Abstract
Background
To determine the effects of dendritic cells (DCs) and cytokine-induced killer (CIK) cells in patients with malignant pericardial effusion.
Material/Methods
All patients underwent pericardial puncture and indwelling catheter insertion. After pericardial drainage, the 16 patients in the treatment group received an infusion of 20 mL DCs and CIK cells (>1.0×1010 cells) and 500,000 U interleukin (IL)-2 for 3 successive days. The 15 control-group patients received 30 mg/m2 cisplatin and 500,000 U IL-2 for 3 successive days. The treatment effects were assessed using imaging data.
Results
The total efficiency and complete remission rates were higher in the treatment group than in the control group at 4 weeks (total efficiency: 87.50% vs. 73.33%; complete remission: 62.50% vs. 46.67%) and 3 months after the treatment (total efficiency: 81.25% vs. 66.67%; complete remission: 50.00% vs. 40.00%; P<0.05 for all). In both groups, the Karnofsky scores for quality of life improved after treatment. However, the curative effects were better in the treatment group than in the control group (P<0.05). The following adverse reactions occurred: fever, 6 treatment-group patients and 3 control-group patients; chest pain, 2 treatment-group patients and 7 control-group patients; gastrointestinal reactions, 1 treatment-group patient and 6 control-group patients; and bone marrow suppression, 1 treatment-group patient and 5 control-group patients. The between-group differences in adverse reactions were significant (P<0.05).
Conclusions
The combination of DCs and CIK cells effectively treated malignant pericardial effusion, produced few side effects, and improved the patients’ quality of life.
MeSH Keywords: Cytokine-Induced Killer Cells, Dendritic Cells, Pericardial Effusion
Background
Malignant pericardial effusion is a common complication in patients with advanced cancers. Patients with this condition may experience chest tightness, shortness of breath, and other symptoms that severely affect their quality of life and often indicate a poor prognosis. Malignant pericardial effusion is commonly associated with lung cancer, breast cancer, ovarian cancer, stomach cancer, colon cancer, lymphoma, and other malignancies.
Currently, adoptive immunotherapy has become an important anticancer treatment that is administered in addition to conventional treatment methods [1]. In particular, cytokine-induced killer (CIK) cell immunotherapy is being increasingly used because of its effectiveness and low toxicity [2–4]. CIK cells form CD3+CD56+ and CD3+CD8+ based heterogenetic cell colonies; these cells are immune effectors with powerful killing activity and have been considered to be good prospects for inducing adoptive immunotherapy [5,6]. Several clinical trials have investigated the use of CIK cells for the treatment of malignant solid tumors and have confirmed that these cells have good curative effects [7–11].
At present, minimally invasive closed drainage of the pericardial cavity and intrapericardial injection of drugs is the preferred treatment for malignant pericardial effusion. The injected drugs often include chemotherapy drugs, auxiliary anticancer drugs, and biologic agents. The current study summarizes the curative effects of dendritic cells (DCs) combined with CIK cells in patients with advanced tumors and malignant pericardial effusion who were treated between October 2009 and June 2015 in our hospital.
Material and Methods
Clinical data
A total of 31 untreated patients with pathologically diagnosed advanced malignant tumors associated with malignant pericardial effusion were selected. The patients consisted of 17 men and 14 women. They were randomly assigned to a control group (n=16) or a treatment group (n=15), using a random number table. The mean ages of the patients in the control and treatment groups were 49.5±3.8 years and 48.9±4.1 years, respectively, and the male-to-female ratios were 9:7 and 8:7, respectively. There were no significant differences between the 2 groups. in age and sex The end-diastolic thickness of malignant pericardial effusion was 1.5 to 4.5 cm on semisitting echocardiography, and all patients showed different degrees of pericardial tamponade as indicated by symptoms and signs, such as expiratory dyspnea, inability to lie flat, palpitation, cyanosis, and cardiac enlargement. The Karnofsky performance scores of the patients were ≥50, and no patient suffered severe complications. The findings of electrocardiography, liver- and kidney-function tests, and routine blood and electrolyte examinations were in the normal range. The expected survival of the patients was ≥3 months. Further, the primary tumors were pathologically diagnosed in all patients and cancer cells were found in the pericardial effusion fluid. This study was approved by the ethics committee of our hospital, and all the patients and their families signed informed consent forms.
Collection and culture of DCs and CIK cells
Approximately 100-mL samples of autologous peripheral blood mononuclear cells (PBMCs) were collected from the cubital vein using an MCS+ blood cell separator (Haemonetics Corporation, United States). Mononuclear cells were isolated from the PBMC samples via culture in lymphocyte separation medium in the GMP laboratory of our hospital. The cells were then added to a 50-mL centrifugal tube and centrifuged at 2000 rpm for 20 min. The supernatant plasma was collected for subsequent experiments. Next, 60 mL saline solution was added to the cells, and the solution was mixed carefully. Following this, 12 mL medical-grade lymphocyte separation medium at 20°C was added to four 50-mL centrifugal tubes, and to each of these tubes, approximately 20 mL of the saline/cell mixture was slowly added along the wall. The mixture was divided into 4 layers by centrifugation and standing. Mononuclear cells were collected, added to phosphate-buffered saline solution at pH 7.4 to 7.6, centrifuged at 1600 rpm for 5 min, rinsed 3 times, and resuspended in 20-mL serum-free medium (supplemented with autologous serum with 2% volume concentration). Then, 100 ng/mL recombinant human granulocyte macrophage colony-stimulating factor (rhGM-CSF) and 30 ng/mL recombinant human interleukin-4 (rhIL-4) were added to stimulate the cells to differentiate into DCs. Half of the solution was replaced on the third day, Tn+ tumor antigen was added on the fifth day, and the cytokine tumor necrosis factor-α was added on the sixth day. On the seventh day, adherent DCs were removed with a cell scraper and co-cultured with CIK cells; the solution was replaced every 1 to 2 days.
Nonadherent CIK cells were collected for the co-culture with DCs. The CIK cells were suspended in 20 mL serum-free medium and added to a 75-cm2 cell-culture flask. Then, 1000 U/mL interferon (IFN)-γ was added to the flask, and 1000 U/mL rhIL-2, 50 ng/mL CD3, and 100 U/mL IL-1a were added 24 h later. On the fourth day, the cells were transferred to a 175-cm2 cell-culture flask supplemented with IFN-γ, rhIL-2, and IL-1a. After this, the solution was replaced every 1 to 2 days, depending on the cell number and medium color, and supplemented with IFN-γ, rhIL-2, and IL-1a. From the seventh day, CIK cells and DCs were co-cultured, and the co-culture solution was replaced every 1 to 2 days.
Before the end of the culture, bacteria, fungi, mycoplasma, and endotoxins were tested for, using the bidirectional method in the laboratory and blood screening test department (bacteria and fungi were tested for by using the appropriate plate assays; mycoplasma was tested for using an IST kit; endotoxins were monitored using tachypleus amebocyte lysate; and viruses were monitored using polymerase chain reaction assays).
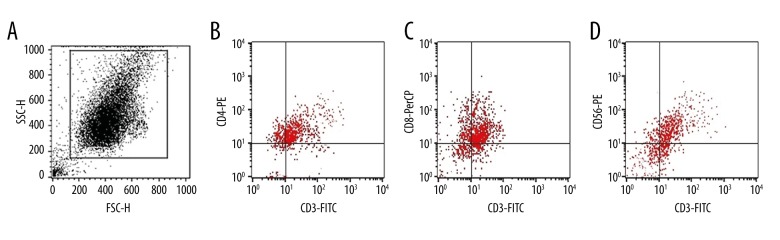
More than 1×1010 DC-CIK cells could be obtained after culture for 13 days. These cells were analyzed using CD3-FITC, CD4-PE, CD8-PerCP, and CD56-PE kits and a Calibur flow cytometer (BD, United States). The main effector cells for DC-CIK cells were double-positive cells: CD3+CD8+ and CD3+CD56+. The culture medium was subjected to a contamination test with Gram staining before the CIK cells were reinfused into the patients (Figure 1).
Figure 1.
Results of flow cytometry (performed 9 times). (A) The expression rate of CD3+ was 90.76±4.32%. (B) The ratio of cells with CD3+CD4+ coexpression was 36.13±1.32. (C) The ratio of cells with CD3+CD8+ coexpression was 39.12±1.43. (D) The ratio of cells with CD3+CD56+ coexpression was 23.42±2.21.
Pericardial effusion treatment
All patients underwent pericardial puncture with an indwelling catheter. After drainage for 3 to 5 days, all patients underwent an ultrasound review, which revealed that the pericardial effusion fluid had been almost completely drained (volume of pericardial effusion, <50 mL). Then, the relevant drugs, according to the patient group, were injected into the pericardial cavity. In the treatment group, 20 mL DCs and CIK cells and 500,000 U rhIL-2 in 10 mL of 0.9% saline were continuously injected into the pericardial cavity through the indwelling catheter for 3 successive days. In the control group, 500,000 U rhIL-2 in 10 mL of 0.9% saline and 30 mg/m2 cisplatin in 20 mL of 0.9% saline were injected over 3 days. The control of pericardial effusion, variation in Karnofsky scores, and adverse reactions at 4 weeks and 3 months after the treatment, as well as the survival duration until the follow-up on October 1, 2015, were compared between the 2 groups.
Determination of pericardial effusion
The curative effects of the treatments were assessed using the World Health Organization (WHO) criteria. Complete remission (CR) referred to the disappearance of pericardial effusion and complete remission of symptoms, lasting for more than 4 weeks. Partial remission (PR) referred to a >50% reduction in pericardial effusion fluid and an improvement in symptoms lasting for more than 4 weeks. No remission (NR) meant that the pericardial effusion was reduced by <50% or was increased, and the patient required repeated pumping solution within 4 weeks. Changes in the amount of pericardial effusion fluid were determined using B-ultrasound examinations. Total efficiency was calculated as CR+PR. The functional status was assessed using the Karnofsky criteria, and side effects were classified as grades 1 to 4, according to WHO criteria.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 software. Intergroup comparisons were conducted using the χ2 test and t test, where P<0.05 was considered statistically significant.
Results
Control of pericardial effusion
At 4 weeks after the treatment, in the treatment group, CR, PR, and NR were achieved in 9, 5, and 2 patients, respectively, yielding a total efficiency of 87.50% (14/16) and a CR rate of 62.50% (10/16). In the control group, CR, PR, and NR were achieved in 7, 4, and 4 patients, respectively, yielding a total efficiency of 73.33% (11/15) and a CR rate of 46.67% (7/15). The differences between the 2 groups were statistically significant (P<0.05).
At 3 months after the treatment, in the treatment group, CR, PR, and NR were achieved in 8, 5, and 3 patients, respectively, yielding a total efficiency of 81.25% (13/16) and a CR rate of 50.00% (8/16). In the control group, CR, PR, and NR were achieved in 6, 4, and 5 patients, respectively, yielding a total efficiency of 66.67% (10/15) and a CR rate of 40.00% (6/15). The differences between the 2 groups were statistically significant (P<0.05).
Side effects
In both groups, there were no severe, grade 4 reactions, as evaluated using the WHO toxicity criteria. The main side effects included grade 1 to 2 chest pain, fever, bone marrow suppression, and grade 1 to 3 gastrointestinal reactions. All of these toxicities were alleviated after symptomatic treatment. Fever occurred in 6 of the 16 patients in the treatment group and 3 of the 15 patients in the control group, with a highest temperature of 38.2°C. Chest pain occurred in 2 patients in the treatment group and 7 patients in the control group. Grade 1 to 3 gastrointestinal reactions occurred in 1 patient in the treatment group and 6 patients in the control group, while bone marrow suppression occurred in 1 treatment group patient and 5 control group patients. The incidence of fever was higher in the treatment group than in the control group, while the incidences of the other three adverse reactions were higher in the control group. The differences between the 2 groups were statistically significant (P<0.05).
Quality of life
After treatment, chest tightness and shortness of breath were alleviated in both groups, and the Karnofsky score was improved to varying degrees. The difference in the symptoms before and after the treatment was statistically significant in both groups (P<0.05; Table 1).
Table 1.
Comparison of Karnof sky scores before and after treatment.
| Group | Patients | Pretreatment Karnofsky score | Post-treatment Karnofsky score | ||||
|---|---|---|---|---|---|---|---|
| 50–70 | 70–80 | ≥80 | 50–70 | 70–80 | ≥80 | ||
| Treatment | 16 | 10 | 5 | 1 | 2 | 3 | 11 |
| Control | 15 | 9 | 4 | 2 | 4 | 6 | 5 |
Discussion
Malignant pericardial effusion is a common clinical issue affecting the quality of life of patients with malignant tumors. Thus, finding an effective method to control malignant pericardial effusion, improve quality of life, and prolong survival is a vital concern.
DCs can recognize tumor antigens and activate immune responses to specific antigens, reducing immune evasion by tumor cells. CIK cells tend to express both CD3 and CD56 membrane proteins and possess the antitumor activity of T lymphocytes and the non-MHC-restricted tumoricidal effect of natural killer cells, which can kill tumor cells via the toxic effect of autologous cells and secretion of cytokines. CIK cells have the advantages of fast growth, high tumoricidal activity, broad tumoricidal spectrum, and few side effects. In recent years, many clinical studies have confirmed that DC/CIK cell therapy is a preferred protocol for a new generation of antitumor adoptive immunotherapy [12], and DCs and CIK cells are 2 important components of tumor immunotherapy [13].
DCs, as the main powerful antigen-presenting cells in the human body, can induce antigen-specific cytotoxic T lymphocytes [14], affect the proliferation of B cells, and activate humoral immune responses [15]. The combination of CIK cells and DCs for the treatment of malignant tumors can relieve the immune incompetence of T cells in tumor patients and has a synergistic antitumor effect.
In this clinical trial, DCs combined with CIK cells were used to treat patients with malignant tumors and pericardial effusion, observing curative effects, side effects, and improvement in quality of life, in an attempt to provide a new therapeutic measure for patients with malignant pericardial effusion. The long-term curative effects of DCs and CIK cells combined with IL-2 treatment on malignant pericardial effusion have not yet been reported. Therefore, more in-depth clinical trials are needed to observe the efficiency and long-term effects of this treatment.
Conclusions
In short, treatment with DCs and CIK cells in patients with malignant pericardial effusion showed high efficiency and low toxicity and is worthy of clinical application.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors declare that they have no actual or potential conflicts of interest.
References
- 1.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6(5):383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Helms MW, Prescher JA, Cao YA, et al. IL-12 enhances efficacy and shortens enrichment time in cytokine-induced killer cell immunotherapy. Cancer Immunol Immunother. 2010;59(9):1325–34. doi: 10.1007/s00262-010-0860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28(6b):3997–4002. [PubMed] [Google Scholar]
- 4.Kimura H, Iizasa T, Ishikawa A, et al. Prospective phase II study of post-surgical adjuvant chemo-immunotherapy using autologous dendritic cells and activated killer cells from tissue culture of tumor-draining lymph nodes in primary lung cancer patients. Anticancer Res. 2008;28(2b):1229–38. [PubMed] [Google Scholar]
- 5.Linn YC, Hui KM. Cytokine-induced NK-like T cells: from bench to bedside. J Biomed Biotechnol. 2010;2010:435745. doi: 10.1155/2010/435745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi M, Zhang B, Tang ZR, et al. Autologous cytokine-induced killer cell therapy in clinical trial phase I is safe in patients with primary hepatocellular carcinoma. World J Gastroenterol. 2004;10(8):1146–51. doi: 10.3748/wjg.v10.i8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutella S, Iudicone P, Bonanno G, et al. Adoptive immunotherapy with cytokine-induced killer cells generated with a new good manufacturing practice-grade protocol. Cytotherapy. 2012;14(7):841–50. doi: 10.3109/14653249.2012.681038. [DOI] [PubMed] [Google Scholar]
- 8.Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer. 2011;2:363–68. doi: 10.7150/jca.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin V, Pizzitola I, Agostoni V, et al. Cytokine-induced killer cells for cell therapy of acute myeloid leukemia: improvement of their immune activity by expression of CD33-specific chimeric receptors. Haematologica. 2010;95(12):2144–52. doi: 10.3324/haematol.2010.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Zhu L, Wei J, et al. The effects of cytokine-induced killer cells for the treatment of patients with solid tumors: A clinical retrospective study. J Cancer Res Clin Oncol. 2012;138(6):1057–62. doi: 10.1007/s00432-012-1179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang JT, Shen YP, Wu CP, et al. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16(48):6155–62. doi: 10.3748/wjg.v16.i48.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg SA, Restifo NP, Yang JC, et al. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren J, Di L, Song G, et al. Selections of appropriate regimen of high-dose chemotherapy combined with adoptive cellular therapy with dendritic and cytokine-induced killer cells improved progression-free and overall survival in patients with metastatic breast cancer: reargument of such contentious therapeutic preferences. Clin Transl Oncol. 2013;15(10):780–88. doi: 10.1007/s12094-013-1001-9. [DOI] [PubMed] [Google Scholar]
- 14.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 15.Zoll B, Lefterova P, Ebert O, et al. Modulation of cell surface markers on NK-like T lymphocytes by using IL-2, IL-7 or IL-12 in vitro stimulation. Cytokine. 2000;12(9):1385–90. doi: 10.1006/cyto.2000.0733. [DOI] [PubMed] [Google Scholar]