Abstract
Background
Amygdala-prefrontal cortex (PFC) functional connectivity may be influenced by anxiety and development. A prior study on anxiety found age-specific dysfunction in the ventromedial PFC (vmPFC), but not amygdala, associated with threat-safety discrimination during extinction recall (Britton et al., 2013). However, translational research suggests that amygdala-PFC circuitry mediates responses following learned extinction. Anxiety-related perturbations may emerge in functional connectivity within this circuit during extinction recall tasks. The current report uses data from the prior study to examine how anxiety and development relate to task-dependent amygdala-PFC connectivity.
Methods
Eighty-two subjects (14 anxious youths, 15 anxious adults, 25 healthy youths, 28 healthy adults) completed an extinction recall task, which directed attention to different aspects of stimuli. Generalized psychophysiological interaction analysis tested whether task-dependent functional connectivity with anatomically-defined amygdala seed regions differed across anxiety and age groups.
Results
Whole-brain analyses showed significant interactions of anxiety, age, and attention task (i.e., threat appraisal, explicit threat memory, physical discrimination) on left amygdala functional connectivity with the vmPFC and ventral anterior cingulate cortex (Talairach XYZ coordinates: −16, 31, −6 and 1, 36, −4). During threat appraisal and explicit threat memory (vs. physical discrimination), anxious youth showed more negative amygdala-PFC coupling, whereas anxious adults showed more positive coupling.
Conclusions
In the context of extinction recall, anxious youths and adults manifested opposite directions of amygdala-vmPFC coupling, specifically when appraising and explicitly remembering previously learned threat. Future research on anxiety should consider associations of both development and attention to threat with functional connectivity perturbations.
Keywords: Anxiety/Anxiety disorders, Child/Adolescent, Functional MRI, GAD/generalized anxiety disorder, SAD/social anxiety disorder/social phobia
Introduction
Translational research implicates amygdala-prefrontal cortex (PFC) circuitry in anxiety and development(1–5). Moreover, because dysfunction in this circuit may provide a target for exposure therapy, such research could inform therapeutics(6,7). A previous functional magnetic resonance imaging (fMRI) study of extinction recall found that patients with an anxiety disorder, relative to healthy individuals, showed age-specific dysfunction in the ventromedial PFC (vmPFC), but not amygdala(8). Of note, translational research suggests that extinction recall is sustained by activity in the amygdala-PFC circuit, and that aberrant functional connectivity in this circuit mediates anxiety-related perturbations(5,9,10). Such findings are consistent with other human studies linking anxiety, development, and amygdala-PFC functional connectivity(11–14). However, Britton et al.(8) did not examine functional connectivity. Using data from this previous report, the current study examines task-based variation in amygdala-PFC connectivity as a function of anxiety and development.
While ample fMRI research examines anxiety-related perturbations in regional activation(3,15–17), circuitry-level dysfunction is now a central research interest(1,18,19). In particular, current research focuses on a circuit connecting the amygdala, involved in fear acquisition and expression(20–22), to the vmPFC, involved in extinction recall(23–29). Prior fMRI studies demonstrate that the amygdala is activated during extinction learning, whereas the vmPFC, but not the amygdala, is activated during the recall of extinction(24,25). While prior studies find anxiety-related group differences in the vmPFC, but not amygdala, during extinction recall(8,30,31), amygdala dysfunction may manifest during extinction recall in the form of aberrant patterns of task-based connectivity with the vmPFC(5).
Task-dependent connectivity studies probe anxiety-related dysfunction in amygdala-PFC circuitry when participants process emotional stimuli(14,19,32–36). For example, studies on adult social anxiety disorder show amygdala-PFC connectivity differences associated with negative emotion stimuli(15), with findings of both greater(36,37) and less(32) connectivity across studies. Of note, most work on anxiety-related differences in amygdala-PFC interactions examines adults. The few relevant studies in youth find complex patterns of amygdala-PFC connectivity, which vary across studies (11,38,39). Such inconsistencies could reflect cross-study differences in task paradigms. Studies employing tasks where there is strong relevance from both the basic and clinical literature may clarify the nature of amygdala-PFC dysfunction in anxiety. Translational research suggests that extinction recall and threat-safety discrimination tasks hold promise in this regard(1).
Development influences both the structure and function of the PFC and amygdala, as well as the functional coupling between the two regions(12,19,40–46). A recent study found that young healthy children showed positive amygdala-mPFC connectivity, whereas adolescents showed negative connectivity when viewing fearful faces compared to a baseline fixation condition(12). Other studies find greater resting-state amygdala-mPFC connectivity in adults than adolescents(47). To our knowledge, no study compares task-based connectivity among healthy and anxious adolescents and adults. There is a particular need for developmental studies of task-based connectivity associated with fear learning and extinction(48–52).
Prior research suggests that associations among development, anxiety, and amygdala-PFC circuitry dysfunction manifest in tasks that focus attention on threat features. This research suggests that group differences in functional connectivity vary with both development(12,43,44) and anxiety(11,19,32,38,53). Such differences may be expected specifically in anxious individuals when attention is directed to threat features, a clinically relevant psychological state(8,53–56). In Britton et al.(8), anxious and non-anxious youths and adults completed difficult threat-safety discrimination tasks during extinction recall, and task conditions modulated between-group differences in vmPFC but not amygdala function. Of note, this prior study only targeted regional activation differences. No other study compares amygdala-vmPFC connectivity during a threat-safety discrimination task among healthy and anxious adolescents and adults.
In this secondary analysis using data from Britton et al.(8), we compare anxious and non-anxious adolescents and adults in their functional connectivity during threat-safety discrimination tasks. Using a factorial design, we test how attention to threat modulates amygdala functional connectivity as a function of anxiety diagnosis and development. Prior findings show both shared and age-specific dysfunction in vmPFC regions among anxious youths and adults during extinction recall(8), as well as anxiety-related differences in amygdala-PFC connectivity(11,15,32,38,53); these inform study hypotheses. This study tests the hypothesis that healthy participants, as compared to anxious participants, exhibit greater amygdala-vmPFC connectivity when attention is directed to threat features, relative to the non-threat control condition. In addition, given prior findings of age-specific vmPFC dysfunction, we also tested the hypothesis that anxious youths and adults differ in amygdala-vmPFC connectivity during threat appraisal.
Materials and Methods
Participants
The current report examines functional connectivity for the extinction recall task reported in Britton et al.(8). Participants completed a two-visit study involving fear acquisition/extinction in a psychophysiology laboratory (Visit 1) and extinction recall in an MRI scanner (Visit 2). For Visit 1, 143 individuals completed fear acquisition/extinction procedures, but 29 participants discontinued these procedures. Of the 114 participants who completed Visit 1, 32 participants were excluded from Visit 2 for various reasons (e.g., scheduling difficulties, MRI contraindications, excessive motion, and technical problems; see (8) for details). The final MRI sample included 14 anxious youths, 15 anxious adults, 25 healthy youths, and 28 healthy adults (Table 1). In the current investigation, anxiety and age group differences in functional connectivity were tested using the MRI data from this sample.
Table 1.
Sample Characteristics
| Anxious | Healthy | |||
|---|---|---|---|---|
|
|
||||
| Youths (14) | Adults (15) | Youths (25) | Adults (28) | |
| Age (Years) | 14.76 (2.82) | 32.90 (6.97) | 14.42 (2.62) | 29.10 (7.51) |
| IQ | 108.00 (10.86) | 122.33 (9.97) | 111.68 (10.50) | 120.36 (10.43) |
| Diagnosis (N) | ||||
| GAD | 11 | 11 | – | – |
| Social Phobia | 9 | 7 | – | – |
| SAD | 2 | 0 | – | – |
| Panic Disorder | 1 | 1 | – | – |
| MDDa | 4 | 0 | – | – |
| Specific Phobia | 4 | 0 | – | – |
| ADHD | 1 | 0 | – | – |
| PARS | 13.70 (3.56) | – | – | – |
| STAI state | 34.64 (5.61) | 42.27 (6.09) | 28.52 (4.03) | 25.41 (5.38) |
| SCARED (Parent) | 35.17 (16.62) | – | 2.90 (3.18) | – |
| SCARED (Child) | 36.17 (18.70) | – | 8.48 (4.99) | – |
| CDI/BDI | 14.00 (8.03) | 6.90 (4.01) | 2.27 (2.93) | 0.85 (1.26) |
| Days between visits | 18.50 (6.80) | 20.93 (6.64) | 22.08 (12.57) | 17.61 (9.10) |
Data reported as mean (SD) unless otherwise specified.
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BDI, Beck Depression Inventory; CDI, Child Depression Inventory; GAD, generalized anxiety disorder; MDD, major depressive disorder; PARS, Pediatric Anxiety Rating Scale; SAD, separation anxiety disorder; SCARED, Screen for Child Anxiety Related Emotional Disorders; SD, standard deviation; STAI, Spielberger State-Trait Anxiety Inventory.
Anxious youths and anxious adults significantly different in rate of comorbid MDD (Fisher’s exact test: p=.042).
Based on the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID)(57) for adults and the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (KSADS-PL)(58) for youths, anxious individuals met DSM-IV-TR criteria for current primary diagnosis of generalized anxiety disorder or social phobia. Anxious individuals were permitted to have comorbid major depressive disorder (MDD) and additional anxiety disorders, but obsessive-compulsive disorder, posttraumatic stress disorder, and other comorbid disorders were exclusionary. Participants in the anxious groups were required to have not received medication for their current episode of anxiety; in practice, this translated to a six-month or greater medication-free period. Healthy volunteers were free of any current Axis I disorders. For all groups, IQ <70 (ascertained by the Vocabulary and Matrix Reasoning subscales of the Wechsler Abbreviated Scale of Intelligence), current physical health problems, and current psychotropic medication use were exclusionary.
Procedures
After receiving complete description of the study, adult participants and parents of youth participants provided written informed consent, and youth provided written assent. All procedures were approved by the National Institute of Mental Health Institutional Review Board.
Participants completed two visits involving (1) a differential fear acquisition and extinction paradigm conducted in the psychophysiological laboratory, followed by (2) a threat-safety discrimination paradigm conducted in the MRI scanner three weeks later (mean=19.7 days, SD = 9.7). The procedures for both paradigms are described in detail in Britton et al.(8) and briefly summarized here.
Fear Acquisition/Extinction Task
During differential fear acquisition, participants passively viewed two neutral female faces, which served as the conditioned stimuli (CS: CS+ and CS−). Counterbalanced across participants, one neutral face (CS+) predicted the unconditioned stimulus (US), i.e., a fearful face of the same identity paired with a loud (95dB), aversive scream. The CS− was never paired with the US. Across all four groups, psychophysiological measures (skin conductance response and startle electromyography) and self-reported anxiety demonstrated robust differential fear acquisition. Compared to both healthy groups, both anxious groups reported higher subjective ratings of anxiety for both the CS+ and CS−, but did not differ in psychophysiological responding or rates of conditioning. Following fear acquisition, participants underwent fear extinction procedures in which both CS+ and CS− were presented without the US. Although the anxious groups exhibited deficits in extinction based on subjective anxiety ratings, anxiety-related differences were absent in psychophysiological responses during extinction(8).
Extinction Recall Task
During fMRI scanning, participants made threat-safety discrimination decisions. To probe psychological processes underlying clinical anxiety and basic discrimination processes, three different yes/no questions were asked in separate blocks, reflecting the following three attention conditions. First, the “threat appraisal” condition probed subjective fear (i.e., “Are you afraid now?”). Second, the “explicit memory” condition assessed participants’ recollections of the conditioned stimuli (i.e., “Did she scream in the past?”). Third, the “physical discrimination” condition directed attention to perceptual discrimination of the facial image (i.e., “Is her hair jet black?”). For each stimulus, participants answered the specific yes/no question via button press.
Participants completed two runs of 4 blocks per attention condition presented in random order, resulting in 8 blocks per condition (24 total blocks). Within each block, participants viewed the two neutral faces (CS+ and CS−) previously presented during acquisition/extinction in Visit 1, nine morphed images created by combining features of these faces, and two blank images, providing an implicit-baseline condition. Trials were presented in random order and lasted 3 seconds with a 0.5 second inter-stimulus interval. MRI data were acquired using two 3T General Electric Signa scanners (Waukesha, WA) with an 8-channel head coil. The MRI acquisition parameters and preprocessing procedures are described in the supplemental material and reported in detail elsewhere(8).
Generalized Psychophysiological Interaction (gPPI) Analysis
Task-dependent changes in functional connectivity were tested using a generalized form of context-dependent psychophysiological interaction analysis (gPPI)(59,60). Context-dependent functional connectivity approaches, such as PPI (59–61), measure changes in functional connectivity modulated by task conditions. The goal of gPPI was to identify brain regions that differ in their functional coupling with the amygdala depending on attention condition, and then to determine whether this condition-specific functional coupling varied by anxiety and development.
Functional connectivity analysis was conducted with AFNI software (Analysis of Functional NeuroImages, http://afni.nimh.nih.gov/afni/). To conduct gPPI analysis, three components are needed: (1) the time series from a seed region, (2) separate regressors for each of the task conditions, and (3) separate interaction terms for the product of the seed time series and each task regressor (i.e., psychophysiological interaction [PPI] term). In the current analysis, we used the amygdala as the seed region given our hypotheses on group differences in amygdala-PFC connectivity, the ability to easily and objectively define the amygdala based on clear anatomical boundaries, and the common use of amygdala seeds in prior studies of anxiety disorders using PPI methods(11,38,39,62). We utilized the whole amygdala as opposed to amygdala subregions for three reasons. First, the spatial resolution of our voxels (i.e., 2.5×2.5×2.5mm) limits our ability to quantify connectivity for relatively small amygdala sub-nuclei. Moreover, we wanted to minimize Type I errors, which could arise with multiple statistical tests being performed for the connectivity maps for each of the amygdala subnuclei. Finally, prior research relies on whole amygdala connectivity(38,63). The anatomical amygdala seed regions were created from binary masks derived from the Talairach Daemon atlas in AFNI and resampled to the post-processed functional imaging data (i.e., isotropic 2.5mm voxels). Separate gPPI analyses were conducted for the left and right anatomical amygdala seeds. After removing motion effects, the time series within each seed was extracted from the scaled preprocessed fMRI data, and then detrended and deconvolved with the gamma variate hemodynamic response function (HRF). Three task regressors corresponding to the threat appraisal, explicit memory, and physical discrimination conditions were generated. For each attention condition, times points corresponding to the particular attention condition (e.g., threat appraisal) were coded as +1, and all other time points (e.g., explicit memory, physical discrimination conditions) were coded as 0. Finally, the PPI regressor for each of the three attention conditions was generated as the product of the deconvolved-seed time series and the task regressor. Given that the stimulus onset times were not synchronized with the acquisition TR (repetition time) grids, both the seed and task regressors were up-sampled prior to generating the PPI regressors. Additionally, all regressors were initially generated within each run separately. As a final step, PPI regressors were then reconvolved using the gamma variate HRF, down-sampled, and concatenated across all runs.
Separate individual-level gPPI general linear model (GLM) analyses were conducted for each amygdala seed using AFNI 3dDeconvolve. Each model included the three PPI regressors, the seed time series (left or right amygdala), and the regressors of the original model (i.e., 33 task condition regressors for each image type [3 attention conditions, 11 morphs per condition], six motion parameters, and regressors modeling linear trends across time for each run). Including both seed and task regressors of the original model in the GLM allows us to test the PPI term as the interaction effect above and beyond the main effects. To capture differences in functional connectivity with the amygdala seed depending on the attention condition, the three PPI regressors are tested in the group level as the within-subject effect.
Group-level analyses were conducted using AFNI 3dMVM(64). This model included between-subjects factors for anxiety (anxious and healthy) and age group (adolescent and adult), a within-subjects factor for the PPI (threat appraisal, explicit memory, and physical discrimination), and nuisance regressors for days between visits and scanner (2 levels). To test our hypotheses regarding anxiety and age effects on amygdala-cortical connectivity, we examined the three-way interaction of PPI-by-anxiety-by-age group, as well as both two-way interactions of PPI-by-anxiety and PPI-by-age group.
We applied a statistical correction for multiple comparisons to the omnibus analysis as reported in Britton et al.(8), using a whole-brain corrected threshold. Using a family-wise-error approach at the cluster level, AFNI AlphaSim determined the spatial extent threshold (number of voxels) using 10,000 Monte Carlo simulations with the cluster probability set to .05, the voxel-wise p-value of .005, and smoothness of 9.11mm, 9.03mm, and 8.21mm (x, y, and z axes, respectively). Smoothness was based on the average blur estimates of the residuals calculated by the AFNI 3dFWHMx program at the individual level, and then averaged across participants. At p<0.005, the whole-brain corrected cluster threshold was determined to be cluster size >90 voxels. Coordinates of peak voxels from the significant clusters are reported in Talairach space using LPI reference frame.
To decompose complex interactions, the mean beta-coefficient values for each PPI regressor in each individual participant were extracted from significant clusters using AFNI 3dROIstats and plotted. Extracted values were inspected for signal dropout in these regions, and values within all voxels were non-zero. Post-hoc tests were conducted in SPSS (IBM SPSS Statistics, Version 22.0). For post-hoc comparisons, significance was set to α=0.05. When Levene’s Test indicated the variances were not equal, we report t-test statistics with equal variances not assumed. We conducted post-hoc correlations between extracted PPI value difference scores and anxiety symptoms in the anxious youth and adult groups, separately, using the Screen for Child Anxiety Related Emotional Disorders (SCARED, average parent and child forms; n=11 anxious youths) and the Spielberger State-Trait Anxiety Inventory (STAI; State form: n=15; Trait form: n=9). Finally, exploratory post-hoc analyses of covariance controlled for the effect of IQ on significant clusters given the significant age difference in IQ (Table 1).
Results
Voxelwise gPPI results for the 3-way interaction of anxiety, age group, and attention condition are reported in Table 2.
Table 2.
Whole-brain Psychophysiological Interaction (PPI) Results
| Talairach Coordinates (LPI) | Cluster size | F (2, 152) | |||
|---|---|---|---|---|---|
| x | y | z | # voxels | ||
| 3-way Interaction of Diagnosis, Age Group, and Attention Condition | |||||
| Left Amygdala Seed | |||||
| Ventromedial PFC | −16 | 31 | −6 | 130 | 10.2 |
| Ventromedial PFC/Ventral ACC | 1 | 36 | −4 | 93 | 10.5 |
| Right Amygdala Seed | |||||
| None | |||||
| 2-way Interaction of Diagnosis and Attention Condition | |||||
| None | |||||
| 2-way Interaction of Age Group and Attention Condition | |||||
| Left Amygdala Seed | |||||
| Left dorsolateral PFC/caudate | −26 | −19 | 36 | 177 | 9.4 |
| Right Amygdala Seed | |||||
| Left Insular Cortex | −34 | −6 | 19 | 152 | 11.2 |
Abbreviations: ACC, anterior cingulate cortex; PFC, prefrontal cortex
Whole-brain corrected threshold: p<.005, cluster size >90 voxels.
Left amygdala seed
For the left amygdala seed, the whole-brain corrected PPI analysis for the 3-way interaction revealed one significant cluster in the vmPFC (130 voxels; Figure 1a) and a second in the vmPFC/ventral anterior cingulate cortex (vmPFC/vACC; 93 voxels; Figure 1b). To decompose these interactions, post-hoc analyses were conducted on the extracted beta-coefficient values for PPI (i.e., connectivity strength), comparing each of the two threat conditions to the non-threat condition. Specifically, post-hoc analyses used difference scores to compare the connectivity strength for threat appraisal and explicit threat memory (i.e., threat conditions) relative to the physical discrimination condition (i.e., non-threat condition).
Figure 1.
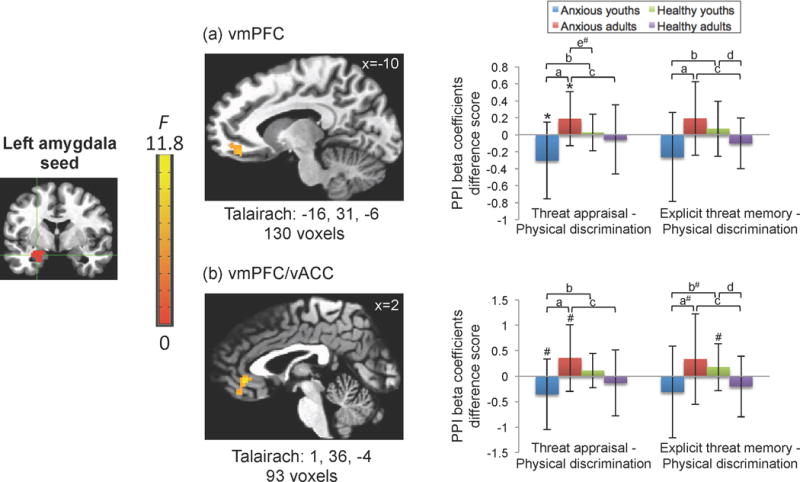
Anxious youths and adults exhibit opposite patterns of left amygdala-prefrontal cortex connectivity in threat relative to non-threat conditions.
Generalized psychophysiological interaction (gPPI) analysis using an anatomically-defined left amygdala seed revealed a significant 3-way interaction of diagnosis, age group, and attention condition in two clusters. Two clusters appear in the (a) vmPFC (Talairach coordinates: −16, 31, −6) and (b) the vmPFC/vACC (Talairach coordinates 1, 36, −4) survived whole-brain correction. Images are shown in neurological convention (i.e., left is left) and thresholded at F(2,152) > 5.48, p<.005, cluster size > 90 voxels.
To decompose these complex interaction effects, the mean beta coefficient values were extracted from significant clusters for each of the three PPI regressors: threat appraisal, explicit threat memory, and physical discrimination and extracted values were averaged across participants in each group. In the graphs, the x-axis (from left to right) represents the difference scores for threat appraisal minus physical discrimination and explicit threat memory minus physical discrimination.
The y-axis shows the beta coefficient values for PPI regressor difference scores, in which negative values reflect more negative functional connectivity and positive values reflect more positive functional connectivity associated with the threat appraisal or explicit threat memory conditions relative to the physical discrimination condition.
Post-hoc pairwise comparisons are denoted as follows:
ap<.05, Anxious only: youth vs. adult;
bp<.05, Youths only: anxious vs. healthy;
cp<.05, Adults only: anxious vs. healthy;
dp<.05, Healthy only: youth vs. adult;
ep<.05, Anxious adults vs. healthy youths;
*p<.05; #p<.08.
Error bars represent +/− standard deviation.
As shown in Figure 1, for these scores, group differences among youth and adult anxiety patients emerged. In the PFC clusters, anxious youths and anxious adults showed opposite patterns of amygdala-PFC task-related connectivity differences. These differences manifested for both the threat appraisal (vmPFC: t(27)= −3.41, p=.002; vmPFC/vACC: t(27)= −2.84, p=.009) and explicit threat memory conditions (vmPFC: t(27)= −2.56, p=.016), relative to the physical discrimination condition. Specifically, as shown in Figure 1, anxious youths showed more negative PFC connectivity with the amygdala for the comparison of threat appraisal and physical discrimination (vmPFC: t(13)= −2.50, p=.027). However, anxious adults showed the opposite pattern of more positive PFC connectivity for threat appraisal compared to physical discrimination (vmPFC: t(14)=2.31, p=.037).
The supplemental material includes graphs of the extracted values for each of the three conditions separately (Figure S1). As shown in Figure 1 and S1, the anxious youth on average showed more negative connectivity to the threat appraisal and explicit threat memory conditions compared to more positive connectivity to the physical discrimination condition, albeit non-significant. In contrast, the anxious adults showed more positive connectivity to the threat appraisal and explicit threat memory conditions compared to more negative connectivity to the physical discrimination condition, albeit non-significant. These patterns generated the opposite connectivity signs for the difference scores between each of the threat conditions and the non-threat condition, accounting for the three-way interaction.
Additionally, differences were found between diagnostic groups and within the healthy groups. The amygdala-PFC connectivity in both anxious groups differed from that in their age-matched healthy counterparts for threat appraisal (all ps<0.05) and explicit threat memory difference scores (all ps<0.02), except for the vmPFC/vACC for youths (p=.076). Moreover, in contrasting explicit memory and physical discrimination conditions, healthy youths showed more positive PFC connectivity with the amygdala, whereas the healthy adults showed more negative connectivity with the amygdala (vmPFC: t(51)=2.01, p=.049; vmPFC/vACC: t(51)=2.58, p=.013).
No significant group connectivity differences between the two threat conditions were detected in any of the PFC clusters (all ps > .20). Finally, there were no significant diagnosis-by-attention task interactions, but there was an age group-by-attention task interaction in the connectivity between the left amygdala seed and the left dorsolateral cortex extending to left caudate (177 voxels).
Right amygdala seed
For the right amygdala seed, no regions survived the whole-brain corrected cluster threshold for the three-way interaction.
There was a significant two-way interaction revealing age group differences in the connectivity between right amygdala and left insular cortex as a function of attention condition (152 voxels). Youths showed more negative connectivity whereas adults showed more positive connectivity for threat appraisal (t(80)=−2.96, p=.004) and explicit threat memory (t(80)=−2.66, p=.009), relative to physical discrimination. However, there was no significant age group difference between the two threat conditions in the left insula (t(80)=−.56, p=.58). Similar to the left amygdala seed, the right amygdala seed showed no significant diagnosis-by-attention condition interactions.
Exploratory post-hoc analyses
We examined associations between connectivity and various continuous measures. There were no significant correlations of the extracted PPI difference scores with SCARED scores in the anxious youth (all ps>.25) or STAI scores in the anxious adult (all ps>.14) groups.
All clusters showing three-way or two-way interactions remained significant after controlling for IQ (all p’s ≤.001).
Discussion
The current study found amygdala-cortical connectivity to vary as a function of anxiety, development, and attention to threat vs. non-threat features, in the context of extinction recall. Specifically, anxious youth and adults exhibited opposite patterns of task-dependent amygdala-vmPFC connectivity. This finding suggests that anxiety- and development-related differences in functional connectivity vary with features of threat-related tasks.
Opposite directions of coupling occurred specifically in amygdala-vmPFC circuitry for the two anxious groups as a function of the task. Thus, a developmental switch manifested in amygdala-vmPFC connectivity, with more negative connectivity between ages 11–19 and more positive connectivity between ages 24–48 when anxious patients directed their attention to threat vs. non-threat features. Such age-related differences are consistent with prior imaging research in humans and more invasive studies in rodents and non-human primates(8,48,49,65). These data suggest that the relationship between amygdala-PFC connectivity and anxiety varies from adolescence to adulthood. Moreover, in the current study, both anxious groups tended to differ from their age-matched healthy counterparts, consistent with prior evidence of anxiety-related perturbations in amygdala-cortical connectivity during emotional processing(11,15,32,38,53).
While prior research consistently shows some form of anxiety-related perturbation, the nature of such perturbations varies across studies. Studies of resting-state fMRI link anxiety to perturbed intrinsic amygdala-PFC connectivity in adults(32,66–69) and youth(13,70,71). However, some studies only find anxiety-related differences in the magnitude of amygdala-PFC connectivity(32,66,69,70), while others find differences in direction(67,71). Methodological factors may contribute to these inconsistencies in resting-state fMRI studies, which are not designed to engage disorder-relevant psychological processes or within-subject reference states(72). However, similar inconsistencies arise in task-based functional connectivity studies; some studies find increased, and others decreased, amygdala-PFC connectivity(15). As such, methodological factors could influence our findings and elucidate inconsistencies in the literature. In fact, unique features of the current study provide an opportunity to ground the observed connectivity differences in behavioral and psychophysiological findings.
One relevant feature concerns levels of fear evoked by stimuli. More consistent cross-study patterns of amygdala-PFC connectivity may emerge when studies are categorized according to whether stimuli elicit between-group differences in reported fear. Differences in amygdala-PFC connectivity manifested when participants viewed stimuli that, as shown in Britton et al.(8), elicited such differences in reported fear. During the conditioning visit three weeks before scanning, both patient groups, relative to the healthy groups, reported higher levels of fear to neutral faces used as conditioned stimuli(8). These conditioned stimuli, and morphed images resembling the conditioned stimuli, were used during neuroimaging. Anxiety-related differences emerged when participants’ attention was directed to the threat features of the stimuli, relative to physical features. Patterns in the current study may occur when subjects engage disorder-relevant processes to stimuli that evoke more fear in patients than healthy participants.
Considerable research in healthy individuals finds age differences in amygdala-PFC circuitry(12,40,47). Specifically, as reviewed by Casey and colleagues, this work suggests that healthy adolescents express unique neural patterns during extinction, as compared to healthy children and adults(42,65). While some suggest that this research impacts treatment, the current findings emphasize the need for direct comparisons in patients and healthy individuals at each age group.
Methods in the current study may enhance anxiety-related between-group differences while minimizing task-related variation in healthy subjects. For example, while the current study found differences in connectivity among healthy and anxious participants, connectivity did not vary in healthy participants when attention was directed to threat content, relative to physical features. Thus, an attention task manipulation effective for probing diagnostic group differences may be less well suited for detecting task-related connectivity variations in healthy populations. However, methods commonly used in studies on age-related variation in healthy participants may have the opposite effect, maximizing task-related variation in healthy participants while minimizing anxiety-related between-group differences. Translational research studies should use tasks that enhance anxiety-related between-group differences, given that research on the development of biomarkers for medical illnesses suggests that direct impact on treatment follows when studies directly compare patients and healthy participants before and after patients receive treatment.
A second methodological feature provides another opportunity to ground observations on functional connectivity in other findings. Although no anxiety-related perturbations were detected in psychophysiological measures during the visit three weeks prior to scanning, age- and anxiety-related differences in subjective fear ratings were observed. Specifically, while both anxious youth and anxious adults differed from their age-matched healthy comparison group in levels of extinction quantified based on reported fear to the CS+ and CS−, the pattern of results differed by age group. Thus, anxiety-related differences in connectivity across age groups may occur in studies finding similar patterns of between-group differences in reported fear. In future work, more consistent age-specific patterns of amygdala-PFC connectivity also may emerge when studies are grouped according to whether stimuli elicit similar or different anxiety-related differences across age groups.
The observed anxiety-related differences in amygdala-vmPFC connectivity may inform clinical thinking, particularly with regard to therapeutics. The best-established behavioral treatment for anxiety disorders, cognitive-behavioral therapy (CBT), applies largely the same principles and techniques to the treatment of adult and pediatric anxiety. In general, for all age groups, CBT produces sizable benefits, though up to half of all patients receiving CBT still require additional treatment(73). Evidence suggests that successful response to CBT may arise through alterations in amygdala-vmPFC connectivity, given the role of this circuitry in the maintenance of extinction(5,10,23). The current findings inform attempts to examine more directly relationships among anxiety, brain function, and age differences in response to CBT.
For example, one promising line of research might inform therapeutics by assessing amygdala-vmPFC connectivity in pediatric and adult anxiety before and after CBT. These findings could inform future research directions. One set of findings might replicate age-specific amygdala-vmPFC dysfunction but reveal no relationship between CBT and baseline or post-treatment connectivity. In this instance, the specific pattern of persistently perturbed amygdala-vmPFC connectivity might generate insights on treatments for individuals failing to benefit from CBT. Alternatively, another set of findings could demonstrate an overall similar pattern of clinical change across age groups treated with CBT, in tandem with age-specific relationships among clinical response and baseline/post-treatment amygdala-vmPFC connectivity. This result would suggest that CBT produces similar clinical changes across age groups, albeit through age-specific mechanisms. This pattern in turn might encourage further attempts to elucidate such age-specific mechanisms. These are only two of the many possible ways in which amygdala-vmPFC connectivity findings could inform novel treatment.
Such attempts to extend the current findings should be considered in light of study limitations. First, the current analyses did not reveal group differences in extinction recall of conditioned stimuli per se. Our task-dependent functional connectivity analyses compared threat-relevant attention conditions, while controlling for conditioned stimuli and morph conditions. Analyses did not compare functional connectivity differences to CS+, CS−, or morph conditions specifically, given the small number of trials per condition. Future studies could use a larger number of event replicates to quantify amygdala-PFC connectivity differences during extinction recall and other aspects of threat/fear and safety learning. Second, the sample sizes were relatively small, particularly in the two anxious groups. Nonetheless, the relatively conservative omnibus test showed the hypothesized three-way interaction effect. Future research is needed to test whether these patterns replicate in larger samples. Third, the current study is cross-sectional, and anxious youths represent a heterogeneous group in which some will remain anxious in adulthood, whereas most will not(74). Therefore, the anxious youths do not necessarily represent the same phenotype as the anxious adults. Future longitudinal/cross-sequential research might investigate developmental trajectories of threat-specific functional connectivity in risk for anxiety. Fourth, although IQ did not differ between age-matched anxious and healthy groups, IQ differed between youths and adults. However, post-hoc analyses of covariance showed the interaction effects remained significant after statistically controlling for IQ. Fifth, to be consistent with prior research, comorbid MDD was permitted in the patient groups. Prevalence of comorbid MDD differed between the anxious youths and anxious adults, which may reflect a potential confound for our between-group differences. Future anxiety research should aim to match clinical groups on comorbid MDD. Finally, given that the amygdala is a functionally and anatomically heterogeneous structure, the use of a whole-amygdala seed reflects a potential limitation. Future studies using high-resolution fMRI and amygdala subregions might examine associations among anxiety, development, and task-dependent connectivity.
Conclusion
In this context-dependent functional connectivity study, anxious youths and adults manifested opposite directions of amygdala-vmPFC coupling. Moreover, both anxious groups generally differed from their healthy counterparts, during threat vs. non-threat conditions. Although both pediatric and adult anxiety disorders exhibit perturbed amygdala-vmPFC coupling, age-specificity emerged in the direction of the functional connectivity differences. These findings highlight potential developmental influences on threat-dependent functional connectivity underlying clinical anxiety.
Supplementary Material
Generalized psychophysiological interaction (gPPI) analysis using an anatomically-defined left amygdala seed revealed a significant 3-way interaction of diagnosis, age group, and attention condition in three clusters. Two clusters appear in the (a) vmPFC (Talairach coordinates: −16, 31, −6) and (b) the vmPFC/vACC (Talairach coordinates 1, 36, −4) survived whole-brain correction. Images are shown in neurological convention (i.e., left is left) and thresholded at F(2,152) > 5.48, p<.005, cluster size > 90 voxels.
Mean beta coefficient values were extracted from significant clusters for each of the three PPI regressors: threat appraisal, explicit threat memory, and physical discrimination and extracted values were averaged across participants in each group. In the graphs, the x-axis (from left to right) represents the mean extracted values for the threat appraisal, explicit threat memory, and physical discrimination conditions.
The y-axis shows the beta coefficient values for PPI regressor, in which negative values reflect more negative functional connectivity and positive values reflect more positive functional connectivity associated with each condition.
Post-hoc pairwise comparisons are denoted as follows:
ap<.05, Anxious only: youth vs. adult;
bp<.05, Youths only: anxious vs. healthy;
cp<.05, Adults only: anxious vs. healthy;
dp<.05, Healthy only: youth vs. adult;
ep<.05, Anxious adults vs. healthy youths;
fp<.05, Anxious youths vs. healthy adults;
*p<.05; #p<.08.
Error bars represent +/− standard deviation.
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute of Mental Health, ZIAMH002781 (DSP), and K99/R00MH091183 (JCB).
Footnotes
All authors report no conflicts of interest.
References
- 1.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–51. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britton JC, Lissek S, Grillon C, et al. Development of anxiety: the role of threat appraisal and fear learning. Depress Anxiety. 2011;28:5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KD, Den ML, Graham BM, Richardson R. A window of vulnerability: Impaired fear extinction in adolescence. Neurobiol Learn Mem. 2014;113:90–100. doi: 10.1016/j.nlm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Shin LM, Davis FC, Vanelzakker MB, et al. Neuroimaging predictors of treatment response in anxiety disorders. Biol Mood Anxiety Disord. 2013;3:15. doi: 10.1186/2045-5380-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden DEJ. How psychotherapy changes the brain–the contribution of functional neuroimaging. Mol Psychiatry. 2006;11:528–38. doi: 10.1038/sj.mp.4001816. [DOI] [PubMed] [Google Scholar]
- 8.Britton JC, Grillon C, Lissek S, et al. Response to Learned Threat: An fMRI Study in Adolescent and Adult Anxiety. Am J Psychiatry. 2013;170:1195–1204. doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanElzakker MB, Dahlgren MK, Davis FC, et al. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–76. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birn RM, Shackman AJ, Oler JA, et al. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014;19:915–22. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Etkin A, Prater KE, Hoeft F, et al. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brühl AB, Delsignore A, Komossa K, Weidt S. Neuroimaging in social anxiety disorder—a meta-analytic review resulting in a new neurofunctional model. Neurosci Biobehav Rev. 2014;47:260–80. doi: 10.1016/j.neubiorev.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Mochcovitch MD, da Rocha Freire RC, Garcia RF, Nardi AE. A systematic review of fMRI studies in generalized anxiety disorder: evaluating its neural and cognitive basis. J Affect Disord. 2014;167:336–42. doi: 10.1016/j.jad.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Gentili C, Cristea IA, Angstadt M, et al. Beyond emotions: A meta-analysis of neural response within face processing system in social anxiety. Exp Biol Med (Maywood) doi: 10.1177/1535370215603514. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 21.Ledoux JE. The emotional brain, fear, and the amygdala. Cell Mol Neurobiol. 2003;23:727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaBar KS, Gatenby JC, Gore JC, et al. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 23.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 24.Milad MR, Wright CI, Orr SP, Pitman RK, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 26.Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Curr Opin Neurobiol. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sotres-Bayon F, Bush DEA, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem. 11:525–35. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- 30.Milad MR, Pitman RK, Ellis CB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garfinkel SN, Abelson JL, King AP, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–43. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prater KE, Hosanagar A, Klumpp H, et al. Aberrant amygdala-frontal cortex connectivity during perception of fearful faces and at rest in generalized social anxiety disorder. Depress Anxiety. 2013;30:234–41. doi: 10.1002/da.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Britton JC, Gold AL, Deckersbach T, Rauch SL. Functional MRI study of specific animal phobia using an event-related emotional counting stroop paradigm. Depress Anxiety. 2009;26:796–805. doi: 10.1002/da.20569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–16. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Demenescu LR, Kortekaas R, Cremers HR, et al. Amygdala activation and its functional connectivity during perception of emotional faces in social phobia and panic disorder. J Psychiatr Res. 2013;47:1024–31. doi: 10.1016/j.jpsychires.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Danti S, Ricciardi E, Gentili C, Gobbini MI, et al. Is Social Phobia a “Mis-Communication” Disorder? Brain Functional Connectivity during Face Perception Differs between Patients with Social Phobia and Healthy Control Subjects. Front Syst Neurosci. 2010;4:152. doi: 10.3389/fnsys.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair K, Geraci M, Devido J, et al. Neural response to self- and other referential praise and criticism in generalized social phobia. Arch Gen Psychiatry. 2008;65:1176–84. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardee JE, Benson BE, Bar-Haim Y, et al. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry. 2013;74:273–9. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guyer AE, Lau JYF, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–9. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 42.Casey BJ, Glatt CE, Lee FS. Treating the Developing versus Developed Brain: Translating Preclinical Mouse and Human Studies. Neuron. 2015;86:1358–68. doi: 10.1016/j.neuron.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hare TA, Tottenham N, Galvan A, et al. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biol Psychiatry. 2008;63:927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J Exp Child Psychol. 2011;108:607–20. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducharme S, Albaugh MD, Hudziak JJ, et al. Anxious/depressed symptoms are linked to right ventromedial prefrontal cortical thickness maturation in healthy children and young adults. Cereb Cortex. 2014;24:2941–50. doi: 10.1093/cercor/bht151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Newman E, Thompson WK, Bartsch H, et al. Anxiety is related to indices of cortical maturation in typically developing children and adolescents. Brain Struct Funct. doi: 10.1007/s00429-015-1085-9. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabard-Durnam LJ, Flannery J, Goff B, et al. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King EC, Pattwell SS, Sun A, et al. Nonlinear developmental trajectory of fear learning and memory. Ann N Y Acad Sci. 2013;1304:62–9. doi: 10.1111/nyas.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pattwell SS, Duhoux S, Hartley CA, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–23. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pattwell SS, Casey BJ, Lee FS. Altered Fear in Mice and Humans. Curr Dir Psychol Sci. 2013;22:146–51. doi: 10.1177/0963721412471323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lau JY, Britton JC, Nelson EE, et al. Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A. 2011;108:4500–5. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCallum J, Kim JH, Richardson R. Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology. 2010;35:2134–42. doi: 10.1038/npp.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 54.Beesdo K, Lau JYF, Guyer AE, et al. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry. 2009;66:275–85. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monk CS, McClure EB, Nelson EE, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–8. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- 56.Pérez-Edgar K, Roberson-Nay R, Hardin MG, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–46. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) [Google Scholar]
- 58.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 59.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cisler JM, Bush K, Steele JS. A comparison of statistical methods for detecting context-modulated functional connectivity in fMRI. Neuroimage. 2014;84:1042–52. doi: 10.1016/j.neuroimage.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friston KJ, Buechel C, Fink GR, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 62.Gorka SM, Fitzgerald DA, Labuschagne I, et al. Oxytocin Modulation of Amygdala Functional Connectivity to Fearful Faces in Generalized Social Anxiety Disorder. Neuropsychopharmacology. 2014;40:1–31. doi: 10.1038/npp.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolf RC, Herringa RJ. Prefrontal-Amygdala Dysregulation to Threat in Pediatric Posttraumatic Stress Disorder. Neuropsychopharmacology. doi: 10.1038/npp.2015.209. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen G, Adleman NE, Saad ZS, et al. Applications of multivariate modeling to neuroimaging group analysis: a comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–88. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Casey BJ, Lee FS. Optimizing treatments for anxiety by age and genetics. Ann N Y Acad Sci. 2015;1345:16–24. doi: 10.1111/nyas.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, VanDerKlok RM. Amygdala-cingulate intrinsic connectivity is associated with degree of social inhibition. Biol Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim MJ, Gee DG, Loucks RA, et al. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–73. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Etkin A, Prater KE, Schatzberg AF, et al. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 69.Hahn A, Stein P, Windischberger C, et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage. 2011;56:881–9. doi: 10.1016/j.neuroimage.2011.02.064. [DOI] [PubMed] [Google Scholar]
- 70.Hamm LL, Jacobs RH, Johnson MW, et al. Aberrant amygdala functional connectivity at rest in pediatric anxiety disorders. Biol Mood Anxiety Disord. 2014;4:15. doi: 10.1186/s13587-014-0015-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–9.e2. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinberger DR, Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am J Psychiatry. doi: 10.1176/appi.ajp.2015.15060753. In Press. [DOI] [PubMed] [Google Scholar]
- 73.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359:2753–66. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pine DS, Cohen P, Gurley D, et al. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generalized psychophysiological interaction (gPPI) analysis using an anatomically-defined left amygdala seed revealed a significant 3-way interaction of diagnosis, age group, and attention condition in three clusters. Two clusters appear in the (a) vmPFC (Talairach coordinates: −16, 31, −6) and (b) the vmPFC/vACC (Talairach coordinates 1, 36, −4) survived whole-brain correction. Images are shown in neurological convention (i.e., left is left) and thresholded at F(2,152) > 5.48, p<.005, cluster size > 90 voxels.
Mean beta coefficient values were extracted from significant clusters for each of the three PPI regressors: threat appraisal, explicit threat memory, and physical discrimination and extracted values were averaged across participants in each group. In the graphs, the x-axis (from left to right) represents the mean extracted values for the threat appraisal, explicit threat memory, and physical discrimination conditions.
The y-axis shows the beta coefficient values for PPI regressor, in which negative values reflect more negative functional connectivity and positive values reflect more positive functional connectivity associated with each condition.
Post-hoc pairwise comparisons are denoted as follows:
ap<.05, Anxious only: youth vs. adult;
bp<.05, Youths only: anxious vs. healthy;
cp<.05, Adults only: anxious vs. healthy;
dp<.05, Healthy only: youth vs. adult;
ep<.05, Anxious adults vs. healthy youths;
fp<.05, Anxious youths vs. healthy adults;
*p<.05; #p<.08.
Error bars represent +/− standard deviation.


