to direct nucleotide sequencing reaction in both directions using the Big Dye Terminator v 3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) in a 3100 Automated DNA Sequencer (Applied Biosystems, Foster City, CA, USA).
The HTLV1-LTR sequences were aligned using the ClustalW program, implemented in the MEGA version 5.2 software package 14 , and edited using the BioEdit v5.0.9 program (Department of Microbiology, North Carolina State University, USA). A neighbor-joining (NJ) tree was constructed by PAUP* software version 4.0b10 15 . The Hasegawa, Kishino & Yano (HKY) model with gamma distribution was selected using the Modeltest 3.7 software 16 . The reliability of the NJ tree was evaluated by bootstrap analysis of 1,000 replicates. Novel nucleotide sequences identified from the present study were deposited in GenBank under accession numbers KM875549 to KM875552.
HTLV-positive samples were tested for hepatitis B virus (HBV), presence of hepatitis B surface antigen (HBsAg), using the Hepanostika HBsAg Ultra (BioMérieux, Marcy l'Étoile, Lyon, France); antibody to hepatitis B core antigen (anti-HBc), Hepanostika anti-HBc Uni-form (BioMérieux, Marcy l'Étoile, Lyon, France) and hepatitis C virus (HCV, anti-HCV-ELISA, Hepanostika Ultra, Biomedical, Shanghai, China).
This study was approved by the Ethics Committee of the Anuar Auad Tropical Disease Hospital, Goiânia, Goiás, Brazil.
RESULTS
A total of 520 treatment-naïve HIV1-infected patients were eligible for inclusion in this study. Of these, 505 (97.1%) agreed to participate. As shown in Table 1, most patients were male (60.2%). The mean age was 37.6 years (standard deviation (SD): 10.2). Almost half of the study population (45.4%) was single, 78.6% were non-Caucasian (Afro-descendants 77% and Asian 1.6%), 69.7% reported a monthly income of US$ 700 or less and 60.8% had less than 10 years of education.
Table 1. Sociodemographic characteristics of HIV1-infected patients in Midwestern Brazil (n = 505).
| Characteristics | n | % |
| Age (mean ± SD: 37.6 ± 10.2) | ||
| < 30 years | 116 | 23.0 |
| 30-39 years | 201 | 39.0 |
| 40-49 years | 117 | 23.2 |
| ≥ 50 years | 71 | 14.0 |
| Gender | ||
| Male | 304 | 60.2 |
| Female | 201 | 39.8 |
| Marital status | ||
| Single | 229 | 45.4 |
| Married | 201 | 39.8 |
| Divorced/widowed | 75 | 14.8 |
| Ethnicity | ||
| Caucasian | 108 | 21.4 |
| Non-caucasian | 397 | 78.6 |
| Monthly incomea | ||
| < 1 minimum wage | 178 | 35.2 |
| 1-2 minimum wages | 174 | 34.5 |
| > 2 minimum wages | 121 | 30.3 |
| Education | ||
| < 5 years | 73 | 14.5 |
| 5-9 years | 234 | 46.3 |
| 10-12 years | 153 | 30.3 |
| > 12 years | 45 | 8.9 |
SD - standard deviation. aMinimum monthly wage was approximately equal to US$ 350.
Behavioral characteristics such as history of blood transfusion with blood bags that were not screened for anti-HTLV (before November 1993; 5.7%), IDU (2.2%), multiple sexual partners (> 10 during lifetime, 61.8%), non-use or occasional use of condoms (during lifetime, 89.9%) and history of sexually transmitted infections (at least one STI in lifetime, 36.6%) were reported by the study population.
Of the 505 study participants, four were found to be HTLV1/2-positive by ELISA. After confirmatory testing (LIA and PCR targeting the tax and LTR regions), these patients were confirmed to be positive for HTLV1. The prevalence of HTLV1/HIV1 coinfection was 0.79% (4/505; 95% CI: 0.25-2.16). No cases of HTLV2 infection were observed.
Although the number of coinfected individuals was small, a higher mean age and percentage of females were observed in this group. The four coinfected patients reported unprotected sex with multiple partners. Of these, two reported STIs and had also a positive serology to HBV. One of them reported IDU and had also a positive serology to HCV. Mean CD4+ T cell counts for HIV1 mono-infected and HTLV1/HIV1 coinfected patients were 526.6 cells/µL and 599.7 cells/µL, respectively. Mean HIV1 viral load (log10) were 4.6 copies/ml and 4.9 copies/mL, respectively (Table 2).
Table 2. Characteristics of HIV1 mono-infected and HIV1/HTLV1 coinfected patients in Midwestern Brazil (n = 505).
| Variables | HIV-mono-infected (n = 501) | HIV1/HTLV1 coinfected (n = 4) |
| Age (years) | ||
| Mean (range) | 37.5 (18-74) | 44 (36-53) |
| Gender, n (%) | ||
| Male | 303 (60.5) | 1 (25) |
| Female | 198 (39.5) | 3 (75) |
| Risk behavior (lifetime), n (%) | ||
| Number of sexual partners (>10) | 308 (61.5) | 4 (100) |
| Unprotected sex | 450 (89.8) | 4 (100) |
| Previous STI | 183 (36.5) | 2 (50) |
| Intravenous drug use | 10 (2.0) | 1 (25) |
| Blood transfusion* | 29 (5.8) | 0 |
| CD4 counts (cells/µL) | ||
| Mean (range) | 526.6 (6-1800) | 599.7 (264-916) |
| HIV viral load (log10 copies/mL) | ||
| Mean (range) | 4.6 (1.3-5.7) | 4.9 (2.5-5.5) |
| HCV seropositivity, n (%) | 22 (4.4) | 1 (25) |
| HBV seropositivity, n (%) | 122 (24.3) | 2 (50) |
*Blood transfusion not screened for anti-HTLV (before November 1993), STI: sexually transmitted infections, HBV: hepatitis B virus; HCV: hepatitis C virus.
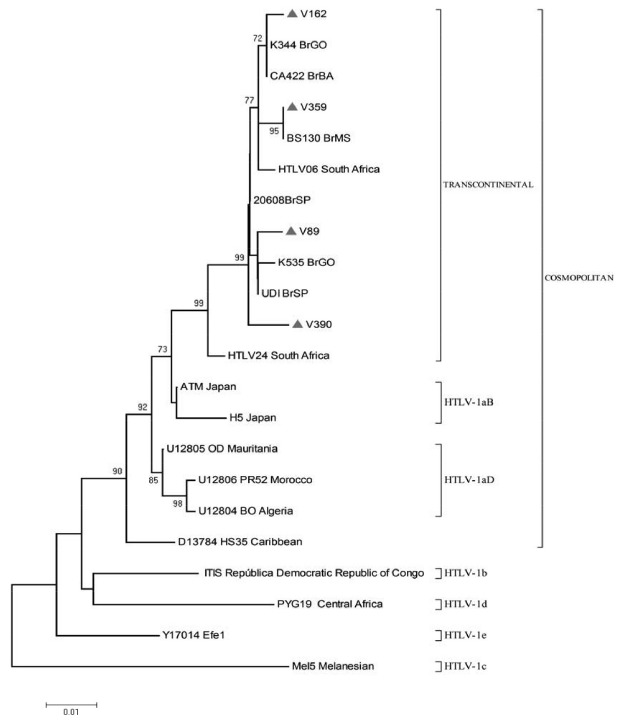
The phylogenetic analysis of the HTLV1-LTR region (Fig. 1) revealed that the four HTLV1 isolates (V-89, V-162, V-359 and V-390) belonged to the Transcontinental (A) subgroup of the Cosmopolitan (1a) subtype.
Fig. 1. Phylogenetic tree of HTLV1 isolates based on the 640 bp fragment of the LTR region, including four sequences from treatment-naïve HIV1-infected patients (V) in Midwestern Brazil and 18 GenBank sequences of subtypes a-d. The phylogenetic tree was constructed using the neighbor-joining method based on the Hasegawa, Kishino & Yano model and g-distribution. The number on the tree represents bootstrap values.
DISCUSSION
In this study, we reported for the first time the HTLV seroprevalence in a population of HIV1-infected patients in Midwestern Brazil. The prevalence of HTLV1 was 0.79%, six times higher than the percentage found in local blood donors (0.13%) (A G Kozlowski, unpublished data). Nevertheless, relative to other Brazilian data regarding HIV1-infected patients published between 2010 and 2015, this prevalence was lower than those reported in Piauí (1.12%) 6 , São Paulo (1.55%) 10 , Porto Alegre (1.9%)9 and Feira de Santana, Bahia (3.74%) 17 . Differences in regional endemicity, ethnic origin of the population, risk behaviors and study designs are the possible reasons for these differences in the observed rates.
These factors may also reflect the wide variation in HTLV1 and HTLV2 distribution in Brazilian HIV1-infected patients. In this study, HTLV1 was identified in the four HTLV/HIV1 coinfected patients. No case of HTLV2 infection was observed. Similarly, HTLV1 was also identified in the majority of HTLV/HIV1 coinfected patients in Piauí 6 , Porto Alegre 9 and Feira de Santana 17 , unlike those from São Paulo, where similar distribution of HTLV1 and HTLV2 was reported 10 . By contrast, some previous studies have demonstrated high HTLV2/HIV1 coinfection rates in Belém 18 , 19 , where HTLV2 infection is known to be endemic, as well as in Londrina 20 , Ribeirão Preto and São Paulo 21 where this coinfection was associated with IDU.
Sexual risk behaviors such as history of multiple sexual partners and non-use or occasional use of condoms were frequently reported among the study participants. These data are consistent with data reported in the Epidemiological Bulletin by the Ministry of Health, showing that the majority of HIV/Aids cases in 2013 (94.9% and 97.4% in men and women, respectively) among individuals older than 12 years old resulted from sexual transmission 22 . It is also an important route of HTLV1 transmission 1 . In fact, the four HTLV1/HIV1 coinfected patients reported unprotected sex with multiple partners. Two of them reported history of STIs and were also exposed to HBV. It is important to emphasize that sexual transmission is a major route for HBV infection in our region 23 . In this study, one HTLV1/HIV1 coinfected patient was seropositive to HCV and reported IDU. This behavior has been reported by others authors among HTLV1/HIV1/HCV coinfected patients 20 , 21 , 24 , reflecting the high transmissibility of these viruses through direct blood contact resulting from the sharing of syringes and needles.
HTLV1 may impact the clinical course of HIV1 and vice-versa. In spite of evidence of increased morbidity, coinfected patients have normal or elevated CD4+ T cell counts. Even if the CD4 levels are elevated, most of the cells may be functionally impaired 5 . Although the design of this study does not allow us to evaluate the impact of HTLV1 on the clinical course of HIV1, the four coinfected individuals were asymptomatic and three of them had normal CD4+ T cell counts (higher than 500 cells/ mm 3 , data not shown). In addition, similar CD4+ T counts were recorded between HTLV1/HIV1 coinfected and HIV1 mono-infected patients (599.7 versus 526.6 cells/µL, respectively; p > 0.05). Therefore, further clinical prospective investigations are necessary to elucidate the interaction of HTLV1 and HIV1 in coinfected patients.
The four HTLV1 isolates from this study were classified as Transcontinental (A) subgroup of the Cosmopolitan (1a) subtype. These data was in accordance with those reported in HIV1-infected patients in Brazil in whom this HTLV1 subgroup is predominant 6 , 17 , 19 , 21 .
This study has limitations that should be taken into account. This was a hospital-based investigation and, therefore, the study population does not represent all treatment-naive HIV1 patients in the state of Goiás. Another limitation of this study is the small number of patients studied, which may limit the statistical power of the results to detect differences between the groups (mono and coinfected) and the detection of HTLV2. On the other hand, only treatment-naïve HIV1 patients were included in the study because antiretroviral therapy may cause fluctuations in the HTLV proviral load and, thus, this condition may interfere with HTLV proviral DNA detection 25 . In addition, due to the lack of available data on the serological and molecular epidemiology of HTLV/HIV1 coinfection in Midwestern Brazil, this study provides the first data on HTLV1/2 in HIV1-infected patients in this region.
In spite of the importance of this coinfection, this status is underdiagnosed 5 . In fact, no HTLV1/HIV1 coinfected patients knew their HTLV status (data not shown). On the other hand, guidelines for the clinical management of HIV patients recommend the HTLV1/2 test, as well as the serology for viral hepatitis (B and C) before the initiation of the antiretroviral therapy 26 . Therefore, it is important to integrate and provide regularly diagnostic tests for these infectious diseases in the Brazilian public health services.
Although the public health system in Brazil provides prevention programs and free and universal access to antiretroviral treatment for HIV/AIDS, HTLV infection is considered a neglected infectious disease 22 , 27 . It is important to emphasize that HTLV infection is also lifelong and, although most of the infected patients remain asymptomatic for many years, these carriers are potential disseminators. In addition, there is no effective treatment or immunization for the HTLV infection and its complications. Thus, more research on HTLV infection is imperative for the elaboration of public policies on educational and prophylactic measures to increase the awareness of the infection and reduce the viral transmission and infection-related diseases.
In conclusion, this study shows a low prevalence of HTLV1/2 in HIV1-infected patients in Midwestern Brazil. In addition, the Transcontinental (A) subgroup of the Cosmopolitan (1a) subtype was detected in all the coinfected patients, highlighting the predominance of this subtype in Brazil.
ACKNOWLEDGEMENTS
The authors would like to thank Pollyanne S. Lemes and Nativa H. A. Del-Rios for their work in the sample and data collection; and Koko Otsuki and Ágabo Macedo da Costa e Silva for their technical assistance. They would also like to thank the staff of the Hospital de Doenças Tropicais (HDT) Dr. Anuar Auad for the fruitful collaboration. This study was supported by the Fundação de Amparo à Pesquisa do Estado de Goiás (FAPEG, process number 201210267001093/2012). Brian Ream edited this manuscript in English.
REFERENCES
- 1.Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23:577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388–388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paiva A, Casseb J. Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev Inst Med Trop Sao Paulo. 2015;57:1–13. doi: 10.1590/S0036-46652015000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roucoux DF, Murphy EL. The epidemiology and disease outcomes of human T-lymphotropic virus type II. AIDS Rev. 2004;6:144–154. [PubMed] [Google Scholar]
- 5.Dhasmana D, Taylor GP. Human T-lymphotropic virus/HIV co-infection a clinical review. Curr Opin Infect Dis. 2014;27:16–28. doi: 10.1097/QCO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira EH, Oliveira-Filho AB, Souza LA, da Silva LV, Ishak MO, Ishak R. Human T-cell lymphotropic virus in patients infected with HIV-1 molecular epidemiology and risk factors for transmission in Piauí, Northeastern Brazil. Curr HIV Res. 2012;10:700–707. doi: 10.2174/1570162x11209080700. [DOI] [PubMed] [Google Scholar]
- 7.Silva MT, Neves ES, Grinsztejn B, de Melo Espíndola O, Schor D, Araújo A. Neurological manifestations of coinfection with HIV and human T-lymphotropic virus type 1. AIDS. 2012;26:521–523. doi: 10.1097/QAD.0b013e32834c4a3e. [DOI] [PubMed] [Google Scholar]
- 8.Travassos AG, Brites C, Netto EM, Fernandes SA, Rutherford GW, Queiroz CM. Prevalence of sexually transmitted infections among HIV-infected women in Brazil. Braz J Infect Dis. 2012;16:581–585. doi: 10.1016/j.bjid.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Galetto LR, Lunge VR, Béria JU, Tietzmann DC, Stein AT, Simon D. Prevalence and risk factors for human T cell lymphotropic virus infection in Southern Brazilian HIV-positive patients. AIDS Res Hum Retroviruses. 2014;30:907–911. doi: 10.1089/AID.2013.0210. [DOI] [PubMed] [Google Scholar]
- 10.Caterino-de-Araujo A, Sacchi CT, Gonçalves MG, Campos KR, Magri MC, Alencar WK. Current prevalence and risk factors associated with human T lymphotropic virus type 1 and human T lymphotropic virus type 2 infections among HIV/AIDS patients in São Paulo, Brazil. AIDS Res Hum Retroviruses. 2015;31:543–549. doi: 10.1089/aid.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu HF, Vandamme AM, Kazadi K, Carton H, Desmyter J, Goubau P. Familial transmission and minimal sequence variability of human T-lymphotropic virus type I (HTLV-I) in Zaire. AIDS Res Hum Retroviruses. 1994;10:1135–1142. doi: 10.1089/aid.1994.10.1135. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa Y, Yamashita M, Usuku K, Izumo S, Nakagawa M, Osame M. Phylogenetic subgroups of human T cell lymphotropic virus (HTLV) type I in the tax gene and their association with different risks for HTLV-I-associated myelopathy/tropical spastic paraparesis. J Infect Dis. 2000;182:1343–1349. doi: 10.1086/315897. [DOI] [PubMed] [Google Scholar]
- 13.Eiraku N, Novoa P, da Costa Ferreira M, Monken C, Ishak R, da Costa Ferreira O. Identification and characterization of a new and distinct molecular subtype of human T-cell lymphotropic virus type 2. J Virol. 1996;70:1481–1492. doi: 10.1128/jvi.70.3.1481-1492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5 molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland: Sinauer Associates; 1998. [Google Scholar]
- 16.Posada D, Crandall KA. MODELTEST testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 17.de Almeida Rego FF, Mota-Miranda A, de Souza Santos E, Galvão-Castro B, Alcantara LC. Seroprevalence and molecular epidemiology of HTLV-1 isolates from HIV-1 co-infected women in Feira de Santana, Bahia, Brazil. AIDS Res Hum Retroviruses. 2010;26:1333–1339. doi: 10.1089/aid.2009.0298. [DOI] [PubMed] [Google Scholar]
- 18.Vallinoto AC, Azevedo VN, Santos DE, Caniceiro S, Mesquita FC, Hall WW. Serological evidence of HTLV-I and HTLV-II co-infection s in HIV-1 positive patients in Belém, state of Pará, Brazil. Mem Inst Oswaldo Cruz. 1998;93:407–409. doi: 10.1590/s0074-02761998000300026. [DOI] [PubMed] [Google Scholar]
- 19.Laurentino RV, Lopes IG, Azevedo VN, Machado LF, Moreira MR, Lobato L. Molecular characterization of human T-cell lymphotropic virus coinfecting human immunodeficiency virus 1 infected patients in the Amazon region of Brazil. Mem Inst Oswaldo Cruz. 2005;100:371–376. doi: 10.1590/s0074-02762005000400006. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto HK, Caterino-de-Araujo A, Morimoto AA, Reiche EM, Ueda LT, Matsuo T. Seroprevalence and risk factors for human T cell lymphotropic virus type 1 and 2 infection in human immunodeficiency virus-infected patients attending AIDS referral center health units in Londrina and other communities in Paraná, Brazil. AIDS Res Hum Retroviruses. 2005;21:256–262. doi: 10.1089/aid.2005.21.256. [DOI] [PubMed] [Google Scholar]
- 21.Kleine W, Neto, Sanabani SS, Jamal LF, Sabino EC. Prevalência, fatores de risco e caracterização genética dos vírus linfotrópico de células T humana tipo 1 e 2 em pacientes infectados pelo vírus da imunodeficiência humana tipo 1 nas cidades de Ribeirão Preto e São Paulo. Rev Soc Bras Med Trop. 2009;42:264–270. doi: 10.1590/s0037-86822009000300006. [DOI] [PubMed] [Google Scholar]
- 22.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, AIDS e Hepatites Virais. Boletim epidemiológico HIV-AIDS: ano III, n° 1, 27ª a 52ª semanas epidemiológicas - julho a dezembro de 2013, 01ª a 26ª semanas epidemiológicas - janeiro a junho de 2014. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 23.Pereira LM, Martelli CM, Merchán-Hamann E, Montarroyos UR, Braga MC, de Lima ML. Population-based multicentric survey of hepatitis B infection and risk factor differences among three regions in Brazil. Am J Trop Med Hyg. 2009;81:240–247. [PubMed] [Google Scholar]
- 24.Etzel A, Shibata GY, Rozman M, Jorge ML, Damas CD, Segurado AA. HTLV-1 and HTLV-2 infections in HIV-infected individuals from Santos, Brazil seroprevalence and risk factors. J Acquir Immune Defic Syndr. 2001;26:185–190. doi: 10.1097/00042560-200102010-00015. [DOI] [PubMed] [Google Scholar]
- 25.Machuca A, Soriano V. In vivo fluctuation of HTLV-I and HTLV-II proviral load in patients receiving antiretroviral drugs. J Acquir Immune Defic Syndr. 2000;24:189–193. doi: 10.1097/00126334-200006010-00017. [DOI] [PubMed] [Google Scholar]
- 26.Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de DST, AIDS e Hepatites Virais . Protocolo clínico e diretrizes terapêuticas para manejo da infecção pelo HIV em adultos. Brasília: Ministério da Saúde; 2015. [Google Scholar]
- 27.Zihlmann KF, de Alvarenga AT, Casseb J. Living invisible HTLV-1-infected persons and the lack of care in public health. PLoS Negl Trop Dis. 2012;6:80. doi: 10.1371/journal.pntd.0001705. [DOI] [PMC free article] [PubMed] [Google Scholar]


