Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- CA 19‐9
carbohydrate antigen 19‐9
- CCA
cholangiocarcinoma
- ERC
endoscopic retrograde cholangiography
- FISH
fluorescent in situ hybridization
- MRC
magnetic resonance cholangiography
- MRI
magnetic resonance imaging
- OLT
orthotopic liver transplantation
- PSC
primary sclerosing cholangitis
The aim of cancer surveillance is to detect cancer or precancerous lesions in asymptomatic, high‐risk individuals when cancer is more likely to be prevented or cured.1 This is a highly germane topic for physicians treating patients with primary sclerosing cholangitis (PSC).
The cause of PSC is unknown, and there are currently no effective therapeutic strategies to prevent adverse outcomes of this progressive liver disease. Malignancies are the cause of death in up to 44% of patients with PSC.2 The risk for cholangiocarcinoma (CCA) development in patients with PSC is approximately 160‐ to 1560‐fold greater than for the general population.3 The absolute risk for CCA in PSC is approximately 9% over 10 years.4 The mean age of CCA diagnosis in patients with PSC is in the fourth decade of life versus the seventh decade in the general population. Hence given the increased risk for CCA in young adults with PSC, this question is frequently posed: Should surveillance strategies be used to detect early CCA in patients with PSC, and if so, how?
Unfortunately, a data‐driven surveillance policy for CCA in patients with PSC does not yet exist (Table 1). The position of the American Association for the Study of Liver Diseases is that “in the absence of evidence based information, many clinicians screen patients with an imaging study plus a CA 19‐9 at annual intervals.”5 The European Association for the Study of Liver Diseases states: “There is at present no biochemical marker or imaging modality which can be recommended for early detection of cholangiocarcinoma. ERCP with brush cytology (and/or biopsy) sampling should be carried out when clinically indicated.”6 Per the American College of Gastroenterology, “Screening for cholangiocarcinoma with regular (every 6–12 months) cross‐sectional imaging with ultrasound or MR and serial CA 19‐9 measures is recommended by experts in this area, for all patients with PSC.”7 Thus, even societal guidelines differ on this topic.
Table 1.
Summary of Societal Guidelines for CCA Surveillance in PSC
| Professional Society | Recommendations |
|---|---|
| American Association for the Study of Liver Diseases |
“Inadequate information exists regarding the utility of screening for CCA in PSC; in the absence of evidence based information, many clinicians screen patients with an imaging study plus a CA 19‐9 at annual intervals.”5
“We recommend evaluation for CCA in patients with deterioration of their constitutional performance status or liver biochemical‐related parameters.”5 |
| European Association for the Study of Liver Diseases | “There is at present no biochemical marker or imaging modality which can be recommended for early detection of cholangiocarcinoma. ERCP with brush cytology (and/or biopsy) sampling should be carried out when clinically indicated.”6 |
| American College of Gastroenterology | “Consider screening for cholangiocarcinoma with regular cross‐sectional imaging with ultrasound or MR and serial CA 19‐9 every 6‐12 months.”7 |
Surveillance for CCA in PSC: Is It Effective?
The following criteria should be met to make surveillance effective: (1) there should be a defined population at risk; (2) testing modalities and treatment for early‐stage disease are available, affordable, and acceptable to the individual and jurisdiction of interest; (3) the process is cost‐effective and standardized; and (4) there are patient survival benefits if cancer is detected early. In the following paragraphs we examine these surveillance criteria in the context of PSC (Table 2).
Table 2.
Principles of Disease Surveillance and Its Applicability to Surveillance of CCA in Patients With PSC
| Criteria for a Successful Surveillance Strategy | Met or Not |
|---|---|
| Defined population at risk |
Yes • Patients with PSC |
| Available, affordable, acceptable surveillance modalities |
Yes • Annual MRI/MRC with CA 19‐9 |
| Available, affordable, acceptable treatment modalities |
Yes, but very limited • Resection for early disease with well‐compensated liver function • Neoadjuvant chemoirradiation followed by liver transplantation for early perihilar CCA in highly specialized centers |
| Cost‐effectiveness of the process |
Unknown • Because the annual incidence rate of CCA in PSC is 1.5%, which is comparable with incidence of hepatocellular carcinoma, surveillance might be justified |
| Standardization of the process | None |
| Patient survival benefits |
Yes • Benefits are limited to patients with resectable and early perihilar disease treated with neoadjuvant chemoirradiation followed by liver transplantation in highly specialized centers |
We do not understand which patients with PSC are at risk for development of CCA. Several environmental and genetic factors have been proposed, but none has been convincingly confirmed in follow‐up studies. The duration of associated inflammatory bowel disease and colectomy for colonic neoplasia may identify a subset of patients with PSC who are at high risk for development of CCA,8 but even this observation needs confirmation. Among patients with PSC diagnosed with CCA, about 50% will be found to have CCA within 2 years of PSC diagnosis. This observation suggests that patients with newly diagnosed PSC may be at highest risk for a CCA diagnosis. However, these data may be confounded by an ascertainment bias, namely, early symptomatic albeit undiagnosed CCA has brought their PSC to medical attention. Nonetheless, if a surveillance strategy is cost‐effective, the incidence of CCA in PSC (1.5% per year)9 is likely sufficient to justify surveillance because it is approximately the same cutoff incidence (1.5%‐2%) that is used to justify surveillance of hepatocellular carcinoma in the cirrhosis patient population.
Magnetic resonance cholangiography (MRC) combined with MRI, ERC, and serum biomarker carbohydrate antigen 19‐9 (CA 19‐9) are the most accepted modalities for CCA surveillance in PSC (Fig. 1). MRI/MRC has 89% sensitivity and 76% accuracy in CCA diagnosis. The CA 19‐9 level ≥20 U/mL enhances MRI/MRC sensitivity to 100% at the expense of the specificity (38%) and accuracy (47%). Biliary brushings obtained by ERC for conventional cytology and/or fluorescent in situ hybridization (FISH) analysis that detects chromosomal alterations (polysomy is an equivalent of aneuploidy) are valuable complementary tests. FISH sensitivity for detection of perihilar CCA is 38% to 58% as compared with 15% sensitivity for conventional cytology. In patients with PSC the combination of CA 19‐9 ≥129 U/mL and polysomy was found to be predictive of cancer (hazard ratio 10.92; P < 0.001), and presence of either of them was associated with cancer diagnosis within 2 years.10 Because an ideal surveillance modality should have high sensitivity and specificity and be noninvasive, in our opinion, endoscopic retrograde cholangiopancreatography is not acceptable because it is too invasive and is fraught with complications (e.g., pancreatitis, cholangitis), having limited performance because of the paucicellular nature of cytological specimens, and missing mass lesions not associated with the large bile duct involvement. CA19‐9 cannot be used alone because it is not specific for CCA and can be elevated with bacterial cholangitis, other gastroenterological and gynecological cancers, and smoking. In addition, more than 30% of patients with PSC with CA 19‐9 ≥129 U/mL are free of cancer long term. CA 19‐9 utility will be influenced by the Lewis blood group phenotype (7% of the population is Lewis‐negative and has the undetectable CA 19‐9 level)9 and by allelic variants of fucosyltransferases 2 and 3.11 Ultrasound sensitivity for CCA detection in PSC is very limited. Traditionally, CCA was thought to be a slowly growing tumor, but its doubling time to guide surveillance intervals is unknown and difficult to define in patients with PSC who most often have non‐mass‐forming perihilar CCA. Cost‐effectiveness of PSC‐CCA surveillance and extent of lead and length time bias are unknown, and affordability depends on the jurisdiction and personal resources. Nonetheless, MRI/MRC and determination of serum CA 19‐9 values are noninvasive and can be used to prompt more invasive and specific studies such as ERC with brush cytology.
Figure 1.
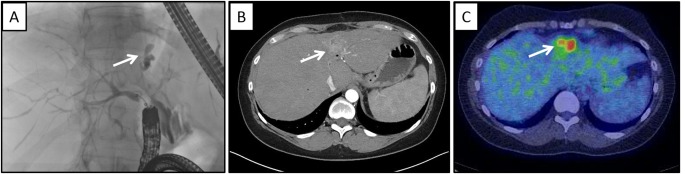
CCA in a 51‐year‐old asymptomatic woman with PSC and an elevated CA 19‐9. (A) ERC; white arrow demonstrates segmental bile duct obstruction worrisome for CCA in this context. (B) MRI; white arrow denotes a mass lesion. (C) Positron emission tomography; white arrow demonstrates avid uptake of 18F‐FDG consistent with a malignancy
Surveillance for CCA in PSC: Is It Justified?
If an early CCA is identified in a patient with PSC, do we have effective therapy? Surgical resection for CCA is associated with a 5‐year survival rate of less than 30% even in patients without PSC who potentially have better baseline liver function. However, neoadjuvant chemoirradiation therapy followed by orthotopic liver transplantation (OLT) available in highly specialized centers is a curative treatment option with 5‐year survival of greater than 70% for the subset of patients with PSC with early perihilar CCA. Liver transplantation outcomes for early perihilar CCA are identical with those for hepatocellular carcinoma for which surveillance policies are well accepted. The protocol for this approach using chemoirradiation plus liver transplantation is resource intensive and not universally available. For example, in the event of nonresectable CCA diagnosis, 37% of transplant centers would perform OLT with neoadjuvant therapy, 33% would resort to palliative treatment, and the remaining 30% would make an outside referral.12
PSC is a rare disease often complicated by infection and cholestasis that require urgent imaging and intervention. It would be unfeasible to conduct randomized, controlled trials on a proper surveillance strategy, because the control group would inevitably be contaminated by imaging studies based on clinical need. Hence it is unlikely that the cost‐effectiveness of surveillance for CCA in patients with PSC will ever be settled by a prospective, randomized trial.
Surveillance for CCA in PSC: What Do Experienced Clinicians Do?
Patients seek medical attention to alleviate current and future suffering and to prolong life. Physicians must respond to these individual goals within the confines of an individual, unique patient–physician relationship, not on the basis of abstract population outcomes. Physicians and patients should participate in shared decision making. In this context, patients with PSC should be informed of their cancer risk, the availability of surveillance strategies, and how positive results would be managed. If the patient opts for surveillance, the authors recommend MRI/MRC combined with serum CA 19‐9 level on an annual basis. If a dominant biliary stricture and/or rise in CA 19‐9 greater than 129 U/mL is observed, an ERC with biliary brushings for conventional and FISH cytological examination should be obtained. A patient with early‐stage perihilar tumor should be evaluated for liver transplantation and referred to a specialized center if deemed to be an acceptable candidate.
Teaching points on surveillance of CCA in PSC include:
Cost‐effectiveness of CCA surveillance in patients with PSC is unknown.
Due to rarity of PSC, the clinical trial assessing cost‐effectiveness of CCA surveillance is unlikely to be feasible.
Fifty percent of patients with newly diagnosed PSC who will experience development of CCA are diagnosed with CCA within the first 2 years since PSC diagnosis and, therefore, should be aggressively screened for this cancer.
Noninvasive MRI/MRC and CA 19‐9 with a cutoff ≥129 U/mL are preferable surveillance modalities for CCA in patients with PSC.
ERC should be reserved for patients with abnormal MRI/MRC and CA 19‐9 findings and combined with FISH cytological evaluation for polysomy or performed when clinically indicated (i.e., jaundice development).
Patients with PSC with early perihilar CCA should be referred to the specialized center for potential liver transplantation.
This work was supported by National Institutes of Health Grant DK59427 (to G.J.G.). Potential conflict of interest: Nothing to report.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Sullivan T, Sullivan R, Ginsburg OM. Screening for cancer: considerations for low‐ and middle‐income countries In: Gelband H, Jha P, Sankaranarayanan R, Horton S, eds. Cancer: Disease Control Priorities. Vol. 3 3rd ed World Bank Publications, Washington, DC; 2015. [PubMed] [Google Scholar]
- 2. Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011;54:1842‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singal AK, Stanca CM, Clark V, Dixon L, Levy C, Odin JA, et al. Natural history of small duct primary sclerosing cholangitis: a case series with review of the literature. Hepatol Int 2011;5:808‐813. [DOI] [PubMed] [Google Scholar]
- 4. Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol 2009;50:158‐164. [DOI] [PubMed] [Google Scholar]
- 5. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51:660‐678. [DOI] [PubMed] [Google Scholar]
- 6. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237‐267. [DOI] [PubMed] [Google Scholar]
- 7. Lindor KD, Kowdley KV, Harrison ME, American College of Gastroenterology . ACG Clinical Guideline: primary sclerosing cholangitis. Am J Gastroenterol 2015;110:646‐659; quiz 660. [DOI] [PubMed] [Google Scholar]
- 8. Gulamhusein AF, Eaton JE, Tabibian JH, Atkinson EJ, Juran BD, Lazaridis KN. Duration of inflammatory bowel disease is associated with increased risk of cholangiocarcinoma in patients with primary sclerosing cholangitis and IBD. Am J Gastroenterol 2016;111:705‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet 2014;383:2168‐2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barr Fritcher EG, Voss JS, Jenkins SM, Lingineni RK, Clayton AC, Roberts LR, et al. Primary sclerosing cholangitis with equivocal cytology: fluorescence in situ hybridization and serum CA 19‐9 predict risk of malignancy. Cancer Cytopathol 2013;121:708‐717. [DOI] [PubMed] [Google Scholar]
- 11. Bonato G, Cristoferi L, Strazzabosco M, Fabris L. Malignancies in primary sclerosing cholangitis ‐ a continuing threat. Dig Dis 2015;33(Suppl 2):140‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trilianos P, Selaru F, Li Z, Gurakar A. Trends in pre‐liver transplant screening for cholangiocarcinoma among patients with primary sclerosing cholangitis. Digestion 2014;89:165‐173. [DOI] [PubMed] [Google Scholar]


