Abstract
There are currently no antiviral therapies available for the tick-borne flaviviruses associated with hemorrhagic fevers: Kyasanur Forest disease virus (KFDV), both classical and the Alkhurma hemorrhagic fever virus (AHFV) subtype, and Omsk hemorrhagic fever virus (OHFV). In this brief study, we describe the in vitro antiviral activity of adenosine analog NITD008 against KFDV, AHFV, OHFV, as well as Tick-borne Encephalitis virus (TBEV). Alongside the well-established activity of NITD008 against mosquito-borne flaviviruses, our results have demonstrated the feasibility of identifying nucleoside analog inhibitors that have pan-flavivirus activity.
Keywords: Flavivirus, NITD008, Antiviral, Nucleoside analog, Tick-borne
Tick-borne flaviviruses within the family Flaviviridae are among the most medically important arboviruses globally, with Tick-borne Encephalitis virus (TBEV) being the best-studied virus within the tick-borne encephalitis (TBE) serocomplex (Dobler, 2010). Despite the availability of several vaccines against TBEV, there are still thousands of human TBE cases detected annually across Eurasia (Amicizia et al., 2013). Moreover there are currently no antiviral therapies approved against TBEV nor against the lesser-known but highly pathogenic tick-borne flaviviruses known to cause hemorrhagic fever symptoms in humans, which include Omsk hemorrhagic fever virus (OHFV), Kyasanur forest disease virus (KFDV) and its close variant Alkhurma hemorrhagic fever virus (AHFV) (Dodd et al., 2011; Gritsun et al., 2003). The lack of antiviral therapies warrant the development of treatments based on specific inhibitors of flavivirus replication. However, antiviral research against these TBVEs has been under performed because of the requirement of high level bio-safety containment. Targeting viral polymerases with nucleoside analogs has been a common approach to antiviral development which has yielded efficacious therapies against several viruses including Hepatitis B, Human Immunodeficiency Virus 1, and Hepatitis C, another member of the Flaviviridae family (Arts and Hazuda, 2012; Asselah, 2014; Menendez-Arias et al., 2014). A recently-characterized adenosine nucleoside analog NITD008 was shown to inhibit replication of mosquito-borne flaviviruses (including West Nile, Dengue, and Yellow Fever viruses) as well as the tick-borne flavivirus (Powassan virus (POWV)) (Yin et al., 2009). Given the activity of NITD008 against POWV, we evaluated the antiviral activity of NITD008 against TBEV (strain Hypr), OHFV (strain Bogoluvovska), KFDV (strain P9605), and AHFV (strain 200300001) from the Centers for Disease Control and Prevention (CDC) Viral Special Pathogens reference collection. All experiments were performed within the CDC Biosafety Level-4 High Containment Laboratory.
We initially assayed NITD008 for its inhibition of virus-induced cytopathic effect (CPE) as previously described (Flint et al., 2014). Briefly, 2 × 104 human lung carcinoma (A549) cells (ATCC, Manassas, VA, USA) in 96-well opaque white plates (Costar, Corning, NY, USA) were pre-treated for 1 h with 3-fold serial dilutions of NITD008 (starting concentration was 100 μM) in quadruplicate and then mock-infected or infected with one of the above mentioned viruses at a multiplicity of infection (MOI) of 0.5. On day three post-infection, cell viability was determined using CellTiter-Glo 2.0 reagent (Promega, Madison, WI, USA). Concentrations of NITD008 that inhibited 50% of the virus-induced cell death (EC50) were calculated from dose–response data fitted to a 4-parameter logistic curve generated using GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA). The 50% cytotoxic concentration (CC50) for the mock-infected cells was derived in similar fashion, and the selectivity index (SI) was calculated by dividing CC50 by EC50. We observed inhibition of CPE against all 4 tick-borne flaviviruses that correlated with increasing concentrations of NITD008, with EC50 values ranging from 0.61 to 3.31 μM (Fig. 1A, Table 1). In contrast, NITD008 showed little to no antiviral activity against a reporter Ebolavirus expressing enhanced Green Fluorescent Protein (EBOV-eGFP) (Towner et al., 2005) in Vero cells (CCL-81, ATCC, Manassas, VA, USA) (Fig. 1B). NITD008 consistently showed lower antiviral activity against AHFV compared to the other three tested viruses across 4 independent experiments (p > 0.0001; Two-way Analysis of Variance of LogEC50 values, Tukey’s multiple comparisons test, Alpha = 0.001; Fig. 1A). The CC50 values derived from both mock-infected A549 and Vero cells treated with 3-fold dilutions of NITD008 was >100 μM (Fig. 1C, Table 1).
Fig. 1.
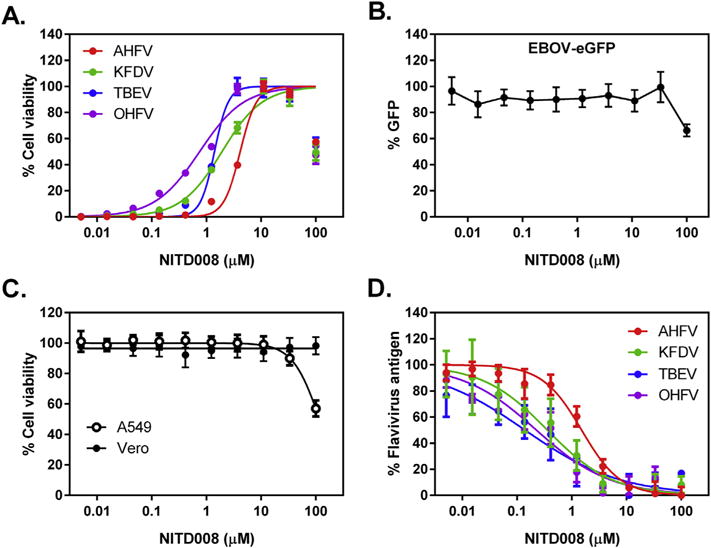
NITD008 inhibits flavivirus-induced cytopathic effect and also reduces levels of flavivirus antigen in infected cells. (A) Cytopathic Effect (CPE) Assay. Representative dose–response curves for AHFV (red), KFDV (green), OHFV (purple), and TBEV (blue) against NITD008. Tick-borne flavivirus Infected A549 cells were incubated with NITD008 at 3-fold serial dilutions for 72 h. Cell viability was measured using CellTiter-Glo 2.0 reagent and presented as a percentage of luminescence detected from the compound-treated cells compared with mock-treated cells. (B) Ebolavirus replication was measured in Vero cells treated with 3-fold serial dilutions of NITD008 by fluorescence levels emitted by the enhanced Green Fluorescent Protein at 48 h post-infection using a plate reader. (C) Cytotoxicity in mock-infected A549 and Vero cells. Mock-infected cells were incubated with NITD008 at 3-fold serial dilutions for 72 h. Cell viability was measured and presented in the same manner as the CPE assay. (D) Cell-based Flavivirus Immunodetection (CFI) assay. Dose-response curves for AHFV (red), KFDV (green), OHFV (purple), and TBEV (blue) against NITD008. Tick-borne flavivirus infected A549 cells were incubated with NITD008 at 3-fold serial dilutions for 24 h, and then fixed and stained with primary anti-flavivirus HMAF and secondary goat anti-mouse antibody conjugated with HRP, respectively. Levels of flavivirus antigen present in infected cells was measured by chemiluminescence, and were presented as a percentage of luminescence detected from the compound-treated cells compared with mock-treated cells. Error bars indicate standard deviation of the means.
Table 1.
Summary of NITD008 results.
| Virus ID | CPEa EC50 (μM) | CFIc EC50 (μM) | VTRd EC50 (μM) | CC50 (μm)a | SI (CPE) | SI (CFI) | SI (VTR) |
|---|---|---|---|---|---|---|---|
| AHFV | 3.31 ± 0.49 | 1.51 | 9.29 | >100 | >30 | >66 | >11 |
| KFDV | 1.42 ± 0.30 | 0.36 | 4.01 | >100 | >70 | >278 | >25 |
| OHFV | 0.61 ± 0.09 | 0.14 | 3.04 | >100 | >164 | >714 | >33 |
| TBEV | 0.90 ± 0.29 | 0.226 | 2.99 | >100 | >111 | >442 | >33 |
| EBOV-GFPb | >100 | ND | ND | >100 | ND | ND | ND |
Effective 50% inhibition and cytotoxic concentrations (EC50 & CC50) were calculated by non-linear regression analysis using GraphPad 6 software. Selectivity index (SI) = CC50/EC50. ND = Not determined.
Cytopathic Effect (CPE) assay. 4 independent experiments were performed for each virus in A549 cells. In each experiment, there were 4 biological replicates for each dilution of NITD008. ± indicates standard deviation from the mean values from the 4 experiments.
Ebolavirus replication was measured indirectly in Vero cells by measuring fluorescence levels emitted by the enhanced green fluorescent protein in a Biotek Synergy H1MD plate reader. One experiment was performed in which there were 4 biological replicates for each dilution of NITD008 treatment.
Cell-based flavivirus immunodetection (CFI) ELISA. One experiment was performed in A549 cells in which there were 3 biological replicates for each dilution of NITD008 against each virus. Chemiluminescence served as the indicator of viral antigen levels present, and was measured in a Biotek Synergy H1MD plate reader.
Virus titer reduction (VTR). One experiment was performed in A549 cells in which there were 4 biological replicates for each dilution of NITD008 against each virus.
We further evaluated the antiviral activity of NITD008 by performing a cell-based flavivirus immunodetection (CFI) enzyme-linked immunosorbent assay (ELISA) that measured the amount of viral protein in infected cells (Yin et al., 2009). As done for the CPE assay, A549 cells were seeded in 96-well opaque white plates, pretreated for 1 h with 3-fold serial dilutions of NITD008, and then infected with either TBE, OHFV, KFDV, or AHFV at MOI = 0.5. At 24 h post-infection, the cells were fixed for 15 min in 10% formalin supplemented with 0.2% Triton-X100 detergent, and assayed for levels of intracellular viral protein by ELISA. Anti-flavivirus hyper immune mouse ascites fluid (HMAF) and goat anti-mouse IgG conjugated with horseradish peroxidase (ThermoFisher, Grand Island, NY, USA) were used as primary and secondary antibodies for the CFI ELISA, respectively. Similarly to the CPE assay, we observed a dose-dependent reduction of flavivirus antigen as the CFI ELISA yielded EC50 values ranging from for 0.14–1.51 μM (Fig. 1D, Table 1).
We next performed an immunofluorescence assay (IFA) to visually verify dose-dependent reduction of flavivirus antigen. A549 cells were pretreated with NITD008, infected with individual viruses for 24 h, and fixed as for the CFI ELISA. Cells were stained with a primary anti-flavivirus HMAF and a secondary donkey anti-mouse antibody conjugated with DyLight 488 (Bethyl Laboratories, Bethesda, MD, USA). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA USA), and images were captured at 40 × magnification using a Nikon Eclipse Ti inverted fluorescence microscope (Nikon, Melville, NY, USA). For all 4 flaviviruses tested, we observed the dose-dependent reduction of flavivirus antigen by NITD008 correlating with the dose–response data obtained from the CPE and CFI assays (Fig. 2, Table 1).
Fig. 2.
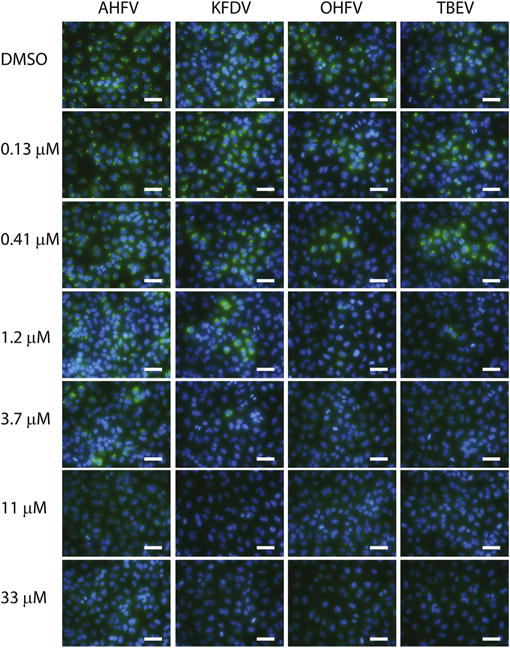
Immunofluorescence Assay (IFA). A549 cells were pretreated with NITD008, infected with indicated viruses for 24 h. Cells were fixed for 15 min in 10% formalin supplemented with 0.2% Triton-X detergent, then stained with a primary anti-flavivirus HMAF and a secondary donkey anti-mouse antibody conjugated with DyLight 488 (green). Cells were counterstained with DAPI (blue), and images were captured at 40 × magnification using a Nikon Eclipse Ti inverted fluorescence microscope. White bar indicates length of 100 μm.
Lastly, we performed viral titer reduction assays against all 4 viruses. A549 cells were infected at MOI 0.5 with either TBE, OHFV, KFDV, or AHFV for 1 h. The virus inoculum was aspirated, and the cells were washed once with PBS before being replenished with growth media containing 3-fold serial dilutions of NITD008. At three days post-infection, virus supernatants were used to determine 50% tissue culture infectious doses (TCID50) using A549 cells (8 wells per dilution). Five days post-infection, the cell monolayers were scored for CPE and endpoint virus titers were calculated using the Reed and Muench method (Reed and Muench, 1938). As shown in Fig. 3, NITD008 inhibited all 4 viruses, with EC50 values ranging from 3 to 9 μM (Table 1). Treatment with 11.1 mM of NITD008 reduced viral titers by 102.5–105.5-fold, which is comparable to the activity observed against mosquito-borne flaviviruses (Yin et al., 2009).
Fig. 3.
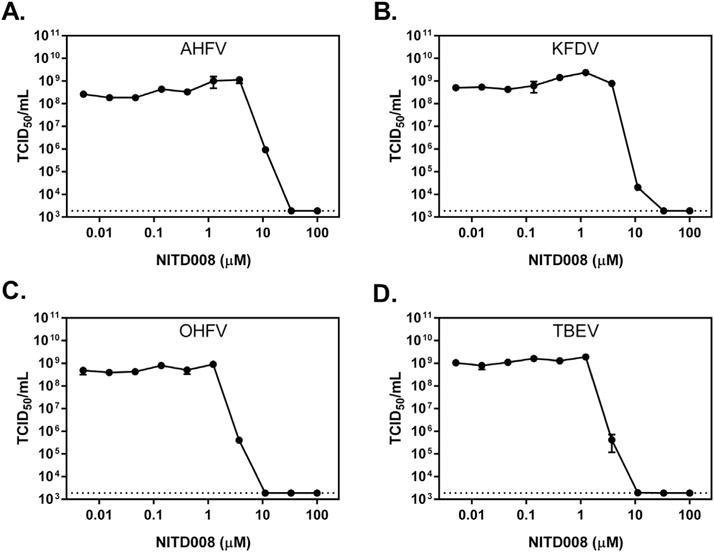
NITD008 inhibits tick-borne flavivirus replication. A549 cells were infected with (A) AHFV, (B) KFDV, (C) OHFV, or (D) TBEV at an MOI of 0.5 for 1 h before being treated with NITD008. Seventy-two h post-infection, the supernatants were harvested and were subjected to one freeze–thaw cycle before median tissue culture infective dose were determined (TCID50/Ml). Mean titers from four biological replicates are depicted, and error bars indicate standard error of the means. Dotted lines indicate the limit of detection.
In summary, our study demonstrates that NITD008 inhibits tick-borne flaviviruses in vitro. Four different assays (CPE, CFI, IFA, and viral titer reduction) were used determine the potency of the compounds against four tick-borne flaviviruses. For each tested virus, the individual assays revealed different EC50 values (Table 1). The discrepancy in EC50 values among the assays was expected because each assay measured different cellular or viral parameters to indicate the compound’s antiviral activity. Despite the variation of EC50 values, the relative ranking of the compound potency against the four viruses remains the same, with the lowest potency being against AHFV and the highest potency being against OHFV and TBEV (Table 1). The difference in potency among the four tested viruses is likely determined by the intrinsic variation of the viral polymerase’s ability to incorporate the triphosphate form of NITD008 into the viral RNA chain during RNA polymerization. At the amino acid level, the NS5 polymerase of AHFV shares approximately 85% identity with the respective TBEV and OHFV polymerases, while sharing almost 98% identity with the KFDV polymerase used in this study. Identifying the residues that account for the lower potency of NITD008 against AHFV may lead to the development of a more effective pan-flavivirus nucleoside analog. Collectively, the results have provided the basis for future evaluation of NITD008 against tick-borne flaviviruses in vivo. It should be noted that since TBEVs are neurotropic, an efficacious compound should be able to penetrate the blood–brain barrier in order to achieve antiviral activity in vivo. Accordingly, a recent study showed that NITD008 treatment protected mice infected with West Nile virus, another neurotropic flavivirus (Nelson et al., 2015). In vivo efficacy studies are needed to demonstrate the utility of NITD008 for TBEV treatment. However, the likelihood of advancing NITD008 into clinical use is low because the therapeutic window of the compound is not more potent against TBEVs (EC50 0.14–9.2 μM) than dengue virus (EC50 0.16–2.6 μM (Yin et al., 2009);). Nevertheless, combined with the well-established activity of NITD008 against mosquito-borne flaviviruses, the current study has demonstrated the feasibility of identifying a nucleoside inhibitor with pan-flavivirus activity (Yin et al., 2009). While vaccines remain the most effective means for preventing flavivirus infections, to date, the administration of both TBEV and KFDV vaccines have not prevented human outbreaks of their respective diseases (Amicizia et al., 2013; Dandawate et al., 1994; Heinz et al., 2013). Development of a pan-flavivirus nucleoside inhibitor for clinical treatment could ameliorate acute disease symptoms, which would likely improve disease outcomes (Bogovic and Strle, 2015; Holbrook, 2012; Ruzek et al., 2010).
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent those of the Centers for Disease Control and Prevention. This work was financially supported by CDC core funding.
References
- Amicizia D, Domnich A, Panatto D, Lai PL, Cristina ML, Avio U, Gasparini R. Epidemiology of tick-borne encephalitis (TBE) in Europe and its prevention by available vaccines. Hum Vaccin Immunother. 2013;9:1163–1171. doi: 10.4161/hv.23802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts EJ, Hazuda DJ. HIV-1 antiretroviral drug therapy. Cold Spring Harb Perspect Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselah T. Sofosbuvir for the treatment of hepatitis C virus. Expert Opin Pharmacother. 2014;15:121–130. doi: 10.1517/14656566.2014.857656. [DOI] [PubMed] [Google Scholar]
- Bogovic P, Strle F. Tick-borne encephalitis: a review of epidemiology, clinical characteristics, and management. World J Clin Cases. 2015;3:430–441. doi: 10.12998/wjcc.v3.i5.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandawate CN, Desai GB, Achar TR, Banerjee K. Field evaluation of formalin inactivated Kyasanur forest disease virus tissue culture vaccine in three districts of Karnataka state. Indian J Med Res. 1994;99:152–158. [PubMed] [Google Scholar]
- Dobler G. Zoonotic tick-borne flaviviruses. Vet Microbiol. 2010;140:221–228. doi: 10.1016/j.vetmic.2009.08.024. [DOI] [PubMed] [Google Scholar]
- Dodd KA, Bird BH, Khristova ML, Albarino CG, Carroll SA, Comer JA, Erickson BR, Rollin PE, Nichol ST. Ancient ancestry of KFDV and AHFV revealed by complete genome analyses of viruses isolated from ticks and mammalian hosts. PLoS Negl Trop Dis. 2011;5:e1352. doi: 10.1371/journal.pntd.0001352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint M, McMullan LK, Dodd KA, Bird BH, Khristova ML, Nichol ST, Spiropoulou CF. Inhibitors of the tick-borne, hemorrhagic fever-associated flaviviruses. Antimicrob Agents Chemother. 2014;58:3206–3216. doi: 10.1128/AAC.02393-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsun TS, Nuttall PA, Gould EA. Tick-borne flaviviruses. Adv Virus Res. 2003;61:317–371. doi: 10.1016/s0065-3527(03)61008-0. [DOI] [PubMed] [Google Scholar]
- Heinz FX, Stiasny K, Holzmann H, Grgic-Vitek M, Kriz B, Essl A, Kundi M. Vaccination and tick-borne encephalitis, central Europe. Emerg Infect Dis. 2013;19:69–76. doi: 10.3201/eid1901.120458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook MR. Kyasanur forest disease. Antivir Res. 2012;96:353–362. doi: 10.1016/j.antiviral.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Arias L, Alvarez M, Pacheco B. Nucleoside/nucleotide analog inhibitors of hepatitis B virus polymerase: mechanism of action and resistance. Curr Opin Virol. 2014;8:1–9. doi: 10.1016/j.coviro.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Nelson J, Roe K, Orillo B, Shi PY, Verma S. Combined treatment of adenosine nucleoside inhibitor NITD008 and histone deacetylase inhibitor vorinostat represents an immunotherapy strategy to ameliorate West Nile virus infection. Antivir Res. 2015;122:39–45. doi: 10.1016/j.antiviral.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Ruzek D, Yakimenko VV, Karan LS, Tkachev SE. Omsk haemorrhagic fever. Lancet. 2010;376:2104–2113. doi: 10.1016/S0140-6736(10)61120-8. [DOI] [PubMed] [Google Scholar]
- Towner JS, Paragas J, Dover JE, Gupta M, Goldsmith CS, Huggins JW, Nichol ST. Generation of eGFP expressing recombinant Zaire ebolavirus for analysis of early pathogenesis events and high-throughput antiviral drug screening. Virol. 2005;332:20–27. doi: 10.1016/j.virol.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Yin Z, Chen YL, Schul W, Wang QY, Gu F, Duraiswamy J, Kondreddi RR, Niyomrattanakit P, Lakshminarayana SB, Goh A, Xu HY, Liu W, Liu B, Lim JY, Ng CY, Qing M, Lim CC, Yip A, Wang G, Chan WL, Tan HP, Lin K, Zhang B, Zou G, Bernard KA, Garrett C, Beltz K, Dong M, Weaver M, He H, Pichota A, Dartois V, Keller TH, Shi PY. An adenosine nucleoside inhibitor of dengue virus. Proc Natl Acad Sci U S A. 2009;106:20435–20439. doi: 10.1073/pnas.0907010106. [DOI] [PMC free article] [PubMed] [Google Scholar]


