Abstract
Disorders of sexual development (DSD) encompass a broad spectrum of urogenital malformations and are amongst the most common congenital birth defects. Although key genetic factors such as the hedgehog (Hh) family have been identified, a unifying postnatally viable model displaying the spectrum of male and female urogenital malformations has not yet been reported. Since human cases are diagnosed and treated at various stages postnatally, equivalent mouse models enabling analysis at similar stages are of significant interest. Additionally, all non-Hh based genetic models investigating DSD display normal females, leaving female urogenital development largely unknown. Here, we generated compound mutant mice, Gli2+/–;Gli3Δ699/+, which exhibit a spectrum of urogenital malformations in both males and females upon birth, and also carried them well into adulthood. Analysis of embryonic day (E)18.5 and adult mice revealed shortened anogenital distance (AGD), open ventral urethral groove, incomplete fusion of scrotal sac, abnormal penile size and structure, and incomplete testicular descent with hypoplasia in male mice, whereas female mutant mice displayed reduced AGD, urinary incontinence, and a number of uterine anomalies such as vaginal duplication. Male and female fertility was also investigated via breeding cages, and it was identified that male mice were infertile while females were unable to deliver despite becoming impregnated. We propose that Gli2+/–;Gli3Δ699/+ mice can serve as a genetic mouse model for common DSD such as cryptorchidism, hypospadias, and incomplete fusion of the scrotal sac in males, and a spectrum of uterine and vaginal abnormalities along with urinary incontinence in females, which could prove essential in revealing new insights into their equivalent diseases in humans.
Introduction
Disorders of sexual development (DSD) are among the most common human birth defects, affecting nearly 3% of all newborns [1]. This high incidence rate can be attributed to the complex set of developmental events that need to be harmoniously synchronized from the onset of sexual differentiation until the emergence of sexually functional adult [2]. Cryptorchidism, hypospadias, and incomplete scrotal sac fusion are the most common congenital complications in males [2, 3]. In females, disorders of sexual development typically present as congenital uterine anomalies, with particular cases such as vaginal duplication threatening child delivery [4, 5]. Despite the clinical significance of such anomalies in both males and females, there is a lack of equivalent animal models.
Male and female urogenital systems arise from the outgrowth, patterning, and differentiation of an ambisexual embryonic bulge called the genital tubercle (GT) [6, 7]. In conjunction, complete cloacal septation is required for correct urogenital and anorectal sinus formation [8–9]. Sonic hedgehog (Shh), a secreted signaling protein, is involved in GT outgrowth and patterning, as well as cloacal septation, whereas Desert hedgehog (Dhh), another hedgehog (Hh) homologue, orchestrates male gonad development and sexual differentiation [6, 8, 10]. While Shh null male and female mice display GT agenesis and persistent cloaca, Dhh null male mice are sterile, and female mice are normal [11–13]. The early and severe genital and cloacal phenotypes of Shh null mice and the sex specific phenotypes of Dhh null mice have limited the generation of a viable mouse model for common DSD affecting both male and females.
Gli transcription factors are downstream mediators of Hh signaling. Gli2 functions predominantly as an activator for Hh target genes, whereas Gli3 undergoes C-terminal truncation to form a potent repressor [14]. The balance between the two is essential for Hh signaling, as gene dosages of Shh, Gli2, and Gli3 modulate the severity of cloacal malformations [15, 16]. Here, we report the generation of a postnatally viable genetic mouse model, Gli2+/–;Gli3Δ699/+, with reduced level of Gli2 activator and constitutive expression of Gli3 repressor (from the Gli3Δ699 mutant allele)[17] for studying common DSD in both male and female animals.
Materials and Methods
The Hospital for Sick Children Research Ethics Board and The Centre for Phenogenomics have approved all animal care and use protocols used in this study. Animal euthanasia was performed via CO2 chambers.
Mutant Generation and Mating
Gli2 mutant mice carry a targeted deletion in the DNA-binding zinc-finger motif of the gene [16]. Gli3Δ699 mutant mice contain a targeted deletion 3’ of the zinc finger motif, rendering it a constitutively active repressor [17]. Intercrosses of Gli2+/–and Gli3Δ699/+ mice were used to generate Gli2–/–and Gli3Δ699/Δ699 mice, respectively. Crosses between Gli2+/–and Gli3Δ699/+ were used to generate Gli2+/–, Gli3Δ699/+, and Gli2+/–;Gli3Δ699/+ mice. All mice were of CD-1 background. Genotypes and sexual identities of the mice were determined by Polymerase Chain Reaction analysis of ear notches (postnatal 4 months) and yolk sac (E18.5) DNA using Gli2, Gli3Δ699, and Sry primers. All protocols were approved by the Institution’s Animal Care and Use Committee.
Fertility Examination
To test male fertility, 12 cages were set up, each with one Gli2+/–;Gli3Δ699/+male mouse, and three normal female mice (Gli2+/–and Gli3Δ699/+). Female mice were checked for plugs every morning for three months. To test female fertility, 7 cages were set up with one normal male (Gli2+/–or Gli3Δ699/+) and 2–3 female mice per cage, with at least one Gli2+/–;Gli3Δ699/+ female. Female mice were checked for plugs every morning for three months. To test fertility of Gli2+/–and Gli3Δ699/+ mice, 5 cages, each with one male and 2–3 female mice of mixed genotypes of Gli2+/–, Gli3Δ699/+, and wild type CD-1 mice were set up. Male and female Gli2+/–and Gli3Δ699/+ mice display wild type like fertility.
Morphological Analysis
Midday of the day of vaginal plug was considered embryonic day (E) 0.5 in embryo collection. Since Gli2–/–and Gli3Δ699/Δ699 mutant mice die at birth, and average gestation age of mice is between 19–21 days, E18.5 was chosen as the last checkpoint for prenatal genitalia development. Pregnant mice were sacrificed via CO2 euthanasia, and embryos were harvested, washed with PBS, and fixed in 4% paraformaldehyde overnight at 4°C for overall urogenital sinus and anogenital distance (AGD) examination under low magnification microscope, with Gli2+/+ or Gli3+/+ mice as controls. To examine the internal openness of female reproductive tract, ink was injected into the distal uterus horns, and the dark color of the ink in contrast with the pink tubules, allowed identification of tubule openness.
Dissection, Paraffin Embedding, and Histology
Adult control mice as well as Gli2+/–;Gli3Δ699/+ mice were used for gross dissection of lower abdomen, and digital photographs were obtained for visual assessment of testicular location, penile/urethral size and position in males and internal genitalia in females. Pairs of control and mutant mice testes were placed side-by-side on petri dish for visual comparison of size. The longest length of each testis was measured under low power microscope and averages for both control and mutant mice were calculated.
Tissue/organs were dissected out from adult mice, and fixed in 4% paraformaldehyde overnight at 4°C. They were dehydrated, processed, and embedded in paraffin wax before sectioning at 5μm. Slides were then dewaxed, rehydrated, and stained with hematoxylin and eosin. All measurements of male external genitalia were performed according to methods described in Rodriguez et al. 2011 [18]. Seminiferous tubule count was performed via manual counting of randomized low magnification images of wild type and Gli2+/–;Gli3Δ699/+ testes stained with H&E.
Immunofluorescence Staining
Immunofluorescence staining was performed on paraffin sections. After quenching the endogenous peroxidases with 3% H2O2 in 10% methanol, targeted antigens were retrieved by boiling the slides in an antigen-unmasking solution (H-3300, Vector Laboratories). Sections were treated with blocking reagent (all supplied by Roche) before application of primary antibodies for Sertoli and Leydig cell detection: GATA-1 (1:200, Cell Signaling Technology), and P450scc (1:100, Cell Signaling Technology), respectively. The number of Sertoli cells were quantified per seminiferous tubule by averaging the number of positive GATA-1 signals for 20 tubules within each testes (n = 5). Leydig cell signals were quantified via ImageJ software by measuring fluorescence intensities of interstitial space P450scc signals and normalized to background (n = 6). Cell proliferation was assayed by immunofluorescence staining using Ki-67 antibodies (1:100, Thermo Scientific) and apoptosis was examined using immunofluorescence staining of caspase-3 antibody (1:200, Cell Signaling Technology). Dhh expression was analyzed using Dhh antibody (1:100, Santa Cruz Biotechnology Inc.). MVH staining (1:200, ab13840) was performed in order to stain spermatogonium. All primary antibodies were incubated at 4°C overnight.
Serum Androgen Detection
Serum from both wild type (n = 3) and Gli2+/-;Gli3 Δ 699/+ (n = 4) adult mice were extracted and sent to Aska Pharma Medical Co., LTD, Japan, where they were analyzed via liquid chromatography-tandem Mass Spectrometry (LC-MS/MS) to quantify testosterone and dihydrotestosterone.
Statistical Analyses
All data were expressed as mean ± standard error of the mean (SEM). Possible differences in penile anatomical structure and testicular lengths, testicular weight, and seminiferous count between wild type and Gli2+/–;Gli3Δ699/+ mice were determined by Student’s two tailed t-test, and p-values<0.05 were considered significant.
Results
Male Gli2+/–;Gli3Δ699/+ Mice Exhibit Severely Reduced AGD and Penile Malformations
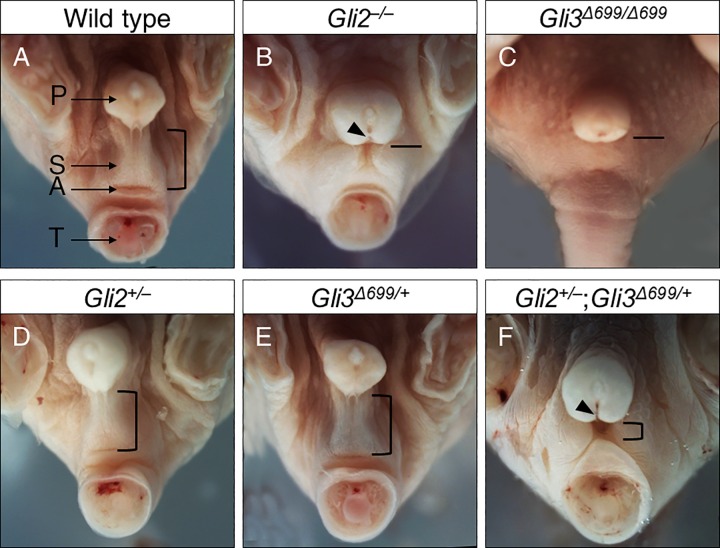
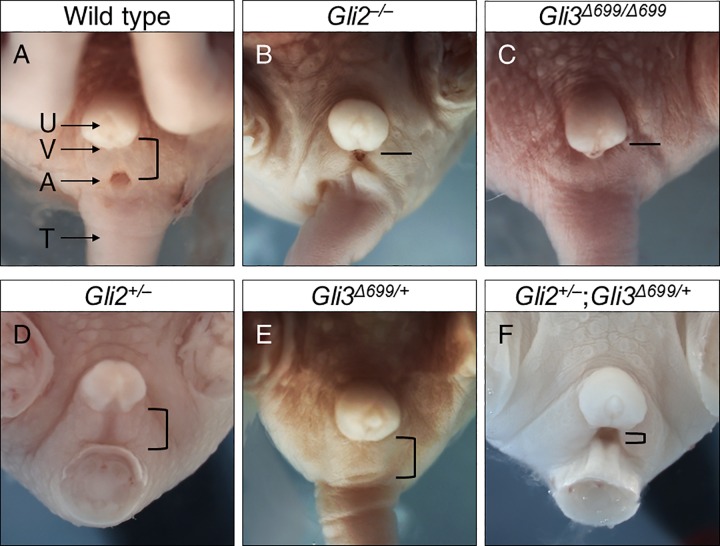
Wild type male mice at E18.5 have proper prepuce development with complete fusion of scrotal sacs, while exhibiting normal AGD and urethral opening (Fig 1A, n = 8). Loss of both alleles of Gli2 resulted in reduced AGD and incomplete fusion of the scrotum with ventrally open urethral groove in Gli2–/–mice (Fig 1B, n = 9) [16]. Gli3Δ699/ Δ699 mice, with two copies of constitutive repressor allele of Gli3, exhibited reduced AGD, a flattened scrotal sac, and imperforate anus (Fig 1C, n = 12) [17]. Loss of one copy of Gli2 in Gli2+/‒ mice (Fig1D, n = 8), or gain of one copy of constitutive Gli3 repressor in Gli3+/Δ699 mice (Fig 1E, n = 8) did not result in any obvious phenotype [16, 17]. In contrast double heterozygous Gli2+/–;Gli3Δ699/+ mice exhibited prominent genitalia malformations, characterized by reduced AGD and incomplete fusion of the scrotum (Fig 1F, n = 15). Although it appeared that these mice displayed cloaca formation, adult stage analysis proved otherwise. In addition, all Gli2+/–;Gli3Δ699/+ mice displayed an open urethral groove on the ventral aspect of the developing phallus, a reliable early indication of hypospadias (Fig 1D black arrow).
Fig 1. Male genital malformations in E18.5 compound mutant mice of Gli2+/–and Gli3Δ699/+.
Arrows indicate major identifiable structures in the urogenital sinus area. Open bracket approximates distance between the prepuce and the anus in mice of different genotypes, with shorter bracket indicating a shortened AGD. A straight line instead indicates imperforated anus in Gli2–/–and Gli3Δ699/Δ699 mice. Black arrow indicates a ventral opening to the urethra, indicative of hypospadias. A, anus; AGD, anogenital distance; P, prepuce; S, Scrotal sac; T, tail.
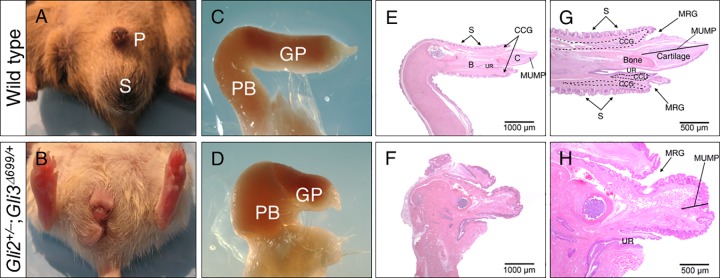
Although male Gli2+/–;Gli3Δ699/+ mice lived past puberty and well into adulthood, they were not able to plug females (n = 12). To characterize these mice in their adult stages of life, we examined their genitals and gonadal phenotypes at 4 months of age. In wild type adult males, mature sexual characteristics, such as proper prepuce size and location, and completely fused scrotal sac, were apparent (Fig 2A). In contrast, all Gli2+/–;Gli3Δ699/+ mice displayed ambiguous or feminized appearance with reduced AGD, bifid phallus, incomplete fusion of scrotal sac, and penile malformations (Fig 2B, n = 20). Isolated penile examination revealed that the mutant phallus is reduced in length and exhibits abnormal shape at both penile body and glans penis (Fig 2C and 2D, n = 12). Histological and morphometric evaluation revealed a reduction in length of several penile features including male urethral mating protuberance (MUMP) and os penis in Gli2+/–;Gli3Δ699/+ mice (Fig 2E, 2F, 2G and 2H, and Table 1). In addition, structures visible dorsally to MUMP, including MUMP ridge groove (MRG), corpus cavernosum glandis (CCG), and surrounding keratinized spines, were disorganized and severely shortened in Gli2+/–;Gli3Δ699/+male mice (Fig 2G and 2H). As a consequence, mutant mice also displayed shortened urethra (UR)(Fig 2G and 2H) [18].
Fig 2. Gli2+/–;Gli3Δ699/+ adult male mice display genital malformations at 4 months of age.
(A-B) Gross morphology of the external genitalia, (C-D) harvested penile structures, and (E-H) H&E mid-sagittal sections of adult penile structures highlighting the significantly reduced length of MUMP, it’s surrounding structures, MCC, and os penis. (CCG, corpus cavernosum glandis; CCU, corpus cavernosum urethrae; GP, Glans penis; GR, glanular ridge; MUMP, male urogenital mating protuberance: MCC, MUMP corpus cavernosum; MRG, MUMP ridge groove; P, Penis; PB, Penile body; S, Scrotum; UR, urethra; UM, urethral meatus).
Table 1. Morphometric data of wild type and Gli2+/–;Gli3Δ699/+ penile structures.
| Feature | Wild type (μm) | Gli2+/–;Gli3Δ699/+ (μm) |
|---|---|---|
| MUMP Length | 1670 ± 170 | 564 ± 90 * |
| Distance (MUMP tip to urethral meatus) | 930 ± 80 | 318 ± 60 * |
| MCC length | 560 ± 70 | 250 ± 50 * |
| Bone Length | 3530 ± 320 | 678 ± 120 * |
| Organ Width | 1980 ± 210 | 843 ± 260 * |
Data represented as SEM
* Significantly different from wild type (P<0.05).
n = 8 for wild type, n = 12 for Gli2+/–;Gli3Δ699/+
Male Gli2+/–;Gli3Δ699/+ Mice Exhibit Undescended, Hypoplastic Testes
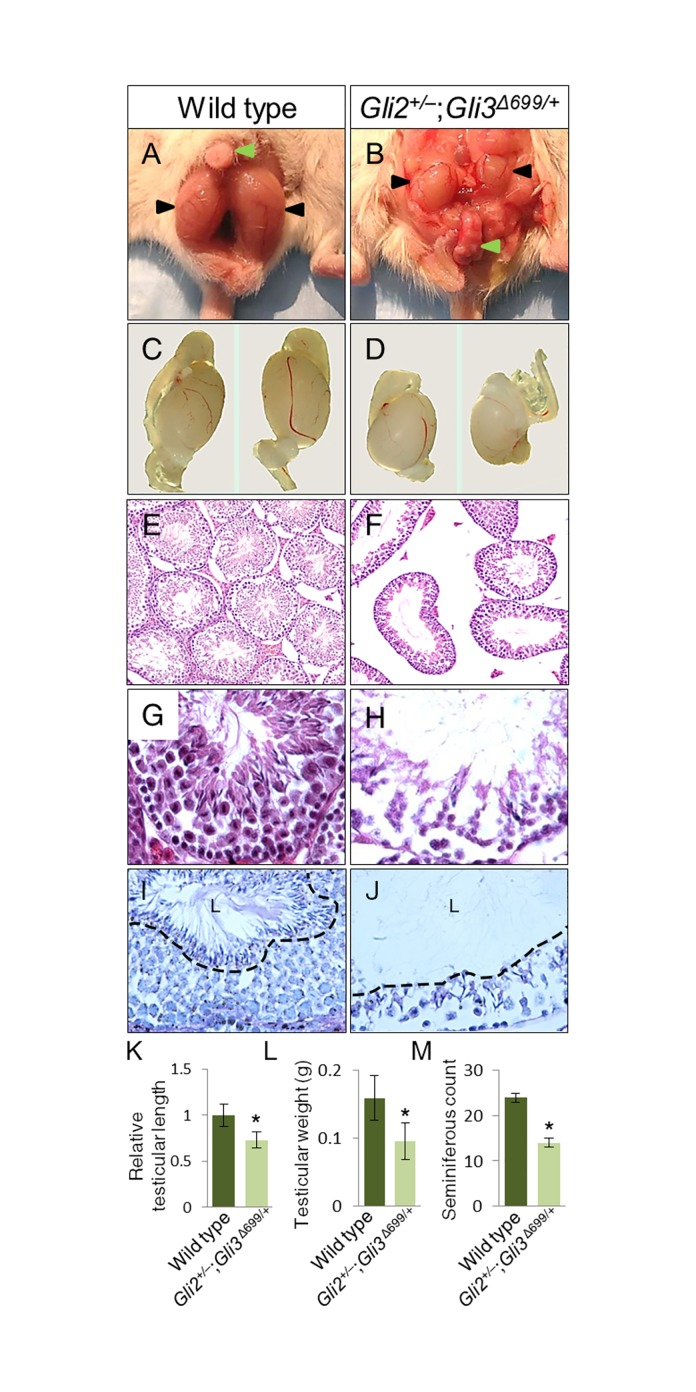
Next, we examined the gonads of Gli2+/–;Gli3Δ699/+ adult male mice. In wild type mice testes lay below the phallus level in their proper scrotal sac position (Fig 3A, n = 8). 80% of Gli2+/-;Gli3Δ699/+ mice showed varying degrees of incomplete testicular descent, of which 70% were bilaterally, and 30% were unilaterally undescended testes (Fig 3B, n = 20). It was readily apparent that testicular size was reduced in Gli2+/–;Gli3Δ699/+(Fig 3C and 3D, n = 20). Histological analyses revealed that Gli2+/–;Gli3Δ699/+ testes were hypoplastic, with enlarged seminiferous tubule luminal space, expanded interstitial space, reduced cellular density, and detached interstitial cells from the basal membrane (Fig 3E–3H, n = 20). PAS staining revealed maturation arrest as no mature spermatozoa were observed in the luminal space of Gli2+/–;Gli3Δ699/+, indicated via black dashed line (Fig 3I and 3J). Morphometric analysis revealed that average testicular length was reduced by 27% in Gli2+/–;Gli3Δ699/+ mice (0.866 cm vs. 0.636 cm, n = 20, P<0.05) (Fig 3C, 3D, and 3K) and average testicular weight was reduced by 40% (0.159g vs. 0.095g, n = 20, P<0.05) (Fig 3L). Gli2+/–;Gli3Δ699/+ mice had a 43% reduction of seminiferous tubule count compared to wild type testes (Fig 3M; n = 20, P<0.05). Analysis of epididymis, seminal vesicle, and prostate also revealed reduced size in Gli2+/–;Gli3Δ699/+ mice (data not shown).
Fig 3. Adult Gli2+/–;Gli3 Δ699/+ male mice display undescended, hypoplastic testes, and sperm maturation arrest.
(A-B) Gross dissection of the lower abdomen and genital region. (C-D) Isolated testes comparison of 4 months old wild type and Gli2+/–;Gli3Δ699/+ mutant, respectively. (E-F) H&E staining of testes sections under 10X magnification. (G-H) 40X magnification. (I-J) PAS staining of seminiferous tubules, black dashed line separating luminal space labeled “L”. (K-M) A comparison between wild type and Gli2+/–;Gli3Δ699/+ testicular size (n = 20, P<0.05) and weight (n = 20, P<0.05) respectively. (K) Relative seminiferous tubule count among wild type and Gli2+/–;Gli3Δ699/+mice (n = 20, P<0.05).
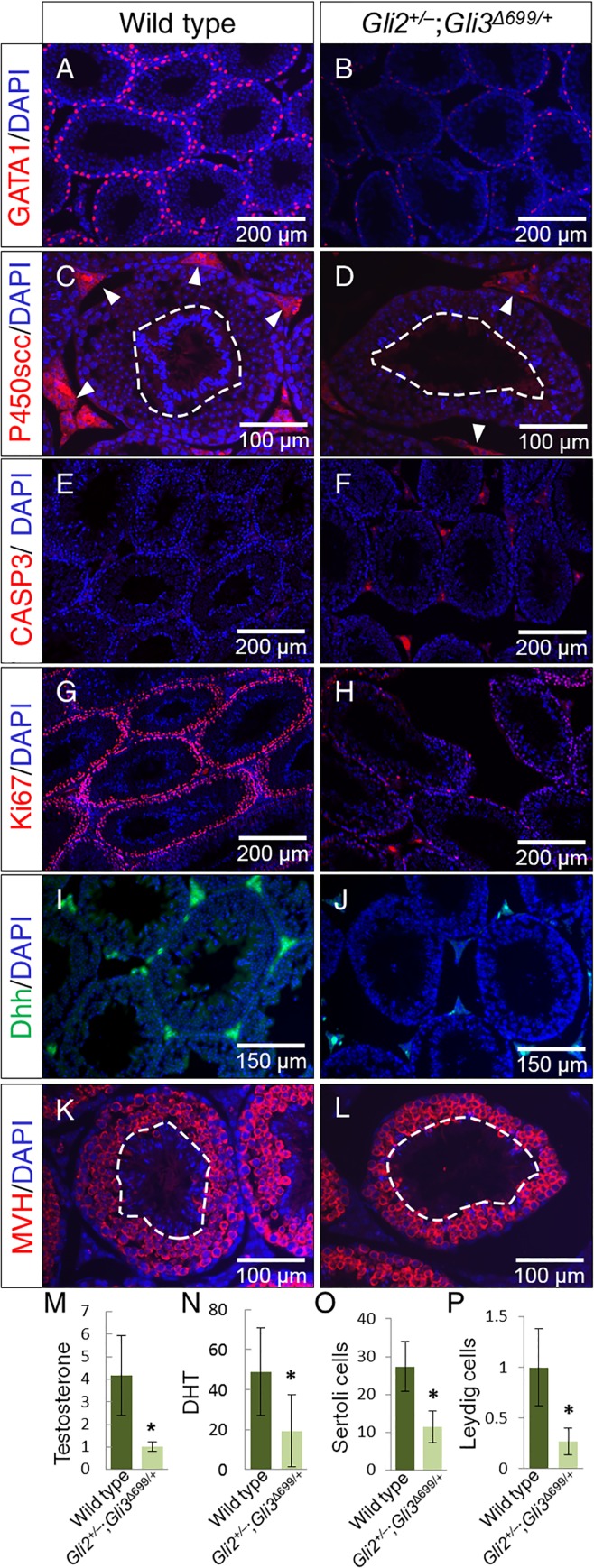
To gain insight into spermatogenesis in Gli2+/–;Gli3Δ699/+ mice, Sertoli and Leydig cell populations were analyzed via GATA1 and P450scc immunofluorescence labeling, respectively. GATA1 staining revealed a reduction of Sertoli cells, the cells responsible for Dhh production in the testes (Fig 4A and 4B). P450scc staining revealed a reduction of Leydig cells in the mutant testes (Fig 4C and 4D), and strong DAPI signals from the seminiferous tubule lumen, where mature spermatozoa reside (outlined with white dashed circle in Fig 4C and 4D). Increased Caspase-3 labeling in the interstitial space (Fig 4E and 4F) revealed increased apoptosis. Ki-67 labeling revealed significantly reduced proliferation (Fig 4G and 4H) along the basal seminiferous tubules in Gli2+/–;Gli3Δ699/+ testes. Consequently, Dhh, the main mediator of testicular development, was also significantly reduced in the mutant testes (Fig 4I and 4J). MVH staining revealed the presence of spermatogonia in Gli2+/–;Gli3Δ699/+ testes, however, no DAPI staining corresponding to mature spermatozoa were observed in the luminal space outlined by white dashed circle (Fig 3K and 3L and Fig 4C and 4D). Serum androgen levels were analyzed, and reduced amounts of testosterone and dihydrotestosterone were found in Gli2+/–;Gli3Δ699/+mice (Fig 3M and 3N, n = 4) with respect to control mice (n = 3). Quantification of Sertoli and Leydig cells indicated significantly reduced respective cell signals (Fig 4O and 4P).
Fig 4. Marker analysis of wild type and Gli2+/–;Gli3Δ699/+ testes.
Analysis of testicular specific Sertoli (A-B) and Leydig (C-D) cells responsible for sperm maturation and testosterone production respectively via GATA1 and P450scc immunofluorescence staining. (E-F) Caspase-3 was used as an indicator of apoptosis whereas Ki67 (G-H) was used as a proliferation marker. (I-J) Dhh staining was performed to analyze levels of Desert hedgehog within adult testes. (K-M) MVH staining for spermatogonia. Quantification of serum (M) testosterone (ng/ml) and (N) dihydrotestosterone (pg/ml) (Wild type (n = 3), Gli2+/–;Gli3Δ699/+ (n = 4), p<0.05). (O) Average Sertoli cell count per seminiferous tubule, (P) Relative fluorescence intensity of interstitial spaces of testes.
Female Gli2+/–;Gli3Δ699/+ Mice Display Reduced AGD, Uterine Abnormalities, and Urinary Incontinence
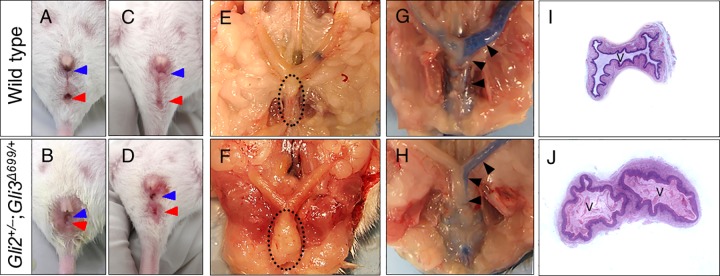
E18.5 wild type females display a well separated vagina and urethra (Fig 5A, n = 8). In contrast, both female Gli2–/–and Gli3 Δ699/ Δ699 mice displayed imperforate anus and were not viable (Fig 5B and 5C, n = 6 and n = 8, respectively) [16, 17]. Gli2+/–and Gli3Δ699/+ female mice exhibited normal external genitalia phenotype (Fig 5D and 5E, n = 6 and n = 8 respectively)[16]. Gli2+/–;Gli3 Δ699/+ female mice showed affected urogenital sinus with reduced AGD (Fig 5F, n = 11). However, unlike Gli2–/–and Gli3 Δ699/Δ699 mice, they survived postnatally. Adult female Gli2+/–;Gli3Δ699/+ mice displayed enlarged clitoris and vaginal openings (Fig 6A–6D, n = 10). Although AGD was severely reduced, it was identified that the urethra, vagina, and anus all remain separate in these mice (Fig 6B and 6D), similar to males. Anatomical and histological evaluations along with dye injection assays revealed vaginal wall hypertrophy in 30% (Fig 6E and 6F), uterine stenosis in 40% (Fig 6G and 6H), and double vaginas in 30% of female mice (Fig 6I and 6J, n = 14). As such, although female Gli2+/–;Gli3Δ699/+ mice were able to become impregnated by wild type males, they were unable to deliver the pups (n = 7). In addition, female Gli2 +/–;Gli3Δ699/+ mice invariably displayed urinary incontinence (data not shown).
Fig 5. E18.5 female Gli2+/–;Gli3Δ699/+ mice display abnormal urogenitalia.
Arrows indicate major structures within the urogenitalia region of female mice. AGD is indicated via the size of an open brackets measuring distance between the vagina and the anus. Straight line indicates imperforate anus in Gli2–/–and in Gli3Δ699/Δ699 respectively. (A, anus; T, tail; U, urethra; V, vagina).
Fig 6. Internal vaginal and uterine phenotype of adult female Gli2+/–;Gli3Δ699/+ mice.
(A-D) Gross external morphology of urogenitalia sinus in female wild type and Gli2+/–;Gli3Δ699/+ mice, blue arrows indicate vaginal opening whereas red arrows indicate anus, and their separation indicates a non-cloacal opening. (E-F) Characteristics of internal reproductive structures, dotted oval indicates the vaginal tube, enlarged in Gli2+/–;Gli3Δ699/+ mice due to double vagina and hypertrophy. (G-H) ink injection assays highlighting the extent of uterine obstruction. (I-J) H&E sectioning of genitalia indicating double vaginas in 30% of Gli2+/–;Gli3Δ699/+ mice (n = 14).
Discussion
Disorders of sexual development cover a spectrum of pathologies ranging from slight genital malformations to complete sex reversals in males and females [1]. Hh signaling has been identified as a key regulator of processes that are vital to proper genital development such as GT outgrowth and patterning, onset of sexual differentiation, and cloacal septation [8, 16]. As such, mice with mutations in the Hh signaling pathway display GT agenesis, imperforate anus, or persistent cloaca, phenotypes too severe for postnatal viability [7, 12– 14]. Although Gli transcription factors have been previously shown to regulate the anatomical sexual dimorphism of the GT, a model with a precise level of Hh signaling low enough to display the spectrum of urogenital malformations, and high enough to maintain viability, has not yet been reported [13, 15, 16, 19]. This is of particular significance, because human equivalent cases are diagnosed and treated postnatally in practice [20,21].
Cryptorchidism, hypospadias, and incomplete scrotal sac fusion are often linked congenital reproductive anomalies in male newborns [3]. To our knowledge, the only viable mouse model which exhibits these three phenotypes is the androgen receptor knockout (ARKO) mouse model [22]. However, although ARKO mice have expanded our understanding of these disorders, their phenotypes including 80% testes size reduction, and agenesis of vas deferens, epididymis, seminal vesicle, and prostate are rather extreme [22]. Here, our Gli2+/–;Gli3 Δ699/+ mouse model displays less severe phenotypes by presenting only a 40% reduction of testes size, with moderate reduction of the epididymis, seminal vesicle, and prostate. As such, Gli2+/–;Gli3 Δ699/+ mice can be used as a relevant animal model for studying DSD.
Male Gli2+/–;Gli3 Δ699/+ mice displayed several genital and gonadal defects, which could all contribute to infertility. However, due to their inability to plug females, the primary reason for their infertility here is attributed to structural malformations of their penis. Of significance, the Gli2+/–;Gli3Δ699/+ phallus displayed reduced length of MUMP and OS penis, two structures that physically facilitate mating [18]. In addition, male Gli2+/–;Gli3Δ699/+ testes displayed significantly reduced seminiferous tubule count with increased interstitial and luminal space. The luminal space of wild type testes displayed a wispy texture, indicative of spermatozoa, which is absent in the luminal space of Gli2+/–;Gli3Δ699/+ testes, suggesting defective spermatogenesis in the mutants [22]. This is supported by both Sertoli and Leydig cell count reductions in the mutant testes. The reduction of Leydig cells, as well as the similarities shared between Gli2+/–;Gli3Δ699/+ and ARKO male mice suggest compromised androgen production in our model. As such we analyzed serum androgen levels, and indeed Gli2+/–;Gli3Δ699/+ mice display reduced testosterone and dihydrotestosterone. This reduction rather than its abolishment as seen in ARKO mice may be the underlying reason behind the milder phenotypes observed.
ARKO mice as well as Dhh, Insl3, and Fkbp52 null mice, which are popular models of male genitalia and/or gonadal malformations, all display normal female counterparts [10, 22–24]. In contrast, Shh null mice, which display severe cases of persistent cloaca, and Gli2 as well as Gli3 null mice, which display imperforate anus with recto-urethral fistula and anal stenosis respectively, reveal the importance of hedgehog signaling in anorectal and urogenital development in both male and female [16]. Here, for the first time, we report a viable mouse model, which displays urogenital sinus anomalies, vaginal duplication, and urinary incontinence in its females. All Gli2+/–;Gli3 Δ699/+ females displayed uterine abnormalities, with a variety of anatomical disturbances. Although the reason for their inability to successfully deliver pups is not clearly known, internal uterine and vaginal stenosis, vaginal wall hypertrophy, and vaginal duplication may be significant contributing factors. This speculation rests on the results of the dye injection experiments but ongoing tests are currently being performed to elucidate this further. It has been previously shown that Gli2 regulates bladder mesenchymal patterning, which is required for proper smooth muscle formation and ultimately functioning of the bladder [25]. In our Gli2+/–;Gli3 Δ699/+ model, reduced Gli2 levels as well as the constitutive expression of Gli3 repressor could potentially affect mesenchymal patterning within the bladder, resulting in defective bladder wall formation and maturation, ultimately leading to urinary incontinence in adult female mice. Importantly, this urinary bladder anomaly is quite representative of those observed in humans as urinary incontinence is significantly more prevalent in females with cloacal anomalies,anorectal malformation, or urogenital sinus anomalies [26, 27]. Thus, the histological infrastructure and functional characteristics of the urinary bladders in Gli2+/–;Gli3 Δ699/+ female mice warrant further investigation.
Conclusions
Proper levels of Hh signaling are required for normal development. While drastic reduction or complete inactivation of the Hh pathway leads to severe hindgut malformations and embryonic lethality, moderate reduction of Hh pathway activity results in viable mice with urogenital malformations and compromised sexual differentiation. Here, we show that a precisely reduced level of Hh pathway activity in Gli2+/–;Gli3Δ699/+ mice generates a spectrum of urogenital malformations with reduced sexual differentiation in both male and female animals. Importantly, the anomalies observed in these mice strongly resemble those of common human clinical cases of cryptorchidism, hypospadias, and incomplete fusion of the scrotal sac in males, and various cases of genital and uterine abnormalities such as vaginal duplication and urinary incontinence in females. Thus, Gli2+/–;Gli3Δ699/+ mice could serve as excellent animal models for studying the pathogenesis of urogenital malformations for insight into their equivalent diseases in humans.
Data Availability
All relevant data are within the paper.
Funding Statement
This work is supported by an operating grant (FRN: 86628) from the Canadian Institutes of Health Research (PCK).
References
- 1.Tannour-Louet M, Han S, Corbett ST, Louet JF, Yatsenko S, Meyers L, et al. Identification of de novo copy number variants associated with human disorders of sexual development. PLoS One. 2010;5:e15392 10.1371/journal.pone.0015392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol. 2004;270:1–18. 10.1016/j.ydbio.2004.02.018 [DOI] [PubMed] [Google Scholar]
- 3.Pohl HG, Joyce GF, Wise M, Cilento BG Jr. Cryptorchidism and hypospadias. J Urol. 2007;177:1646–51. 10.1016/j.juro.2007.01.058 [DOI] [PubMed] [Google Scholar]
- 4.Ruggeri G, Gargano T, Antonellini C, Carlini V, Randi B, Destro F, et al. Vaginal malformations: a proposed classification based on embryological, anatomical and clinical criteria and their surgical management (an analysis of 167 cases). Pediatr Surg Int. 2012;28:797–803. 10.1007/s00383-012-3121-7 [DOI] [PubMed] [Google Scholar]
- 5.Nahum GG. Uterine anomalies. How common are they, and what is their distribution among subtypes? J Reprod Med 1998;43:877–87. [PubMed] [Google Scholar]
- 6.Blaschko SD, Cunha GR, Baskin LS. Molecular mechanisms of external genitalia development. Differentiation. 2012;84:261–8. 10.1016/j.diff.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic hedgehog signaling from the urethral epithelium controls external genital development. Dev Biol. 2002; 247: 26–46. 10.1006/dbio.2002.0668 [DOI] [PubMed] [Google Scholar]
- 8.Runck LA, Method A, Bischoff A, Levitt M, Peña A, Collins MH, et al. Defining the molecular pathologies in cloaca malformation: similarities between mouse and human. Dis Model Mech. 2014;7:483–93. 10.1242/dmm.014530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark AM, Garland KK, Russell LD. Desert hedgehog (Dhh) gene is required in the mouse testis for formation of adult-type Leydig cells and normal development of peritubular cells and seminiferous tubules. Biol Reprod. 2000;63:1825–38. [DOI] [PubMed] [Google Scholar]
- 10.Warne SA, Hiorns MP, Curry J, Mushtaq I. Understanding cloacal anomalies. Arch Dis Child. 2011;96:1072–6. 10.1136/adc.2009.175034 [DOI] [PubMed] [Google Scholar]
- 11.Lin C, Yin Y, Veith GM, Fisher AV, Long F, Ma L. Temporal and spatial dissection of Shh signaling in genital tubercle development. Development. 2009;136:3959–67. 10.1242/dev.039768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitgood MJ, Shen L, McMahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Curr Biol. 1996;6:298–304. [DOI] [PubMed] [Google Scholar]
- 13.Haraguchi R, Mo R, Hui CC, Motoyama J, Makino S, Shiroishi T, et al. Unique functions of Sonic hedgehog signaling during external genitalia development. Development. 2001;128;4241–50. [DOI] [PubMed] [Google Scholar]
- 14.Hui CC, Angers S. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–37. 10.1146/annurev-cellbio-092910-154048 [DOI] [PubMed] [Google Scholar]
- 15.Kimmel SG, Mo R, Hui CC, Kim PC. New mouse models of congenital anorectal malformations. J Pediatr Surg. 2000;35:227–30. [DOI] [PubMed] [Google Scholar]
- 16.Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 2001;159:765–7. 10.1016/S0002-9440(10)61747-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Böse J, Grotewold L, Rüther U. Pallister-Hall syndrome phenotype in mice mutant for Gli3. Hum Mol Genet. 2002;11:1129–35. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez EJ, Weiss DA, Yang JH, Menshenina J, Ferretti M, Cunha TJ, et al. New Insights on the Morphology of Adult Mouse Penis. Biol Reprod. 2011;85:1216–21. 10.1095/biolreprod.111.091504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyagawa S, Matsumaru D, Murashima A, Omori A, Satoh Y, Haraguchi R, et al. The role of sonic hedgehog-Gli2 pathway in the masculinization of external genitalia. Endocrinology. 2011;152:2894–903. 10.1210/en.2011-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrocco G, Vallasciani S, Fiocca G, Calisti A. Hypospadias surgery: a 10-year review. Pediatr Surg Int. 2004;20:200–3. 10.1007/s00383-004-1147-1 [DOI] [PubMed] [Google Scholar]
- 21.Kokorowski PJ, Routh JC, Graham DA, Nelson CP. Variations in timing of surgery among boys who underwent orchidopexy for cryptorchidism. Pediatrics. 2010;126:e576–82. 10.1542/peds.2010-0747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh S, Tsai MY, Xu Q, Mu XM, Lardy H, Huang KE, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99:13498–503. 10.1073/pnas.212474399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nef S, Parada LF. Cryptorchidism in mice mutant for Insl3. Nat Genet. 1999;22:295–9. 10.1038/10364 [DOI] [PubMed] [Google Scholar]
- 24.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–36. 10.1074/jbc.M609360200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng W, Yeung CK, Ng YK, Zhang JR, Hui CC, Kim PC. Sonic Hedgehog mediator Gli2 regulates bladder mesenchymal patterning. J Urol. 2008;180:1543–50. 10.1016/j.juro.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 26.Nitti VW. The prevalence of urinary incontinence. Rev Urol. 2001;3:S2–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Levitt MA, Peña A. Anorectal malformations. Orphanet J Rare Dis. 2007; 2:33 10.1186/1750-1172-2-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.