Abstract
Emotion regulation is a critical life skill that can facilitate learning and improve educational outcomes. Developmental studies find that the ability to regulate emotion improves with age. In neuroimaging studies, emotion regulation abilities are associated with recruitment of a set of prefrontal brain regions involved in cognitive control and executive functioning that mature late in development. In this review we discuss the regulation of both negative and positive emotions, the role of other people in guiding our emotional responses, and the potential applications of this work to education.
Introduction
How a student feels can profoundly shape how he or she thinks. For example, emotions can promote learning by capturing and holding attention as well as deepening encoding [1–3]. But they can also inhibit learning by blocking these cognitive processes in the face of threat [1,4]. Thus, what emotions are elicited and whether a student can adaptively manage those emotions can have a strong impact on his or her learning. Given the multiple important roles emotion can play in educational contexts, it is essential that we understand how to promote and maintain emotional states that foster optimal learning. The capacity to regulate emotion may be key in this regard. Emotion regulation involves active attempts to maintain or change emotions and is a critical life skill that predicts positive life outcomes in adulthood [5,6]. The ability to regulate one's emotions can serve many purposes: it can both increase emotional arousal or positive valence to enhance learning, and it can help to dampen emotional responses that might be blocking successful encoding of new information. Here we discuss how the neural systems underlying emotion regulation develop and consider their educational implications.
Neural Mechanisms Supporting Emotion Regulation
While there are many strategies that can be used to actively regulate one's emotions (for review see [7], McRae this volume), in brain imaging studies the most commonly studied strategy is reappraisal [8], which involves deliberately changing the way one thinks about the meaning of an emotionally evocative stimulus or situation. There has been increasing interest in the distinction between explicit forms of emotion regulation, like reappraisal, where one has an active goal of regulating and uses effortful control processes to do so, and implicit forms of regulation, where there may be no conscious goal to regulate and automatic processes may support emotion change (see upcoming review by Martin Braunstein, Gross, Ochsner, 2016, and [8,9]). It remains for future work to study how explicit and implicit forms of emotion regulation differ in their developmental trajectories.
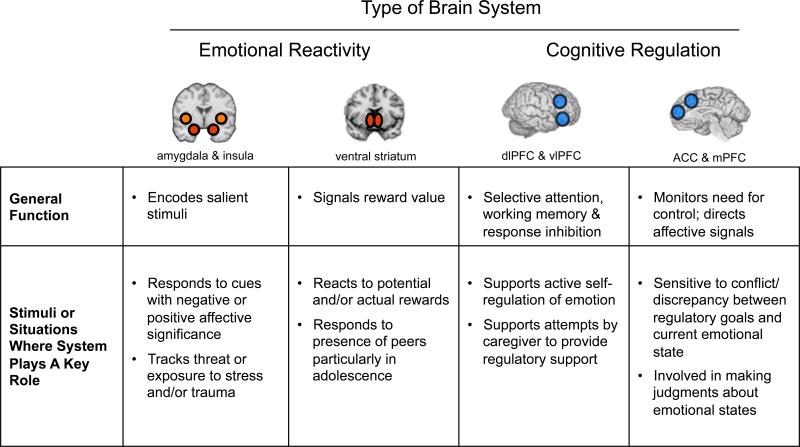
Reappraisal has been shown to be effective at dampening or enhancing responses in systems associated with affective responding. Chief among them is the amgydala, a subcortical structure important for signaling the presence, and modulating the encoding, of affect-relevant stimuli [11]. Also impacted by reappraisal is the ventral striatum, another subcortical structure implicated in signaling the reward value of stimuli [12], and the insula, a cortical region representing information about the body states associated with affective responses [13].
Reappraisal is believed to modulate these regions via recruitment of a network of regions including the dorsolateral prefrontal cortex (dlPFC), posterior parietal cortex (PPC), ventrolateral prefrontal cortex (vlPFC), posterior medial prefrontal cortex (mPFC) and anterior cingulate cortex (ACC) [8,14]. This set of regions is not specific to emotion regulation, but is also commonly activated in tasks involving cognitive control more generally. The dlPFC is commonly active during selective attention and working memory tasks, which may aid in holding emotion-regulation strategies in mind [15]. vlPFC is commonly active during response selection and inhibition, which may help with selecting an appropriate reappraisal tactic [16]. Finally, mPFC and ACC are commonly activated in tasks involving selection among competing responses, and may help in identifying when regulation is needed [17].
Development of the Neural Systems Supporting Emotion Regulation
While neuroscience research on emotion regulation in adults has exploded over the past 10-15 years, developmental research has emerged only more recently. A popular theory is that prefrontal control regions like dlPFC and vlPFC mature at a slower rate relative to affective response regions like the amygdala and ventral striatum [18,19]. This imbalance is represented by a pattern of stronger activations in subcortical relative to cortical regions peaking during adolescence, which may contribute to mood instability and greater emotional reactivity in this age group [20]. The imbalance theory may be an oversimplification of a more complex series of interactions between cognition and emotion taking place during development, where they can mutually inform, help, or hinder one another [21–24]. Thus, more studies investigating the maturational patterns of cortical-subcortical circuitry are undoubtedly needed in order to better understand how affect and mood change with age.
What does this slower maturity mean for a child's ability to manage his or her emotions? The answer to this question depends on the context of the situation including whether a child is responding to a negative or positive situation.
Regulation of Negative Emotions Across Development
To date, only a handful of studies have examined the ability to regulate negative emotion in children as compared to adults. From these few studies, however, two kinds of key findings emerge. The first concern a child's ability to engage prefrontal systems to decrease a current emotion. Data suggest that the behavioral ability to down-regulate negative emotion, decrease amygdala activation [25] and increase activity in lateral prefrontal regions tracks with age [26,27]. Amygdala-prefrontal functional connectivity also increases with age, suggesting that stronger cortical-subcortical relationships underlie age-related increases in successful emotion regulation [27,28].
The second concerns negative environmental influences that can affect one's ability to regulate emotions. For example, in some situations, moderate levels of stress can enhance learning by way of increasing attentional vigilance [29]. However, acute stress (e.g. test anxiety) and outside factors contributing to chronic stress (e.g. poverty) can have deleterious effects on one's cognitive abilities [4,30,31].
Importantly, these decreases in cognitive performance may be mediated by one's ability to regulate emotion. For example, one study found that adolescents who were maltreated demonstrated more reactivity to negative emotional scenes in the amygdala and insula and greater recruitment of dlPFC and ACC regions when reappraising those scenes, suggesting more reactivity and more effortful regulation [32]. Growing up in conditions of poverty also can have a negative effect on the developing brain and one's ability to regulate emotions. Kim et al., found decreased dlPFC and vlPFC activation and increased amygdala activation during a reappraisal task in adults who had experienced poverty during childhood compared to adults whose families had higher incomes during childhood [31]. In both studies, the groups who had experienced greater adversity demonstrated greater neural reactivity to negative images in amygdala and different patterns of activation in prefrontal cortex compared to the groups experiencing less adversity.
At the classroom level, training students on emotion regulation strategies (e.g. distancing, mindfulness, reinterpretation of negative scenarios – for examples see references [33–36]) could be an effective intervention approach particularly for individuals or populations exposed to situations of high stress and adversity. Additionally, teacher development or training programs emphasizing the effects negative environmental influences can have on attention, cognition, and the ability to regulate negative emotions could help teachers build more effective classroom management plans, and perhaps provide more optimal support and scaffolding to struggling students.
Regulation of Positive Emotions Across Development
Another critical, yet less explored, area of research concerns the regulation of positive emotions. While negative emotions are thought to focus attention on and promote encoding of potential threats, positive emotions are believed to broaden one's attentional scope which can then facilitate enhanced learning and memory [37]. While developmental neuroimaging studies testing this hypothesis have yet to be done, there is a related literature on how children and adolescents respond to rewards. The role of ventral striatum in response to rewards across development is complex and in some cases conflicting, with some studies finding increases in activation in this region peaking during adolescence, whereas others find attenuations [20,23,38]. Though few of these studies directly address regulation, in one reward domain - appetitive reactivity to foods -several studies found that application of reappraisal strategies led to decreased craving for rewarding foods and decreased activation in ventral striatum, and this decreased craving and activation improved linearly with age [39,40]. Interestingly, and in contrast to studies of regulating negative emotions, the key developmental differences were found in the degree to which children and adolescents craved the foods at baseline as compared to adults, rather than their skill in reappraising. Similarly, a study examining emotional reactivity to positive and negative scenes across age found that younger children showed greater activation in amygdala, ventral striatum, and vlPFC for positive pictures compared to negative [41]. Together these studies, though only a subset in a larger, more complex body of literature on reward processing, suggest that in certain positive domains, children's reward circuitry may be especially responsive to rewards and positive emotions, although they can successfully attempt to down-regulate these responses when desired.
These conclusions are echoed by studies of responses to feedback during learning, which is a fundamental component of any educational experience. Being rewarded for getting a good grade on an exam or experiencing the negative consequences of getting a bad grade can elicit an affective response and subsequent need for emotional management. Similar to the previously mentioned studies on reactivity to positive or rewarding stimuli, children may also be especially responsive to positive feedback. A study by Duijvenvoorde et al. comparing responses to positive and negative feedback across age found that all age groups performed better on a rule learning task when they received positive feedback compared to negative feedback, and this trend was the largest for eight to nine year olds [42]. At the neural level, children in the eight to nine year old age group demonstrated greater brain activation during positive feedback compared to negative feedback, particularly in dlPFC and parietal regions, whereas adults showed the reverse trend, activating similar regions more than the other age groups when receiving negative compared to positive feedback. Similarly, in a belief-updating task on good and bad news by Moutsiana et al., children were more likely to update accurately for good news compared to bad news, and with age, the differences in updating between good news and bad news decreased and lost significance in adulthood [43]. Responses to positive feedback activate different neural circuitry than responses to negative feedback and may follow different neurodevelopmental trajectories, which may explain why children learn better from positive feedback [44].
In sum, these findings suggest that while all ages are able to regulate positive emotions successfully, younger individuals may be especially responsive to positive rewards and feedback and therefore might need to deploy regulation more often. This knowledge can be harnessed in the classroom in a number of ways. For example, because positive stimuli elicit stronger reward responses in young children and we know that they are capable of regulating them, curricula could be designed with rewards that incentivize learning as well as reminders to regulate when appropriate. And for older children and adolescents who may not be as engaged by rewards, curricula could attempt to teach them to effortfully attend to and elaborate the rewarding aspects of material they are learning (thereby up-regulating positive responses).
The Social Regulation of Emotion
Although our emotions are experienced individually, any parent or teacher knows that they can be heavily influenced by the words and actions of other people. Such social forms of regulation – which clearly are important – have seen little neuroscience research, although interest in them is growing (See, e.g. Reeck, Ames & Ochsner, 2016, and [39]) and related research on the interaction of social cognition and emotion during development is emerging [24,46]. While to date, there are no imaging studies directly examining how other people can help us reappraise – across development or in adults, studies examining other forms of social regulation are beginning to emerge [45,47]. For example, in children, the presence of a caregiver can have a buffering effect, reducing emotional and stress responses to negative stimuli [48,49]. Similarly, in the reward domain, the presence of a caregiver during a risky decision task decreased risky choices and increased activation in control regions including vlPFC and mPFC, and decreased ventral striatum and amygdala activation in adolescents [50]. In each case, the presence of a caregiver modifies the affect-eliciting situation, altering the affective value assigned to stimuli.
In analogous fashion, the presence of peers may influence recruitment of brain regions that trigger negative and positive emotions. In contrast to the role of parents, however, the presence of a similarly aged peer may increase risky decision making as well as ventral striatum and vlPFC activation in adolescents [51,52]. Similarly, in tasks involving peer rejection, adolescents show increased activation of ACC and mPFC, regions which have been associated with depression and social pain; individual difference factors like rejection sensitivity, depression and resistance to peer influence may moderate these effects [53,54]. Taken together, this work shows how social regulation can modulate neural architecture and subsequent behavior.
Discussion
Because emotions can enhance or impede learning, the ability to regulate one's own and others’ emotions can facilitate successful educational outcomes. Emotion regulation is a type of emotion-cognition interaction where cognitive control systems are believed to aid in dampening or enhancing negative and positive emotions (Figure 1). Since brain regions associated with cognitive control structures – such as prefrontal cortex – may have a slower maturational trajectory relative to structures associated with emotional responding – like the amygdala and ventral striatum – children and adolescents may have a harder time regulating their emotions. Weaker or less organized functional and structural connectivity between these brain regions in childhood may also be contributing toward increased difficulty with emotion regulation [55–57]. That said, extant data suggest a dissociation between the negative and positive affective domains: children and adults respond similarly to aversive stimuli and children have greater trouble regulating responses to them; by contrast, children respond more strongly to rewards than do adults and all age groups can regulate responses to them. Of course, dispositional factors such as depression and anxiety and situational factors outside the classroom such as stress or abuse can enhance emotional reactivity or impair prefrontal function, either of which may make emotion regulation more effortful.
Figure 1.
Overview of neural systems supporting emotion reactivity and regulation. Neuroimaging studies identify key brain systems involved in emotional reactivity and regulation along with their proposed function and the environmental stimuli and/or contexts where they may play key roles - affective systems are triggered by the presence of particular stimuli; regulatory systems are being brought online to actively implement strategies for regulating one's own or others’ emotions.
Importantly, these data could be leveraged to influence curricula and policy (Figure 2). For example, because positive emotions, feedback, and rewards may facilitate better learning, using regulation strategies to enhance positive emotions could be an effective approach in educational settings. And more generally, understanding how students regulate (or fail to regulate) emotions given their developmental stage and background can help educators better scaffold and manage their classrooms to enhance learning and successful student outcomes. Finally, a key direction for future work is understanding how other people play a role in helping students manage their emotions. While we know that educators and caregivers can help reduce negative emotions in children – and adolescents may be especially sensitive to the influence of their peers – little is known about how such behavioral effects relate to the development of underlying neural systems.
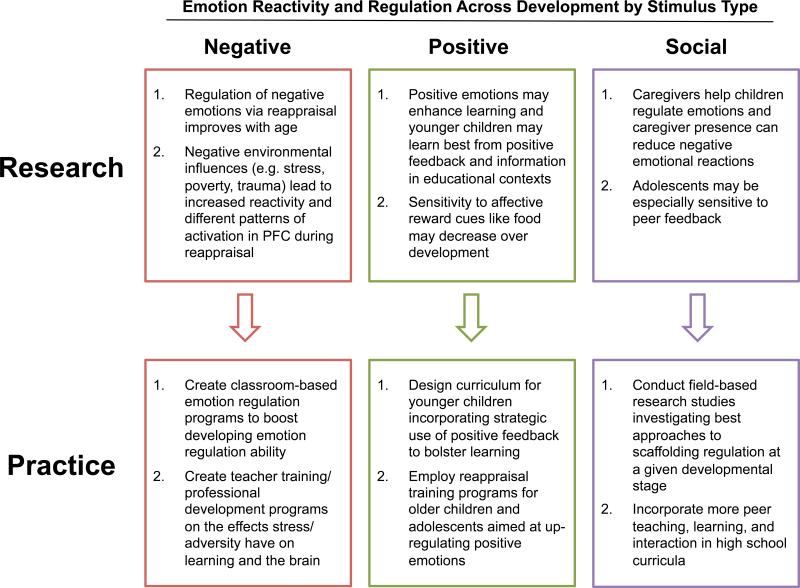
Figure 2.
Linking research and practice. Summary of studies of the development of emotion regulation as a function of the type of emotion triggering stimulus. Top boxes describe key research findings and lower boxes describe potential educational applications based on research findings.
Attention, executive functioning, memory and learning are all cognitive constructs critical in understanding how to improve learning and teaching, however, often before any of these cognitive functions can happen, they have to pass through the filter of an individual's emotional experience. As such, helping students, teachers, and families better understand the mechanics behind emotion regulation development and learn how to employ appropriate strategies could make for a more engaging, dynamic, and effective educational experience for all.
Highlights.
Emotion regulation serves to modify the nature, intensity or duration of emotions
Prefrontal cortex may modulate subcortical structures when regulating emotion
Regulation of positive and negative emotions have different developmental courses
Prefrontal cortex development may improve regulation of negative emotions
Caregivers can help children better regulate emotions
Acknowledgments
Thanks to Daphna Shohamy, Jennifer Silvers, and members of the SCAN Lab at Columbia for thoughtful discussion on the manuscript.
Funding
This work was supported by the National Institutes of Health (R01 HD0691780, R01 AG043463, F31 NIMH 107119).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None declared.
References
- 1.Clore GL, Huntsinger JR. How emotions inform judgment and regulate thought. Trends Cogn. Sci. 2007;11:393–399. doi: 10.1016/j.tics.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Talmi D. Enhanced Emotional Memory Cognitive and Neural Mechanisms. Curr. Dir. Psychol. Sci. 2013;22:430–436. [Google Scholar]
- 3.Yiend J. The effects of emotion on attention: A review of attentional processing of emotional information. Cogn. Emot. 2010;24:3–47. [Google Scholar]
- 4.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 5.Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, Berman MG, Wilson NL, Teslovich T, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc. Natl. Acad. Sci. U. S. A. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duckworth AL, Kim B, Tsukayama E. Life Stress Impairs Self-Control in Early Adolescence. Front. Psychol. 2013;3 doi: 10.3389/fpsyg.2012.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross JJ. Emotion regulation: Conceptual and empirical foundations. Handb. Emot. Regul. 2014;2:3–20. [Google Scholar]
- 8.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann. N. Y. Acad. Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyurak A, Gross JJ, Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cogn. Emot. 2011;25:400–412. doi: 10.1080/02699931.2010.544160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed SP, Bittencourt-Hewitt A, Sebastian CL. Neurocognitive bases of emotion regulation development in adolescence. Dev. Cogn. Neurosci. 2015;15:11–25. doi: 10.1016/j.dcn.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phelps EA, LeDoux JE. Contributions of the Amygdala to Emotion Processing: From Animal Models to Human Behavior. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uddin LQ, Kinnison J, Pessoa L, Anderson ML. Beyond the Tripartite Cognition–Emotion–Interoception Model of the Human Insular Cortex. J. Cogn. Neurosci. 2013;26:16–27. doi: 10.1162/jocn_a_00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb. Cortex. 2013 doi: 10.1093/cercor/bht154. doi:10.1093/cercor/bht154. [This meta-analysis reviewed 48 studies of emotion regulation and reported commonly activated regions across studies and their function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wager TD, Smith EE. Neuroimaging studies of working memory. Cogn. Affect. Behav. Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 16.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn. Sci. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Shenhav A, Botvinick MM, Cohen JD. The Expected Value of Control: An Integrative Theory of Anterior Cingulate Cortex Function. Neuron. 2013;79:217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamnes CK, Walhovd KB, Dale AM, Østby Y, Grydeland H, Richardson G, Westlye LT, Roddey JC, Hagler DJ, Jr, Due-Tønnessen P, et al. Brain development and aging: Overlapping and unique patterns of change. NeuroImage. 2013;68:63–74. doi: 10.1016/j.neuroimage.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey BJ. Beyond Simple Models of Self-Control to Circuit-Based Accounts of Adolescent Behavior. Annu. Rev. Psychol. 2015;66:295–319. doi: 10.1146/annurev-psych-010814-015156. [Comprehensive recent review of developmental imaging studies related to self-control. Self-control for incentives and threats are discussed.] [DOI] [PubMed] [Google Scholar]
- 21.Pfeifer JH, Allen NB. Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends Cogn. Sci. 2012;16:322–329. doi: 10.1016/j.tics.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahl RE. The developmental neuroscience of adolescence: Revisiting, refining, and extending seminal models. Dev. Cogn. Neurosci. 2016;17:101–102. doi: 10.1016/j.dcn.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telzer EH. Dopaminergic reward sensitivity can promote adolescent health: A new perspective on the mechanism of ventral striatum activation. Dev. Cogn. Neurosci. 2016;17:57–67. doi: 10.1016/j.dcn.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crone EA, Dahl RE. Understanding adolescence as a period of social–affective engagement and goal flexibility. Nat. Rev. Neurosci. 2012;13:636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- 25.Stephanou K, Davey CG, Kerestes R, Whittle S, Pujol J, Yücel M, Fornito A, López-Solà M, Harrison BJ. Brain functional correlates of emotion regulation across adolescence and young adulthood. Hum. Brain Mapp. 2015 doi: 10.1002/hbm.22905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Gabrieli JDE, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc. Cogn. Affect. Neurosci. 2012;7:11–22. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 2014 doi: 10.1111/desc.12260. doi:10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlman SB, Pelphrey KA. Developing connections for affective regulation: age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011;108:607–620. doi: 10.1016/j.jecp.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z, Lukowski SL, Hart SA, Lyons IM, Thompson LA, Kovas Y, Mazzocco MMM, Plomin R, Petrill SA. Is Math Anxiety Always Bad for Math Learning? The Role of Math Motivation. Psychol. Sci. 2015 doi: 10.1177/0956797615602471. doi:10.1177/0956797615602471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Supekar K, Iuculano T, Chen L, Menon V. Remediation of Childhood Math Anxiety and Associated Neural Circuits through Cognitive Tutoring. J. Neurosci. 2015;35:12574–12583. doi: 10.1523/JNEUROSCI.0786-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, Liberzon I, Phan KL. Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proc. Natl. Acad. Sci. 2013;110:18442–18447. doi: 10.1073/pnas.1308240110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin KA, Peverill M, Gold AL, Alves S, Sheridan MA. Child Maltreatment and Neural Systems Underlying Emotion Regulation. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:753–762. doi: 10.1016/j.jaac.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denny BT, Ochsner KN. Behavioral effects of longitudinal training in cognitive reappraisal. Emot. Wash. DC. 2014;14:425–433. doi: 10.1037/a0035276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kross E, Duckworth A, Ayduk O, Tsukayama E, Mischel W. The effect of self-distancing on adaptive versus maladaptive self-reflection in children. Emot. Wash. DC. 2011;11:1032–1039. doi: 10.1037/a0021787. [DOI] [PubMed] [Google Scholar]
- 35.Blair C, Raver CC. Closing the Achievement Gap through Modification of Neurocognitive and Neuroendocrine Function: Results from a Cluster Randomized Controlled Trial of an Innovative Approach to the Education of Children in Kindergarten. PLoS ONE. 2014;9:e112393. doi: 10.1371/journal.pone.0112393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zenner C, Herrnleben-Kurz S, Walach H. Mindfulness-based interventions in schools a systematic review and meta-analysis. Front. Psychol. 2014;5 doi: 10.3389/fpsyg.2014.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought action repertoires. Cogn. Emot. 2005;19:313–332. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richards JM, Plate RC, Ernst M. A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: The impact of task design and implications for understanding neurodevelopment. Neurosci. Biobehav. Rev. 2013;37:976–991. doi: 10.1016/j.neubiorev.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvers JA, Insel C, Powers A, Franz P, Weber J, Mischel W, Casey BJ, Ochsner KN. Curbing Craving Behavioral and Brain Evidence That Children Regulate Craving When Instructed to Do So but Have Higher Baseline Craving Than Adults. Psychol. Sci. 2014 doi: 10.1177/0956797614546001. doi:10.1177/0956797614546001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuliani NR, Pfeifer JH. Age-related changes in reappraisal of appetitive cravings during adolescence. NeuroImage. 2015;108:173–181. doi: 10.1016/j.neuroimage.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS. Functional differences in emotion processing during adolescence and early adulthood. NeuroImage. 2014;91:70–76. doi: 10.1016/j.neuroimage.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 42.Duijvenvoorde ACK van, Zanolie K, Rombouts SARB, Raijmakers MEJ, Crone EA. Evaluating the Negative or Valuing the Positive? Neural Mechanisms Supporting Feedback-Based Learning across Development. J. Neurosci. 2008;28:9495–9503. doi: 10.1523/JNEUROSCI.1485-08.2008. [This study is the first to examine neural systems supporting feedback learning during across development and found that younger age groups have more activation in lateral prefrontal regions than adolescents and adults to positive feedback compared to negative feedback. In contrast adults had more activation in frontoparietal regions than children and adolescents to negative feedback compared to positive feedback.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moutsiana C, Garrett N, Clarke RC, Lotto RB, Blakemore S-J, Sharot T. Human development of the ability to learn from bad news. Proc. Natl. Acad. Sci. 2013;110:16396–16401. doi: 10.1073/pnas.1305631110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Bos W, Güroğlu B, van den Bulk BG, Rombouts SARB, Crone EA. Better than expected or as bad as you thought? The neurocognitive development of probabilistic feedback processing. Front. Hum. Neurosci. 2009;3:52. doi: 10.3389/neuro.09.052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reeck C, Ames DR, Ochsner KN. The Social Regulation of Emotion: An Integrative, Cross-Disciplinary Model. Trends Cogn. Sci. 2015 doi: 10.1016/j.tics.2015.09.003. doi:10.1016/j.tics.2015.09.003. [Recent review proposing a process model of social regulation of emotion. Synthesizes research from developmental, dyadic, self-regulation and social support literatures.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCormick EM, Qu Y, Telzer EH. Adolescent neurodevelopment of cognitive control and risk-taking in negative family contexts. NeuroImage. 2016;124(Part A):989–996. doi: 10.1016/j.neuroimage.2015.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blakemore S-J, Mills KL. Is Adolescence a Sensitive Period for Sociocultural Processing? Annu. Rev. Psychol. 2014;65 doi: 10.1146/annurev-psych-010213-115202. [Review of literature related to adolescent social processing including functional and structural development of the “social brain” and educational, legal, and social implications.] [DOI] [PubMed] [Google Scholar]
- 48.Callaghan BL, Tottenham N. The Neuro-Environmental Loop of Plasticity: A Cross-Species Analysis of Parental Effects on Emotion Circuitry Development Following Typical and Adverse Caregiving. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.204. doi:10.1038/npp.2015.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 50.Telzer EH, Ichien NT, Qu Y. Mothers know best: redirecting adolescent reward sensitivity toward safe behavior during risk taking. Soc. Cogn. Affect. Neurosci. 2015;10:1383–1391. doi: 10.1093/scan/nsv026. [This study found that in the presence of their mothers, adolescents were more likely to activate prefrontal control regions during a risky decision making task compared to when they were alone.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Dev. Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith AR, Steinberg L, Strang N, Chein J. Age differences in the impact of peers on adolescents’ and adults’ neural response to reward. Dev. Cogn. Neurosci. 2014 doi: 10.1016/j.dcn.2014.08.010. doi:10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc. Cogn. Affect. Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peake SJ, Dishion TJ, Stormshak EA, Moore WE, Pfeifer JH. Risk-taking and social exclusion in adolescence: Neural mechanisms underlying peer influences on decision-making. NeuroImage. 2013;82:23–34. doi: 10.1016/j.neuroimage.2013.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Silvers JA, Shu J, Hubbard AD, Weber J, Ochsner KN. Concurrent and lasting effects of emotion regulation on amygdala response in adolescence and young adulthood. Dev. Sci. 2015;18:771–784. doi: 10.1111/desc.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saygin ZM, Osher DE, Koldewyn K, Martin RE, Finn A, Saxe R, Gabrieli JDE, Sheridan M. Structural Connectivity of the Developing Human Amygdala. PLoS ONE. 2015;10:e0125170. doi: 10.1371/journal.pone.0125170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: A cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]