Abstract
Rhizoctonia solani represents an important plant pathogenic Basidiomycota species complex and the host of many different mycoviruses, as indicated by frequent detection of dsRNA elements in natural populations of the fungus. To date, eight different mycoviruses have been characterized in Rhizoctonia and some of them have been reported to modulate its virulence. DsRNA extracts of the avirulent R. solani isolate DC17 (AG2-2-IV) displayed a diverse pattern, indicating multiple infections with mycoviruses. Deep sequencing analysis of the dsRNA extract, converted to cDNA, revealed that this isolate harbors at least 17 different mycovirus species. Based on the alignment of the conserved RNA-dependent RNA-polymerase (RdRp) domain, this viral community included putative members of the families Narnaviridae, Endornaviridae, Partitiviridae and Megabirnaviridae as well as of the order Tymovirales. Furthermore, viruses, which could not be assigned to any existing family or order, but showed similarities to so far unassigned species like Sclerotinia sclerotiorum RNA virus L, Rhizoctonia solani dsRNA virus 1, Aspergillus foetidus slow virus 2 or Rhizoctonia fumigata virus 1, were identified. This is the first report of a fungal isolate infected by 17 different viral species and a valuable study case to explore the diversity of mycoviruses infecting R. solani.
Introduction
Rhizoctonia solani Kühn [teleomorph: Thanatephorus cucumeris (Frank) Donk] represents an important species complex of known plant pathogens, which infect a wide variety of plant species including many economically important crops [1]. Due to its soil-borne nature, facultative saprophytism and the wide host-range, this pathogen is difficult to control by conventional agronomic measures like fungicides, crop rotation or plant resistance [2]. Rhizoctonia is considered as ubiquitous in soil, but has limited abilities of long distance spread, because it does not produce asexual spores [1, 3]. To understand the epidemiology of diseases caused by Rhizoctonia, especially regarding the disease suppression observed in association with monoculture of host plants, the interaction of the fungus with its environment and, in particular, the microflora has been studied by many researchers [4]. Today, it is well established that Rhizoctonia is significantly affected by suppressive organisms, but the nature of this biocontrol is still a matter of debate [4]. The finding that hypovirulent isolates of Rhizoctonia can be successfully used for biocontrol purposes, raised also interest in the possible involvement of mycoviruses [5, 6].
Mycoviruses have been identified in all major fungal taxa and the number of newly described species has expanded enormously during the last years [7]. The increasing usage of deep sequencing approaches will further enhance this trend, leading to a better understanding of the diversity of mycoviruses and the interaction with their hosts. To date, thirteen viral families have been recognized by the International Committee for the Taxonomy of Viruses (ICTV) that harbor mycoviruses [8]. Most of the mycoviruses characterized possess an RNA genome consisting either of single-stranded or double-stranded RNA, which in some cases is segmented [7]. In general, mycoviruses are considered as endogenous viral elements without an extracellular phase. An exception from this assumption has been discovered recently by Yu and coworkers, who identified a novel mycovirus species, related to the ssDNA Geminiviridae, which is reported to be transmitted via spray application of purified virions and induces hypovirulence [9]. Nevertheless, in most cases mycoviral infections are cryptic [10, 11] and only little is known about the interaction of these pathogens with their hosts [12]. The advances in the field of mycovirology achieved during the last years indicate that these interactions are rather diverse, ranging from mutualism, e.g. improvement of the adaption abilities of the host towards abiotic stress [13], to severe impairment including irregular growth, diminished reproduction, lack of pigmentation and hypovirulence [14]. Using mycoviruses, which induce such impairments in their host, for the control of plant pathogens in agricultural systems is a tempting approach. Factors, like the homogenous environmental conditions and the low species diversity, which facilitate the development of plant diseases, might also facilitate the spread and development of mycoviral infections of the plant pathogens [15]. Successful examples, like the biocontrol of chestnut blight (Cryphonectria parasitica) caused by hypoviruses, raises hope to exploit also other mycoviruses as biocontrol agents [16]. Nevertheless, the first step towards a mycovirus based biocontrol system is the identification of suitable viral candidates. Numerous studies have been carried out indicating a high diversity among R. solani infecting mycoviruses [17–20]. However, only a small number of different species has been characterized so far, including species of the Partitiviridae, Endornaviridae, Narnaviridae and a few unclassified mycoviruses [21–28]. Deep sequencing analysis, especially of viral metagenomes, is currently the most efficient way to identify unknown viruses. On the one hand, it might allow the identification of promising biocontrol candidates and on the other hand, it can provide important information about the global ecology and evolution of mycoviruses [15, 29]. Until now, studies showed that 60 to 95% of the sequences derived from viral metagenome sequencing do not find homologous sequences in the NCBI database and mycoviruses are less well studied compared to animal or plant viruses [12]. This leads to significant difficulties in the characterization of novel mycoviruses from complex viromes. The RNA-dependent RNA polymerase (RdRp) gene is the only gene that is universally conserved among RNA viruses and represents the key for the identification of evolutionary relationships between the respective viruses [30–32]. The RdRp domain is typically smaller than 400 amino acids in length. Apart from seven conserved motifs, named G, F1-3, A, B, C, D, E, which are essential for its function and represent about 75% of the domain, the RdRp sequences are highly variable [33]. Considering the diversity of RNA viruses, the sequence similarity of the RdRp domain is too low for the construction of evolutionary trees of all viral families, as only a few amino acid residues like a conserved aspartic acid in motif A exist in all viruses [33, 34]. However, the analysis of the conserved RdRp domain allows the identification of evolutionary lineages, named superfamilies, which show a higher level of conservation within this group [33, 35]. Furthermore, phylogenetic analysis of the conserved RdRp domain within these superfamilies enables a classification of RNA viruses on family level. The identification of novel RdRp genes in viral genomes therefore represents an efficient way to estimate the viral diversity in the sample, making first taxonomic assignments possible and permits the fast identification of unknown viruses. This study describes the diversity of a fungal virome consisting of a high number of novel RNA mycoviruses, using the viral hallmark gene RdRp for classification.
Material & Methods
Fungal isolate and culturing
The fungal strain DC17, originally isolated from sugar beet, was derived from the IRS (Institute of Sugar Beet Research, Netherlands) and identified as anastomosis group 2-2IV by sequencing of the internal transcribed spacer region [36]. The isolate showed unusual light pigmentation, slow growth on PDA and hypovirulence in a damping-off assay with sugar beet (data not shown). The strain was stored at -80°C on PDA. For extraction of DNA, RNA and dsRNA, mycelium was grown in PDB at 24°C.
Extraction of dsRNA from fungal mycelium
The dsRNA was extracted using a modified version of the protocol published by Valverde and colleagues [37]. Mycelium (10.3 g) was homogenized in liquid nitrogen in the presence of 0.25 g of polyvinylpolypyrrolidone. The pulverized mycelium was suspended in 10 mL 2xSTE buffer (0.1 M Tris-HCl, pH 6.8, 2 mM EDTA, 0.2 M NaCl2) containing 0.25 g polyvinylpyrrolidone. Nucleic acids were extracted by addition of 2 mL beta-mercaptoethanol, 0.5 mL SDS (20%), 20 mL phenol/chloroform (1:1) and the suspension was incubated on a shaker for 30 min at RT, followed by centrifugation for 20 min at 5000 xg. The aqueous phase was supplemented with pure ethanol to a final concentration of 16%, mixed with 2 g CF-11 cellulose (Whatman) and incubated for another 20 min at RT. Contaminants were removed by three washing steps with STE16 (STE + 16% EtOH) followed by centrifugation for 2 min at 500 xg. The cellulose was resuspended in 20 mL STE16 and transferred into Econo-Columns (Biorad). After an additional washing step with 20 mL STE16, the dsRNA was eluted by the addition of 10 mL 2xSTE. To remove contaminating genomic DNA and RNA, the eluate was treated with T1-RNase (Roche) (1 U/mL) (30 min, 37°C) followed by the addition of 3.3% 1 M MgCl2 and a treatment with DNase I (Roche) (2 U/mL) for 30 minutes at 37°C each. Thereafter the extract was supplemented with EtOH to a final concentration of 16% and 3.3% 0.5 M EDTA was added. The eluate was mixed with 2 g cellulose and treated as described above. The dsRNA was eluted with 8 mL STE. For precipitation, 80% isopropanol was added. The mixture was incubated at -20°C over night and centrifuged for 30 min at 10 000 xg. After a washing step with 70% EtOH, the dsRNA was resolved in TE buffer and checked by gel electrophoresis for quantity and quality control.
cDNA synthesis and library preparation for high-throughput sequencing
The cDNA library was prepared following a modified version of the protocol of Froussard [38]. Four μL of dsRNA were mixed with 100 pmol universal Primer-dN6 (GCCGGAGCTCTGCAGAATTCNNNNNN), incubated for 2 min at 99°C and chilled on ice. An enzyme mix was prepared using 6.25 mM dNTPs, 50 U RevertAid H-Minus Reverse transcriptase (Thermo Scientific) and 5 μL RT buffer in a volume of 20 μL and added to the dsRNA for an incubation of 30 min at 42°C. The cDNA was purified using the NucleoSpin, PCR clean-up, gel extraction kit (Macherey-Nagel) following the manufacturer’s instructions. The purified cDNA (25 μL) was mixed with 100 pmol of Primer-dN6, incubated for 2 min at 99°C and chilled on ice. For second strand synthesis, a mixture containing 3.125 mM dNTPs, 5 U Klenow-fragment (Thermo Scientific) and 5 μL of 10x Klenow-buffer in a total volume of 24 μL was added and incubated at 37°C for 30 min. The generated dscDNA was again purified and 5 μL were used for amplification. The PCR reaction mixture contained 125 nmol MgCl2, 0.15 pmol Primer (GCCGGAGCTCTGCAGAATTC), 12.5 nmol dNTPs, 1 U Dreamtaq (Thermo Scientific) and 5 μL 10x Dreamtaq buffer in a total volume of 50 μL. PCR conditions were as follows: Initial denaturation at 94°C for 1 min, followed by 30 cycles of 20 sec 94°C, 30 seconds 55°C, 2 min 72°C and a final elongation step of 10 min at 72°C. PCR products were analyzed by gel electrophoresis and fragments between 0.5 and 2 kb were excised and extracted from the gel using the NucleoSpin, PCR clean-up, gel extraction kit. Fragments were sheared and adaptors for Illumina sequencing were ligated [39].
High-throughput sequencing on the Illumina MiSeq system and sequence analysis
DNA quality was assessed by gel-electrophoresis and the quantity was estimated by a fluorescence-based method using the Quant-iT PicoGreen dsDNA kit (Invitrogen) and the Tecan Infinite 200 Microplate Reader (Tecan Deutschland GmbH). The R. solani DC17 virome was established on the Illumina MiSeq system by a pair-end sequencing run (2 × 300 bp) with a distance range of about 500 bp. A de novo assembly was performed using the gsAssembler software (version 2.8.) with default settings. Resulting virus sequences were in silico finished [40], imported into the automatic annotation platform GenDB [41] and refined manually. Raw data was deposited the European Nucleotide Archive (ENA) under the accession number PRJEB15275. In addition, all generated contigs were checked for open reading frames using the ORF finder software at the NCBI website (http://www.ncbi.nlm.nih.gov/gorf/gorf.html). Predicted amino acid sequences were subsequently compared to the NCBI database using BLASTP with standard settings and conserved domains were identified using the conserved domain database at the NCBI website (http://www.ncbi.nlm.nih.gov/cdd).
Completion of partial RdRp domains
To complete partial RdRp domains derived from smaller contigs, cDNA was synthesized using a sequence specific primer based on known sequence information targeting the whole RdRp domain (S1 Table). cDNA synthesis followed by dscDNA synthesis was performed as described above. After PCR amplification the resulting fragments were gel purified, fragments between 800 und 2000 bp were excised and extracted. The fragments were cloned using the pJet1.2 cloning Kit (Fermentas) following the manufacturer’s instructions. The resulting clones were sequenced with specific primers by standard Sanger-sequencing (Seqlab).
Confirmation of sequence assembly results
Specific primers were designed according to the results of the Illumina sequencing, flanking the region of the conserved RdRp domains. Primer sequences are listed in S1 Table. For cDNA synthesis total RNA was extracted using the RNeasy Plant Mini kit (Qiagen) according to the manufacturer’s instructions. cDNA synthesis and PCR amplification were done as described above using 2 μL cDNA. PCR products were gel purified and sequenced.
Identification of conserved sequence motifs and phylogenetic analysis
Nucleotide sequences, which were confirmed by resequencing with specific primers, were translated into amino acid sequences and aligned to reference genomes from the NCBI database. Conserved motifs were identified by comparisons with well characterized RdRps based on work published by Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 42–44]. For phylogenetic analysis, the RdRp domains, spanning the identified conserved motifs, were aligned in MEGA 6.06 using MUSCLE with standard settings and Neighbor-joining trees were constructed with 1000 bootstrap replicates [45]. Sequences spanning the conserved motifs A to G were deposited in the NCBI GenBank database under the accession numbers KX291005 and KX349056—KX349071.
Results and Discussion
Virome sequencing of R. solani isolate DC17
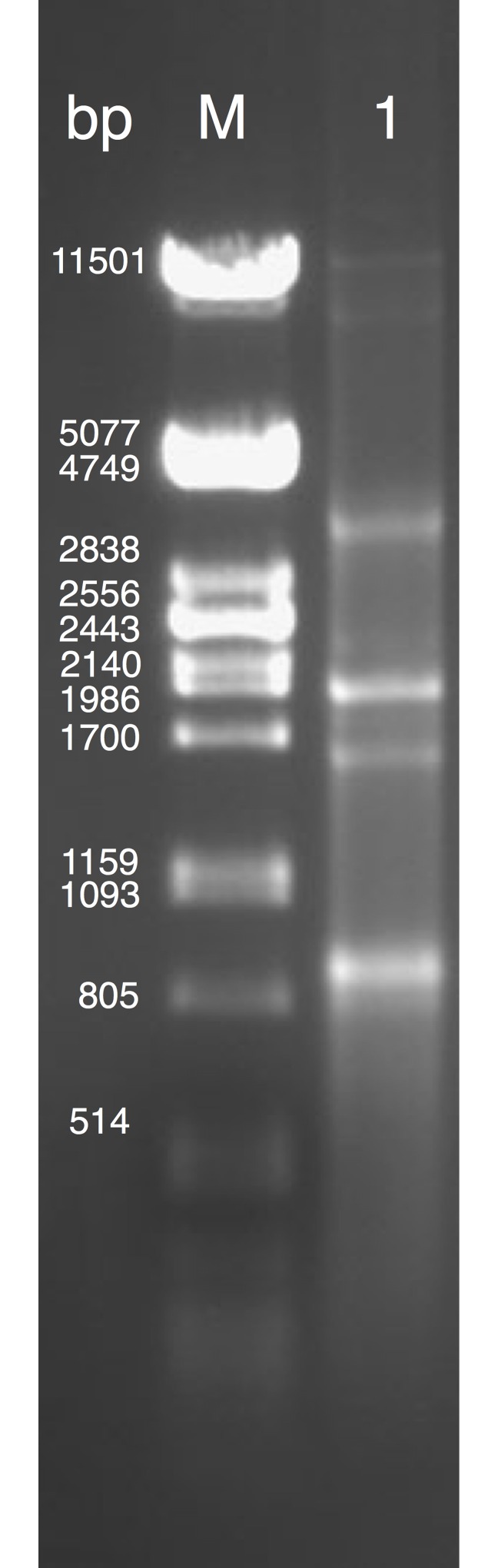
The dsRNA extract of the R. solani isolate DC17 showed a diverse pattern of dsRNA fragments after gel electrophoresis indicating the presence of multiple mycoviruses. At least six dsRNA fragments with a mobility corresponding to the sizes of approximately 0.85 to 12 kb were identified (Fig 1).
Fig 1. Purified dsRNA extracted from R. solani DC17.
Electrophoresis of dsRNA extract from DC17 (1) in a 1% agarose gel stained with ethidium bromide showing several dsRNA fragments ranging from approx. 0.85 to 12 kb in size compared to a size marker of a PstI digested lambda DNA (M).
Sequencing on the Illumina MiSeq System resulted in a total of 930,558 paired-end reads yielding 279 Mb sequence information. After quality analysis, 0.1% of these reads were excluded from further analysis. Based on at least 90% sequence identity over a minimum of 40 bases, reads were assembled into 144 contigs with sizes between 12.8 and 0.4 kb. In total, 40.2% of the contigs were classified as viral sequences based on a BLAST search corresponding to about 84% of the used reads. The average read coverage of the contigs was approx. 270-fold, but great differences between contigs were observed (~15x–4500x). Based on the assembled virome data, it was possible to identify 17 contigs encoding different viral RdRp domains. For detailed analysis, these RdRp domains were re-sequenced based on whole RNA extracts and partial domains were completed. These data were used for taxonomic classification. The identified RdRps belong to four different protein families (Pfam) corresponding to either the alphavirus-like superfamily, the picornavirus-like super family or the Narnaviridae. They showed similarities from 31 to 93% to RdRp domains of viral sequences deposited in the NCBI database.
Putative members of the family Narnaviridae identified in R. solani DC17
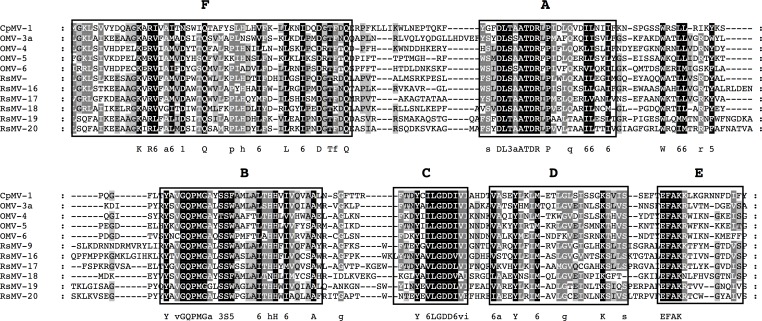
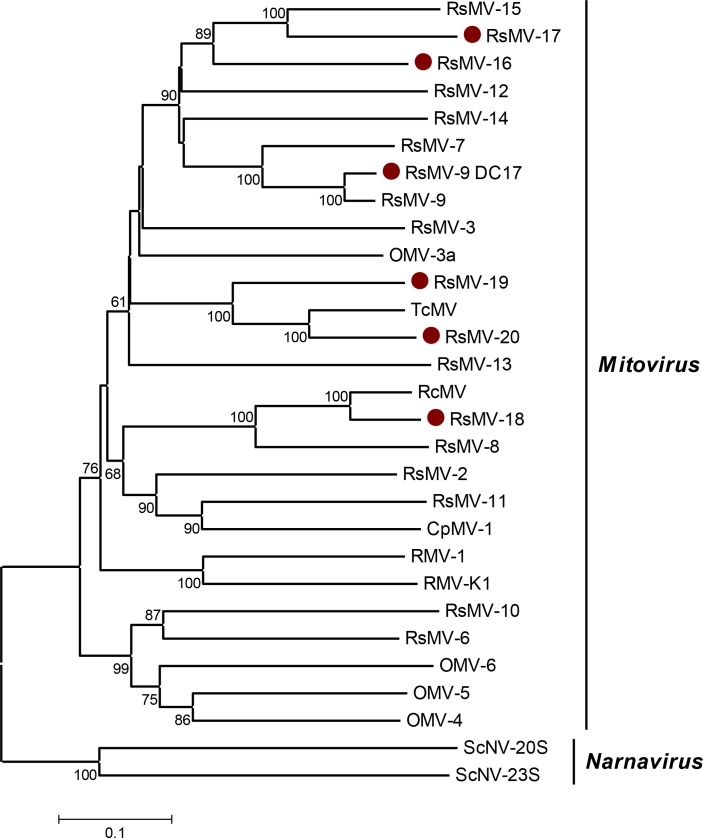
Viruses of the family Narnaviridae are the simplest of all RNA viruses consisting only of one small single-stranded RNA of 2.3 to 3.6 kb, which codes for a single RdRp gene [46]. The family is divided into the two genera Narnavirus and Mitovirus. The members of the Narnaviridae are only distantly related to other RNA viruses infecting eukaryotes and share higher sequence similarity to bacteriophages of the family Leviviridae [47]. The analysis of the conserved RdRp domains revealed that the R. solani isolate DC17 is the host of six different mitoviruses. Sequence comparisons with all recognized members of the genus Mitovirus allowed the identification of the four conserved motifs I to IV previously described for mitoviruses by Kitahara and coworkers [48]. Multiple alignments identified these motifs as motif F to C and, additionally, motif E and D were detected in this study (Fig 2). So far, 18 different mitoviruses have been characterized in the genus Rhizoctonia, the majority of them in R. solani [49, 50]. Analysis of the conserved RdRp domain indicated that five of the mitoviruses found in this study, represent novel species, as they display only limited sequence similarity to the mitoviruses described so far (Fig 3). These mitoviruses were named Rhizoctonia solani mitovirus 16 to 20 (RsMV-16 to 20). The RdRp domain of one mitovirus identified in the analyzed virome shows high sequence similarity of 92% to Rhizoctonia solani mitovirus 9 (KP900918.1). Since only a partial sequence of this virus has been deposited in the NCBI database, no comparison of the complete RdRp protein was possible, to check whether both viruses fulfill the species demarcation criterion of less than 90% amino acid sequence similarity over the complete RdRp protein [8]. This mitovirus was presumably considered as Rhizoctonia solani mitovirus 9 DC17.
Fig 2. Identification of the conserved motifs A to F in the RdRp domain of the genus Mitovirus.
The RdRp domains of putative mitoviruses identified in R. solani DC17 and recognized members of the genus mitovirus [8] were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [47]. Conserved motifs A-F were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [32, 36, 44–46]. CpMV-1, Cryphonectria parasitica mitovirus (NP_660174.1); OMV-3a, Ophiostoma mitovirus 3a (CAJ32468.1); OMV-4, Ophiostoma mitovirus 4 (NP_660179.1); OMV-5, Ophiostoma mitovirus 5 (NP_660180.1); OMV-6, Ophiostoma mitovirus 6 (NP_660181.1), RsMV-9, Rhizoctonia solani mitovirus 9 DC17 (KX349058); RsMV-16, Rhizoctonia solani mitovirus 16 (KX349057); RsMV-17, Rhizoctonia solani mitovirus 17 (KX349059); RsMV-18, Rhizoctonia solani mitovirus 18 (KX349060); RsMV-19, Rhizoctonia solani mitovirus 19 (KX349056); RsMV-20, Rhizoctonia solani mitovirus 20 (KX291005). Shading indicates level of conservation and the consensus sequence is displayed.
Fig 3. Phylogenetic analysis of the members of the family Narnaviridae.
An unrooted phylogenetic tree of the Narnaviridae was calculated based on the alignment of the RdRp domain spanning the conserved motifs A to F using the neighbor-joining method with 1000 bootstrap replicates. The scale represents a genetic distance of 0.1 amino acid substitutions per site. Bootstrap values < 50% are not shown. Analyzed species include mitoviruses identified in Rhizoctonia as well as species of the family Narnaviridae recognized by the ICTV. Species identified in this study are marked with dots. Analysis was conducted using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. CpMV-1, Cryphonectria parasitica mitovirus (NP_660174.1); OMV-3a, Ophiostoma mitovirus 3a (CAJ32468.1); OMV-4, Ophiostoma mitovirus 4 (NP_660179.1); OMV-5, Ophiostoma mitovirus 5 (NP_660180.1); OMV-6, Ophiostoma mitovirus 6 (NP_660181.1); ScNV-20S, Saccharomyces 20S RNA narnavirus (NP_660178.1); ScNV-23S, Saccharomyces 23S RNA narnavirus (NP_660177.1); RsMV-1, Rhizoctonia solani mitovirus 1 (ALD89121.1); TcMV-1, Thanatephorus cucumeris mitovirus 1 (AAD17381.1); RsMV-2, Rhizoctonia solani mitovirus 2 (ALD89121.1); RsMV-3, Rhizoctonia solani mitovirus 3 (ALD89122.1); RsMV-4, Rhizoctonia solani mitovirus 4 (ALD89123.1); RsMV-5, Rhizoctonia solani mitovirus 5 (ALD89124.1); RsMV-6, Rhizoctonia solani mitovirus 6 (ALD89125.1); RsMV-7, Rhizoctonia solani mitovirus 7 (ALD89126.1); RsMV-8, Rhizoctonia solani mitovirus 8 (ALD89127.1); RsMV-9, Rhizoctonia solani mitovirus 9 (ALD89128.1); RsMV-10, Rhizoctonia solani mitovirus 10 (ALD89102.1); RsMV-11, Rhizoctonia solani mitovirus 11 (ALD89116.1); RsMV-12, Rhizoctonia solani mitovirus 12 (ALD89117.1); RsMV-13, Rhizoctonia solani mitovirus 13 (ALD89118.1); RsMV-14, Rhizoctonia solani mitovirus 14 (ALD89119.1); RsMV-15, Rhizoctonia solani mitovirus 15 (ALD89120.1); RsMV-K1, Rhizoctonia solani mitovirus K1 (ALD60243.1); RcMV, Rhizoctonia cerealis mitovirus (AIT71973.1); RsMV-9, Rhizoctonia solani mitovirus 9 DC17 (KX349058); RsMV-16, Rhizoctonia solani mitovirus 16 (KX349057); RsMV-17, Rhizoctonia solani mitovirus 17 (KX349059); RsMV-18, Rhizoctonia solani mitovirus 18 (KX349060); RsMV-19, Rhizoctonia solani mitovirus 19 (KX349056); RsMV-20, Rhizoctonia solani mitovirus 20 (KX349062).
Putative member of the family Endornaviridae identified in R. solani DC17
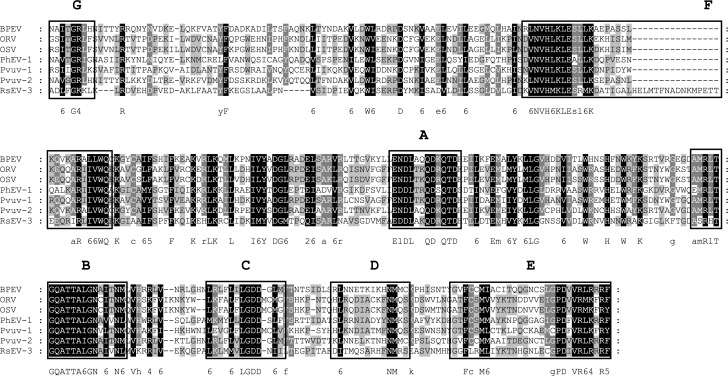
Viruses belonging to the family Endornaviridae are found in algae, plants and fungi [8]. They possess a non-segmented genome of 14 to 17 kb which codes for a single large polyprotein [7]. The polyprotein always carries a conserved RdRp domain (pfam00978), specifying its affiliation to the alphavirus-like superfamily. Other typical conserved replicase motifs like a methyltransferase, helicase or glycosyltransferase are missing in some species [51]. The CDD search of the deduced gene products derived from the deep sequencing analysis of dsRNA revealed the presence of one conserved RdRp domain belonging to the pfam00978 family. It is 45% identical to the conserved RdRp domain of Soybean-associated endornavirus 1, which is the species in the NCBI database showing the highest level of similarity [52]. Seven conserved motifs (A-G), typical for members of the family Endornaviridae were identified by sequence alignments with all recognized members of this family (Fig 4). This indicates the presence of a novel endornavirus, named Rhizoctonia solani endornavirus 3 (RsEV-3).
Fig 4. Identification of the conserved motifs A to G in the RdRp domain of the family Endornaviridae.
The RdRp domains of the recognized members of the family Endornaviridae [8] and Rhizoctonia solani endornavirus 3 (RsEV-3) (KX349065) were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. Conserved motifs A-G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [32, 36, 44–46]. BPEV, Bell pepper endornavirus (AKP92841.1); ORV, Oryza rufipogon endornavirus (YP_438202.1); OSV, Oryza sativa endornavirus (YP_438200.1); PhEV-1, Phytophthora endornavirus 1 (YP_241110.1); Pvuv-1, Phaseolus vulgaris endornavirus 1 (YP_009011062.1); Pvuv-1, Phaseolus vulgaris endornavirus 2 (ALJ56098.1). Shading indicates level of conservation and the consensus sequence is displayed.
This finding was confirmed by a phylogenetic analysis of the conserved RdRp domain within the alphavirus-like superfamily which placed this novel virus within the family Endornaviridae. So far, three mycoviruses belonging to the family of the Endornaviridae were described to infect the genus Rhizoctonia, namely Rhizoctonia solani endornavirus—RS002 (RsEV-1), Rhizoctonia solani endornavirus 2 (RsEV-2) and Rhizoctonia cerealis endornavirus 1 (RcEV-1) [28,50, 53]. Sequence comparisons showed that RsEV-3 and RsEV-1 possess 44.2% amino acid homology across the conserved RdRp domain. Comparison of the conserved motifs revealed that the RdRp domain of RsEV1 is incomplete, as the motifs C to E were missing. Sequence comparison of the remaining RdRp domain shows a homology of 52.5% to RsEV-1. Recently, the genome of RsEV-2 was deposited in the NCBI database [50]. Both viruses share 63.2% sequence identity within the RDRP domain. Due to the high conservation within this region, similarities between different members of the Endornaviridae can also be high, like it is the case for the Bell pepper endornavirus and Phaseoulus vulgaris endornavirus, which share a sequence homology of 74% within their RdRp domains.
Putative members of the order Tymovirales identified in R. solani DC17
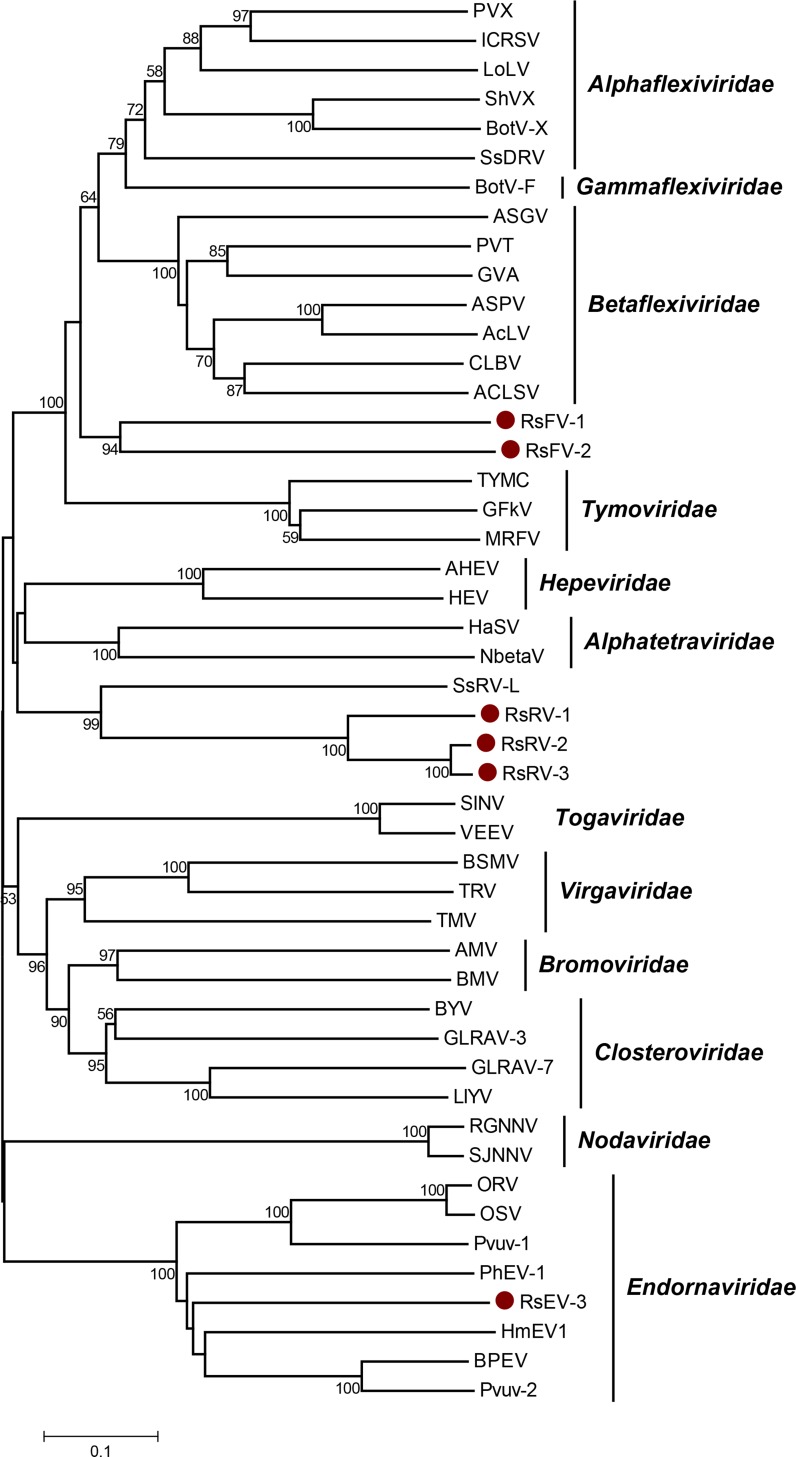
The order Tymovirales comprises four viral families. Viruses belonging to the Alphaflexiviridae, Betaflexiviridae and Gammaflexiviridae feature a filamentous morphology, whereas members of the Tymoviridae are icosahedral. So far, three mycoviruses belonging to the order Tymovirales have been recognized by the ICTV; all of them represent own genera [8]. Whereas Botrytis virus X (Botrexvirus) and Sclerotinia sclerotiorum debilitation-associated RNA virus (Sclerodarnavirus) belong to the Alphaflexiviridae, Botrytis virus F represents the only member of the family Gammaflexiviridae [54–56]. The search for conserved RdRp domains with the deduced gene products of the virome of R. solani DC17 revealed the presence of two domains assigned to pfam00978, which were distantly related to each other and to several members of the order Tymovirales. Sequence comparisons with the type species of all genera within the order allowed the identification of the seven conserved motifs A to G (Fig 5). A phylogenetic analysis of this region including all families of the alphavirus-like super family, placed both viruses within the order Tymovirales (Fig 6). They form a distinct clade, which indicates that they may represent members of a so far uncharacterized family within this order. The novel viruses were named Rhizoctonia solani flexivirus 1 (RsFV-1) and Rhizoctonia solani flexivirus 2 (RSFV-2).
Fig 5. Identification of the conserved motifs A to G in the RdRp domain of the type species of all genera within the order Tymovirales.
The RdRp domains of Rhizoctonia solani flexivirus 1 (RsFV-1) (KX349064), Rhizoctonia solani flexivirus 2 (RSFV-2) (KX349069) and the type species of all genera within the Tymovirales [8] were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [44]. Conserved motifs A to G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 34, 42–44]. ACLSV, Apple chlorotic leaf spot virus (ABY71563.1); AcLV, Aconitum latent virus (NP_116487.1); ASGV, Apple stem grooving virus (BAA98054.1); ASPV, Apple stem pitting virus (AEP02955.1); BotV-F, Botrytis virus F (NP_068549.1); BotV-X, Botrytis virus X (NP_932306.1); CLBV, Citrus leaf blotch virus (NP_624333.1); GFkV, Grapevine fleck virus (NP_542612.1); GVA, Grapevine virus A (AAO17778.1); ICRSV, Indian citrus ringspot virus (NP_203553.1); LoLV, Lolium latent virus (YP_001718499.1); MRFV, Maize rayado fino virus (NP_115454.1); PVT, Potato virus T (BAM16482.1); PVX, Potato virus X (AAF89747.1); SsDRV, Sclerotinia sclerotiorum debilitation-associated RNA virus (YP_325662.1); ShVX, Shallot virus X (NP_620648.1). Shading indicates level of conservation and the consensus sequence is displayed.
Fig 6. Phylogenetic analysis of selected type species within the families of the Alphavirus-like superfamily.
An unrooted phylogenetic tree of the alphavirus-like superfamily was calculated based on the alignment of the RdRp domain spanning the conserved motifs A to G using the neighbor-joining method with 1000 bootstrap replicates. The scale represents a genetic distance of 0.1 amino acid substitutions per site. Bootstrap values < 50% are not shown. Newly identified species are marked with dots and compared to their next relatives and selected type species within families proposed to form the alphavirus-like superfamily [8, 29]. Analysis was conducted using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. BPEV, Bell pepper endornavirus (AKP92841.1); ORV, Oryza rufipogon endornavirus (YP_438202.1); OSV, Oryza sativa endornavirus (YP_438200.1); PhEV-1, Phytophthora endornavirus 1 (YP_241110.1); Pvuv-1, Phaseolus vulgaris endornavirus 1 (YP_009011062.1); Pvuv-1, Phaseolus vulgaris endornavirus 2 (ALJ56098.1); SsRV-L, Sclerotinia sclerotiorum RNA virus L (ACE88957.1); ACLSV, Apple chlorotic leaf spot virus (ABY71563.1); AcLV, Aconitum latent virus (NP_116487.1); ASGV, Apple stem grooving virus (BAA98054.1); ASPV, Apple stem pitting virus (AEP02955.1); BotV-F, Botrytis virus F (NP_068549.1); BotV-X, Botrytis virus X (NP_932306.1); CLBV, Citrus leaf blotch virus (NP_624333.1); GFkV, Grapevine fleck virus (NP_542612.1); GVA, Grapevine virus A (AAO17778.1); ICRSV, Indian citrus ringspot virus (NP_203553.1); LoLV, Lolium latent virus (YP_001718499.1); MRFV, Maize rayado fino virus (NP_115454.1); PVT, Potato virus T (BAM16482.1); PVX, Potato virus X (AAF89747.1); SsDRV, Sclerotinia sclerotiorum debilitation-associated RNA virus (YP_325662.1); ShVX, Shallot virus X (NP_620648.1); BMV, Brome mosaic virus (NP_041197.1); AMV, Alfalfa mosaic virus (YP_053235.1); GLRAV-3, Grapevine leafroll-associated virus 3 (NP_813795.3); BYV, Beet yellows virus (NP_733949.1); LIYV, Lettuce infectious yellows virus (Q83045.2); GLRAV-7, Grapevine leafroll-associated virus 7 (YP_004935919.1); HCV, Hepatitis C virus (ALB38672.1); BVDV-1, Bovine viral diarrhea virus 1 (BAC55961.1); JEV, Japanese encephalitis virus (AEV57608.1); HEV, Hepatitis E virus (ALM55661.1); AHEV, Avian hepatitis E virus (AAL13366.1); SJNNV, Striped Jack nervous necrosis virus (NP_599247.1); RGNNV, Redspotted grouper nervous necrosis virus (ACV07658.1); SINV, Sindbis virus (AGT56188.1); VEEV, Venezuelan equine encephalitis virus (AAM28636.1); TBSV, Tomato bushy stunt virus (ACT67403.1); TNV-A, Tobacco necrosis virus A (ADE10194.1); TMV, Tobacco mosaic virus (AIL54434.1); TRV, Tobacco rattle virus (AHG52750.1); BSMV, Barley stripe mosaic virus (NP_604481.1); RsEV-3, Rhizoctonia solani endornavirus 3 (KX349065); RsFV-1, Rhizoctonia solani flexivirus 1 (KX349064); RsFV-2, Rhizoctonia solani flexivirus 2 (KX349069), RsRV-1, Rhizoctonia solani RNA virus 1 (KX349068); RsRV-2, Rhizoctonia solani RNA virus 2 (KX349067); RsRV-3, Rhizoctonia solani RNA virus 3 (KX349066).
Sclerotinia sclerotiorum RNA virus L -like viruses in R. solani DC17
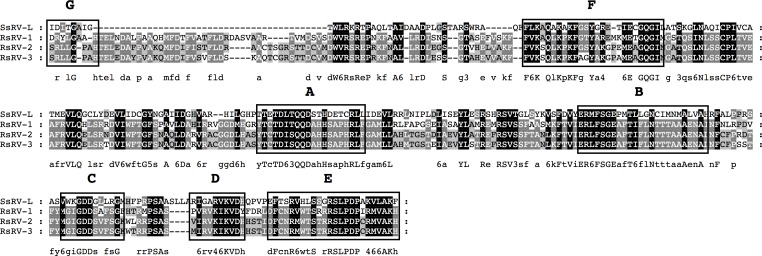
The search for conserved RdRp domains in the R. solani DC17 virome also revealed the presence of three domains, representing the pfam00978 group that were closely rated to each other, but showed no clear affiliation to any of the recognized families of the alphavirus-like superfamily. Instead, they display similarities to the so far unassigned mycovirus Sclerotinia sclerotiorum RNA virus L (SsRV-L), as indicated by BLAST analysis. SsRV-L is a positive-strand RNA virus of 6,043 nts, which carries one large ORF of 1965 aa featuring conserved domains for a methyltransferase, helicase and RdRp [57]. Multiple alignments with other conserved RdRp domains of pfam00978, allowed the identification of the seven conserved motifs A to G (Fig 7). A phylogenetic analysis of this region including different type species from families of the alphavirus-like superfamily showed that the three viruses found in R. solani DC17 form a separate clade together with SsRV-L. This group shows a distant relationship to the families of the Hepeviridae and Alphatetraviridae (Fig 6). Two of the three RdRp domains within this group share a very high sequence similarity of 93% (Table 1), indicating that they possibly belong to the same species. However, comparison of the putative replicase protein spanning the conserved motifs of the methyltransferase, helicase and RdRp reduced this similarity to 77.6% on the amino acid level and to 68.8% on the nucleotide level. Appling the species demarcation criteria of the next suitable relative, the Tymovirales (sequence homology of the RdRp gene < 80% on amino acid or < 72% on nucleotide level) [8], the two RdRp domains can be considered as belonging to different viral species. For this reason, the identified viruses were named Rhizoctonia solani RNA virus 1 (RsRV-1), Rhizoctonia solani RNA virus 2 (RsRV-2), Rhizoctonia solani RNA virus 3 (RsRV-3).
Fig 7. Identification of the conserved motifs A to G in the RdRp domain of a novel group of mycoviruses related to SsRV-L.
The RdRp domains of the three newly identified mycoviruses Rhizoctonia solani RNA virus 1 to 3 (RsRV-1, -2, -3) and Sclerotinia sclerotiorum RNA virus L (SsRV-L) (ACE88957.1) were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. Conserved motifs A to G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 42–44]. Shading indicates level of conservation and the consensus sequence is displayed.
Table 1. Similarity comparison between Rhizoctonia solani RNA virus 1 (RsRV-1), Rhizoctonia solani RNA virus 2 (RsRV-2), Rhizoctonia solani RNA virus 3 (RsRV-3) and Sclerotinia sclerotiorum RNA virus L (SsRV-L) using an 810 bp fragment (nt) and the corresponding amino acid sequence (aa) of the viral RdRps spanning the conserved motifs A to G.
| Similarity on nt level \ Similarity on aa level [%] | SsRV-L | RsRV-1 | RsRV-2 | RsRV-3 |
|---|---|---|---|---|
| SsRV-L | 46.1 | 46.0 | 45.2 | |
| RsRV-1 | 34.8 | 78.9 | 63.4 | |
| RsRV-2 | 34.1 | 93.2 | 66.1 | |
| RsRV-3 | 33.0 | 70.8 | 71.1 |
Putative member of the family Partitiviridae identified in R. solani DC17
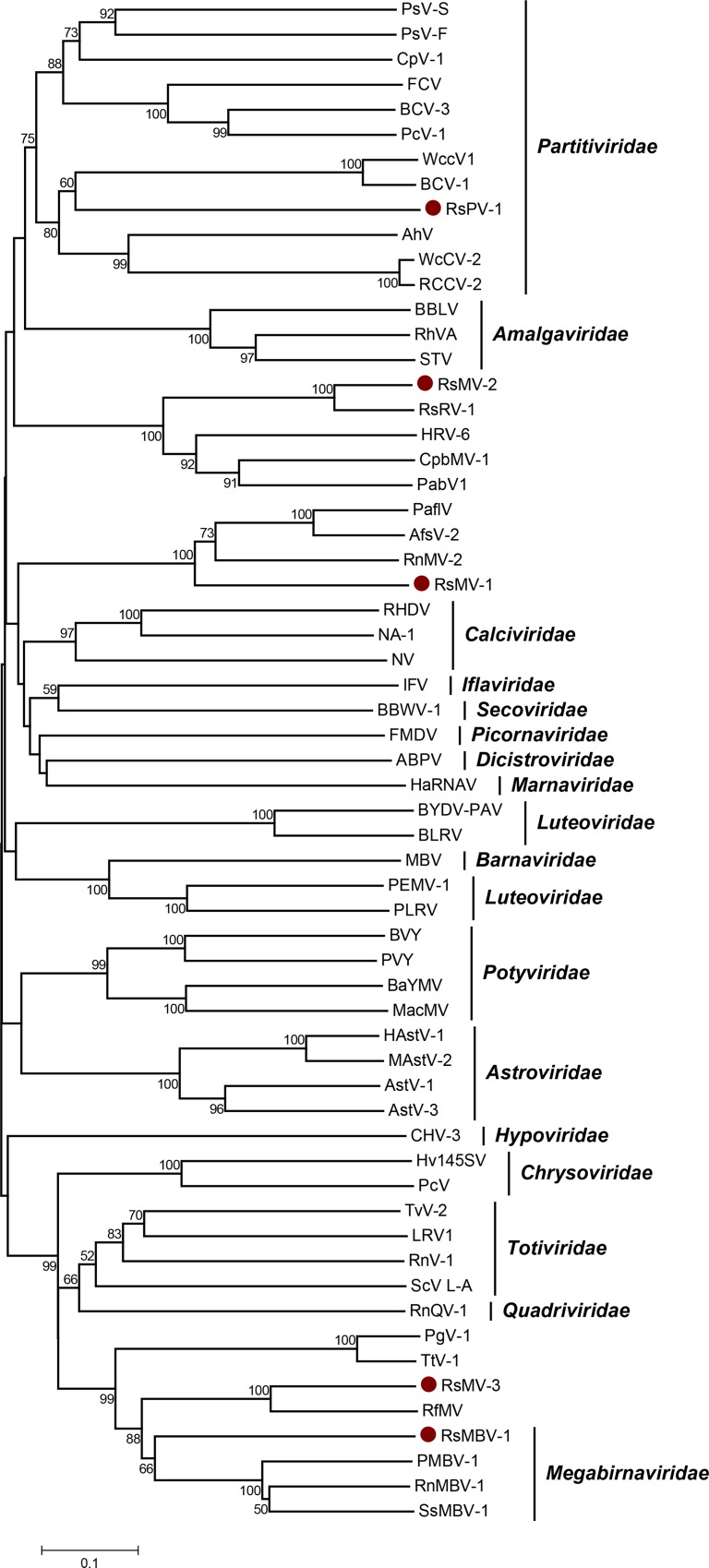
The virome analysis revealed the presence of one contig of 1958 nt, which codes for a putative protein of 633 aa. The protein contains a conserved RdRp domain showing the highest sequence similarity of 56% to the conserved RdRp domain of Rosellinia necatrix partitivirus 7. An alignment with several recognized members of the family Partitiviridae allowed the identification of the conserved domains A to F. It is reported that motif G is not well conserved within this viral family [30]. Nevertheless, an alignment with several other members of the picornavirus-like superfamily indicated the presence of a conserved glycine residue at the N-terminal region of the F motif which we considered as motif G (Fig 8). A phylogenetic analysis of the region spanning these motifs classified this virus as a member the genus Alphapartitivirus (Fig 9). A second contig of 1805 nt carried an ORF coding for a putative protein of 586 aa, which shows highest sequence similarity (24.7%) to the coat protein of Sclerotinia sclerotiorum partitivirus S. To date, three different members of the Partitiviridae have been characterized in the genus Rhizoctonia. Two of them, Rhizoctonia solani dsRNA virus 2 and Rhizoctonia solani virus 717, were isolated from R. solani. The putative partitivirus identified in this study, showed only low sequence similarity of less than 30% within the conserved region of the RdRp to these viruses. It is therefore most likely that this novel virus, referred to Rhizoctonia solani partitivirus 1 (RsPV 1), is a new member of the genus Alphapartitivirus.
Fig 8. Identification of the conserved motifs A to G in the RdRp domain of the family Partitiviridae.
The RdRp domains of the newly identified Rhizoctonia solani partitivirus 1 (RsPV-1) (KX349061) and recognized species within the Partitiviridae, including the type species, [8] were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. Conserved motifs A to G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 34, 42–44]. PsV-S, Penicillium stoloniferum virus S (YP_052856.2); PsV-F, Penicillium stoloniferum virus F (YP_271922.1); BCV-3, Beet cryptic virus 3 (AAB27624.1); PcV1, Pepper cryptic virus 1 (AEJ07890.1); FCV, Fig cryptic virus (YP_004429258.1); CpV-1, Cryptosporidium parvum virus 1 (BAU19336.1); WcCV1, White clover cryptic virus 1 (YP_086754.1); BCV-1, Beet cryptic virus 1 (YP_002308574.1); WcCV-2, White clover cryptic virus 2 (YP_007889821.1); AhV RCCV-2, Red clover cryptic virus 2 (YP_007889823.1); RsV-717, Rhizoctonia solani virus 717 (AJE29742.1). Shading indicates level of conservation and the consensus sequence is displayed.
Fig 9. Phylogenetic analysis of selected type species within the families of the picornavirus-like superfamily.
An unrooted phylogenetic tree of the picornavirus-like superfamily was calculated based on the alignment of the RdRp domain spanning the conserved motifs A to G using the neighbor-joining method with 1000 bootstrap replicates. The scale represents a genetic distance of 0.1 amino acid substitutions per site. Bootstrap values > 50% are not shown. Newly identified species are marked with dots and compared to their next relatives and selected type species within families proposed to form the picornavirus-like superfamily [8, 29]. Analysis was conducted using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. PMBV-1, Pleosporales megabirnavirus 1 (ALO50147.1); RnMBV-1, Rosellinia necatrix megabirnavirus 1/W779 (YP_003288763.1); SsMBV-1, Sclerotinia sclerotiorum megabirnavirus 1 (YP_009143529.1); TtV-1, Thelephora terrestris virus 1 (YP_009209482.1); RfV-1, Rhizoctonia fumigatavirus 1 (AJE29745.1); PgV-1, Phlebiopsis gigantea mycovirus dsRNA 1 (YP_003541123.1); GaRV-6, Gremmeniella abietina RNA virus 6 (AIU98624.1); HRV-6, Heterobasidion RNA virus 6 (AHA82547.1); FgV-4, Fusarium graminearum dsRNA mycovirus 4 (YP_003288790.1); PabV-1, Penicillium aurantiogriseum bipartite virus 1 (YP_009182335.1); CpbMV-1, Cryphonectria parasitica bipartite mycovirus 1 (YP_007985675.1); RsRV-1, Rhizoctonia solani dsRNA virus 1 (AFZ85210.1); AfsV-2, Aspergillus foetidus slow virus 2 (CCD33025.1), PaflV, Penicillium aurantiogriseum foetidus-like virus (YP_009182156.1); RnMV-2, Rosellinia necatrix mycovirus 2 (BAM36407.1); PsV-F, Penicillium stoloniferum virus F (YP_271922.1); BCV-3, Beet cryptic virus 3 (AAB27624.1); PcV1, Pepper cryptic virus 1 (AEJ07890.1); FCV, Fig cryptic virus (YP_004429258.1); CpV-1, Cryptosporidium parvum virus 1 (BAU19336.1); WcCV1, White clover cryptic virus 1 (YP_086754.1); BCV-1, Beet cryptic virus 1 (YP_002308574.1); WcCV-2, White clover cryptic virus 2 (YP_007889821.1); AhV RCCV-2, Red clover cryptic virus 2 (YP_007889823.1); RsV-717, Rhizoctonia solani virus 717 (AJE29742.1); BBLV, Blueberry latent virus (YP_003934623.1); RhVA, Rhododendron virus A (ADM36020.1); STV, Southern tomato virus (ALM54938.1); AstV-1, Avastrovirus 1 (CAB95006.3); AstV-3, Avastrovirus 3 (AAF60952.1); HAstV-1, Human astrovirus 1 (Q67726.1); MAstV-2, Mamastrovirus 2 (AII82242.1); MBV, Mushroom bacilliform virus (NP_042510.2); NAV-1, Newbury agent 1 (YP_529897.1); NV, Norwalk virus (AAC64602.1); RHDV, Rabbit hemorrhagic disease virus (AMQ25852.1); Hv145SV, Helminthosporium victoriae 145S virus (YP_052858.1); PcV, Penicillium chrysogenum virus (YP_392482.1); ABPV, Acute bee paralysis virus (Q9DSN9.1); CHV-3, Cryphonectria hypovirus 3 (NP_613266.1); IFV, Infectious flacherie virus (ADP24157.1); BYDV-PAV, Barley yellow dwarf virus-PAV (CAH18868.1); BLRV, Bean leafroll virus (ALX34940.1); PEMV-1, Pea enation mosaic virus 1 (P29154.2); PLRV, Potato leafroll virus (AHA43772.1); HaRNAV, Heterosigma akashiwo RNA virus (NP_944776.1); FMDV, Foot-and-mouth disease virus—type O (BAU20293.1); BaYMV, Barley yellow mosaic virus (BAG70349.1); BVY, Blackberry virus Y (YP_851006.1); MacMV, Maclura mosaic virus (AAB02823.1); PVY, Potato virus Y (BAN16607.1); RnQV1, Rosellinia necatrix quadrivirus 1 (BAM93353.1); BBWV-1, Broad bean wilt virus 1 (AAX12375.1); GLV, Giardia lamblia virus (NP_620070.1); LRV-1, Leishmania RNA virus 1 (AHJ90430.1); ScV-L-A, Saccharomyces cerevisiae virus L-A (NP_620495.1); TVV-2, Trichomonas vaginalis virus 2 (AKE98370.1), RsPV-1, Rhizoctonia solani partitivirus 1 (KX349061); RsMV-1, Rhizoctonia solani mycovirus 1 (KX349063); RsMV-2, Rhizoctonia solani mycovirus 2 (KX349062); RsMV-3, Rhizoctonia solani mycovirus 3 (KX349070); RsMBV-1, Rhizoctonia solani megabirnavirus 1 (KX349071).
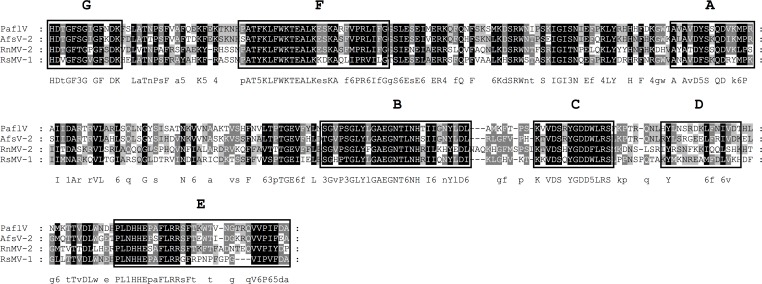
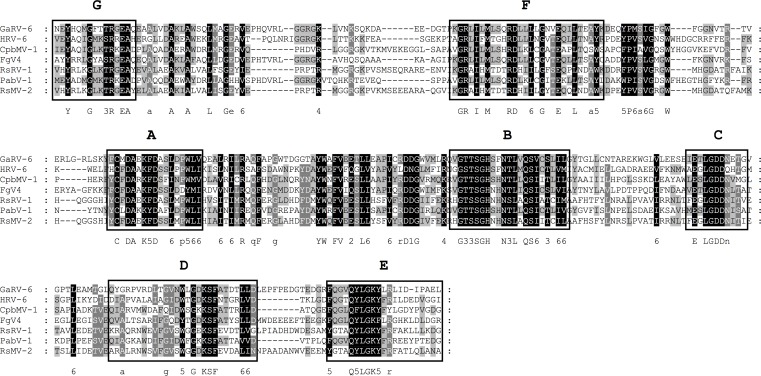
Aspergillus foetidus slow virus 2 -like virus in R. solani DC17
The search for conserved RdRp domains in the R. solani DC17 virome showed the presence of a domain belonging to the cd01699 group. Different members of the picornavirus-like superfamily, like the Claciviridae or the Partitiviridae, share this group of domain. A multiple alignment with different members of the picornavirus-like superfamily allowed the identification of the conserved motifs A to G (Fig 10). A BLAST analysis of the region revealed that the RdRp domain shows significant sequence similarities to three so far unclassified viruses. The closest relative with 54% sequence identity is Aspergillus foetidus slow virus 2 (AfsV-2). The virus present in R. solani DC17 was named Rhizoctonia solani mycovirus 1 (RsMV-1). The phylogenetic analysis grouped RsMV-1 with AfsV-2, Penicillium aurantiogriseum foetidus-like virus (PaflV) and Rosellinia necatrix mycovirus 2 (RnMV-2) into one clade, which is distantly related to the Calciviridae (Fig 9). The contig, carrying the RdRp domain of RsMV-1, is 4,130 nt in size and encodes putative protein is 1376 aa. The conserved RdRp domain is positioned within the N-terminal region of the protein. A similar organization is reported for RnMV-2, also referred to as yado-kari virus 1 [58], whereas AfsV-2 has a smaller genome and the conserved RdRp domain is positioned in the center of the protein [59]. Both, RnMV-2 and AfsV-2 are described to be unique viruses which hijack the capsid protein of an unrelated dsRNA virus [58, 59]. Whether this unusual behavior also holds true for RsMV-1 needs further investigation.
Fig 10. Identification of the conserved motifs A to G in the RdRp domain of Rhizoctonia solani mycovirus-1 (RsMV-1).
The RdRp domains of the newly identified Rhizoctonia solani mycovirus 1 (RsMV-1) (KX349063) and Aspergillus foetidus slow virus 2 (AfsV-2) (CCD33025.1), Penicillium aurantiogriseum foetidus-like virus (PaflV) (YP_009182156.1) and Rosellinia necatrix mycovirus 2 (RnMV-2) (BAM36407.1) were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. Conserved motifs A to G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 34, 42–43]. Shading indicates level of conservation and the consensus sequence is displayed.
Rhizoctonia solani dsRNA virus 1 -like virus in R. solani DC17
Rhizoctonia solani dsRNA virus 1 (RsRV-1) is an unclassified bi-segmented dsRNA virus, which was isolated from R. solani AG-1 IA [23]. Its genome structure is similar to those of the Partitiviridae and both share a RdRp of pfam00680 group. Nevertheless, a phylogenetic analysis indicates that it is only distantly related to the family Partitiviridae [23]. The BLAST analysis of the multiple RdRps, isolated from R. solani DC17 showed that one of the RdRps is very similar to the one of RsRV-1 carrying the same conserved motifs A to G (Fig 11). Although, both viruses share 81.6% identity within the conserved RdRp domain, the comparison of the whole replicase indicated a lower similarity level of 76.8%.
Fig 11. Identification of the conserved motifs A to G in the RdRp domain of Rhizoctonia solani mycovirus-2 (RsMV-2).
The RdRp domains of the newly identified Rhizoctonia solani mycovirus 2 (RsMV-2) (KX349062) and the unassigned mycoviruses Gremmeniella abietina RNA virus 6 (GaRV-6) (AIU98624.1); Heterobasidion RNA virus 6 (HRV-6) (AHA82547.1); Fusarium graminearum dsRNA mycovirus 4 (FgV-4) (YP_003288790.1); Penicillium aurantiogriseum bipartite virus 1 (PabV-1) (YP_009182335.1); Cryphonectria parasitica bipartite mycovirus 1 (CpbMV-1) (YP_007985675.1) and Rhizoctonia solani dsRNA virus 1 (RsRV-1) (AFZ85210.1) were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [46]. Conserved motifs A to G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 34, 42–44]. Shading indicates level of conservation and the consensus sequence is displayed.
Thus, this virus found in R. solani DC17, is novel and was named Rhizoctonia solani mycovirus 2 (RsMV-2). Both viruses are closely related, which was also confirmed by the phylogenetic analysis of the RdRp domain. Both viruses cluster together and form a distinct clade together with some so far unclassified viruses like Heterobasidion RNA virus 6 or Curvularia thermal tolerance virus (Fig 9).
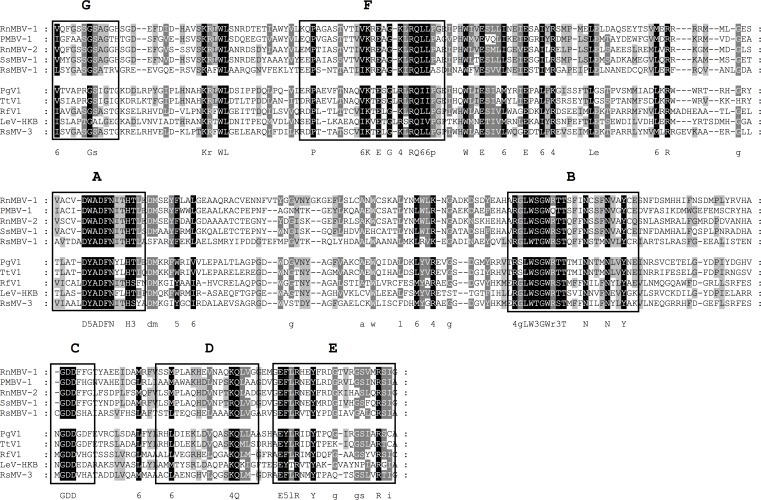
Putative members of the family Megabirnaviridae in R. solani DC17
Currently, Rosellinia necatrix megabirnavirus 1 (RnMBV-1) is the only recognized member of the family Megabirnaviridae. It possesses a bi-segmented double-stranded RNA genome and the segments are encapsidated separately into isometric particles. The first segment carries two ORFs, which code for an RdRp of the pfam02123 protein family and a coat protein. The second segment encodes two proteins of unknown function [60, 61]. In the virome of R. solani DC17, two conserved RdRp domains of the pfam02123 group were detected. One showed significant similarity of 68% to the RdRp of RnMBV-1, whereas the second RdRp domain displayed lower similarity of only 29%. Sequence comparisons with different members of the picornavirus-like superfamily allowed the identification of the conserved motifs A to G in both novel RdRps (Fig 12). A phylogenetic analysis of this region including different type species within the picornavirus-like superfamily as well as some so far uncharacterized viruses, grouped both RdRps into one clade with the families Totiviridae, Chrysoviridae, Quadriviridae and Megabirnaviridae (Fig 9). However, the first RdRp clusters with RnMBV-1 and some other proposed members of the family Megabirnaviridae. The second RdRp shows close a relationship to the recently characterized RdRp domain of the unclassified mycovirus Rhizoctonia fumigate virus 1 (RfV-1) [25]. RfV-1 is a one-segmented dsRNA virus. It is proposed to form a new genus together with Phlebiopsis gigantean large virus-1 (PgV-1) and Lentinula edodes mycovirus (HKB and HKA) [25]. The analysis indicates, that the virome of R. solani DC17 contains two mycoviruses, which are related to the family Megabirnaviridae. The first virus seems to be a member of the genus Megabirnavirus and was named Rhizoctonia solani megabirnavirus 1 (RsMBV-1). The second virus is closely related to a different group of so far unclassified viruses and was named Rhizoctonia solani mycovirus 3 (RsMV-3). To elucidate whether RsMV-3 belongs to a new viral family or a new proposed genus within the Megabirnaviridae [25], further criteria apart from the phylogeny of the RdRp domain need to be considered.
Fig 12. Identification of the conserved motifs A to G in the RdRp domain of Rhizoctonia solani megabirnavirus 1 (RsMBV-1) and Rhizoctonia solani mycovirus 3 (RsMV-3).
The RdRp domains of the newly identified Rhizoctonia solani megabirnavirus 1 (RsMBV-1) (KX349071), Rhizoctonia solani mycovirus 3 (RsMV-3) (KX349070) and proposed members of the family Megabirnaviridae were aligned using MEGA 6.06 and the sequence alignment algorithm MUSCLE [45]. Conserved motifs A to G were marked according to Bruenn 2003, Černý et al. 2014, Koonin et al. 1993, Boehr et al. 2014 and Xu et al. 2003 [30, 34, 42–44]. PMBV-1, Pleosporales megabirnavirus 1 (ALO50147.1); RnMBV-1, Rosellinia necatrix megabirnavirus 1/W779 (YP_003288763.1); RnMBV-2, Rosellinia necatrix megabirnavirus 2-W8 (YP_009227124.1); SsMBV-1, Sclerotinia sclerotiorum megabirnavirus 1 (YP_009143529.1); TtV-1, Thelephora terrestris virus 1 (YP_009209482.1); RfV-1, Rhizoctonia fumigata mycovirus (AJE29745.1); LeV-HKB, Lentinula edodes mycovirus HKB (BAG71788.2); PgV-1, Phlebiopsis gigantea mycovirus dsRNA 1 (YP_003541123.1). Shading indicates level of conservation and the consensus sequence is displayed.
Methodological approach and its implication for virus identification
Our results demonstrate that a deep sequencing approach is the method of choice for the analysis of diverse viromes and that the characterization of the conserved RdRp domain is the best way to get a valid overview about viral diversity in such complex RNA virus viromes. However, our study also showed the two main pitfalls of virus characterization in virome samples. In general, virome analysis faces the problem, that no marker gene for targeted amplification like the ribosomal internal transcribed spacer (ITS) is present in viruses. Instead, other methods for the enrichment of viral sequences are used, which all bring along certain disadvantages. The most popular method is the purification of virus particles, which seems natural, as the viruses used to be defined as capsid-coding organisms [47]. However, the identification of the capsidless hypoviruses and their acceptance by the ICTV changed this [46]. Further progress in the field of mycovirus research revealed that the absence of a coat protein is a common feature among mycoviruses making the approach of particle purification rather unsuitable for virome analysis in fungi. An alternative approach recently used for the characterization of mycoviruses, utilized siRNAs from infected fungi [62]. Even though they were able to detect mycoviruses belonging to the Narnaviridae (ssRNA, unencapsidated), as well as to the Partitiviridae (dsRNA, encapsidated), it is not sure if all mycoviruses are delectable by this method, as interference with RNA silencing seems to be an important trait in mycoviruses [63]. The sequencing of total RNA and DNA is a further popular method but viruses of low a titer might not be detected [63]. Especially in virome analysis of environmental samples, the first level assignment of reads belonging to novel viruses is another problem, when no separation between viral and host sequences has been applied [64]. To overcome the limitations described, we used an extraction of dsRNA, which is a replicative intermediate of all RNA viruses, as starting material for library preparation in order to maximize the number of reads derived from viruses. Still, the coverage between contigs differed enormously, most likely due to differences in the virus titer within the sample, as the whole dsRNA extract was used for analysis, compared to individual dsRNA band excision from the gel. Using equimolar ratios of purified dsRNA fragments would probably improve the uniformity in coverage, but may cause the problem of missing certain species, which, due to low virus titers, are not visualized by gel electrophoresis. However, the coverage can be used for an indication of the dominance of certain viral species in the virome, which is especially interesting, as hypovirulence is reported to be correlated to higher virus titers [65, 66]. Following this hypothesis, RsMV 17 could be of special interest, as its coverage was 17 times higher than the average. Still, some of the contigs representing novel species, like RsFV-1 and RsFV-2 displayed a very low coverage indicating that the contigs do not represent full-length genome components.
The second problem is the limited number of characterized mycoviral sequences, which can be used as reference for species assignment of the generated contigs. Apart from conserved regions, sequence similarities are too low to draw conclusions about the phylogeny of the virus [67]. The RdRp domain is the best available indicator to identify also novel viruses, sharing no other homologies with known viruses and represents the key for the reconstruction of evolutionary relationships between those viruses [29–31]. In general, virus taxonomy is a challenging field since high mutation rates and frequent exchange of whole gene modules lead to a very high sequence divergence, which makes reasonable alignments virtually impossible [67]. Nevertheless, the analysis the RdRp allows clustering above the order level in so-called superfamilies. The identification of the conserved motifs within the RdRp domain is an important point, since only the comparison of homologous sequences will lead to a valid taxonomic assignment [67]. We identified the conserved motifs A to G of the novel viruses, as well as in reference sequences derived from the NCBI database and used an alignment of this region for a first taxonomic classification.
This approach enabled us to get a first and valid impression of the viral diversity of the species inhabiting R. solani DC17 and allowed an identification of novel viruses which might be of special interest in the fields of biocontrol, virus biology and taxonomy. For example, the analysis revealed the presence of the novel mitovirus RsMV-20, which is closely related to Thanatephorus cucumeris mitovirus (TcMV). An infection of Rhizoctonia with TcMV resulted in decreased virulence of the host [21, 68]. Studies using this hypovirulent isolate of Rhizoctonia (AG-3), infected with TcMV, demonstrated its potential use as biocontrol agent [6]. This makes RsMV-20 an interesting candidate for further analysis in regard to hypovirulence induction. Also, a further characterization of RsMBV-1 is promising, since, so far, all described megabirnaviruses, apart from Pleosporales megabirnavirus 1, which was detected in the virome of marine fungi, were associated with hypovirulence of their hosts [61, 69–71]. As well, the identification of RsMV-1, which is closely related to AfV-S2 and RnMV-2 is an interesting finding. AfV-S2 and RnMV-2 are reported to feature a rather unusual lifestyle since they possess a one-segmented ssRNA genome, which hijacks the capsid protein (CP) of an unrelated dsRNA virus [57, 58]. The coat protein of RnMV-2 originates from Rosellinia necatrix mycovirus 1, which belongs to a group of novel viruses distantly related to the Totiviridae [57]. In case of AfV-S2, the capsid protein is derived from a member of the Victoriviruses (Totiviridae) [58]. The close relationship between those viruses and the newly identified virus RsMV-1 raises the hypothesis that also RsMV-1 might hijack a CP of another virus. As no totivirus or close relative is present in the virome of R. solani DC17, RsMV-1 would have to use the CP of another virus, like Rhizoctonia solani partitivirus-1 or Rhizoctonia solani megabirnavirus-1. Either way, a finding like this would be interesting in regard to virus interactions and evolution.
Still, for a full-value assignment into viral families and a biological characterization of the novel viruses, more criteria than the phylogeny of the RdRp have to be analyzed, especially when the viruses belong to novel viral groups. The next steps need to be the determination of full-length sequences and the isolation of single virus species to study their biological effects by e.g. purification and transfection of viral particles, the construction of infectious full-length cDNA-clones, or the elimination of certain virus by mycelial fragmentation, hyphal tipping or protoplastation [72–75].
Conclusion
This is the first study characterizing a complex virome from a hypovirulent isolate of R solani to explore the diversity of mycoviruses in this fungus. By the characterization of the conserved RdRp domains, we identified 17 different mycoviruses of which 16 domains represent novel species not characterized before. To our knowledge no comparable number of virus species belonging to at least eight different families, has been previously reported to infect a single fungal isolate. The research conducted here is a step forward in exploring the mycoviruses of R. solani. The enormous diversity identified in this study raises questions about the interactions of viruses as well as the dynamics of viral communities in fungi. More detailed analysis of the hypovirulent isolate DC17 and its inhabiting viruses might help to get insights into this still unexplored field of mycovirus research. Work, like the one presented here, will help to further populate public databases with viral sequences, which in turn will help to solve difficulties in virus identification and classification in the future [64].
Supporting Information
(DOCX)
Data Availability
All viral sequences used for analysis are available from the NCBI database; accession numbers are listed in the manuscript. Raw data is deposited at ENA under the Acc PRJEB15275.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Cubeta MA, Vilgalys R. Population biology of the Rhizoctonia solani complex. Phytopathology. 1997; 87: 480–484. 10.1094/PHYTO.1997.87.4.480 [DOI] [PubMed] [Google Scholar]
- 2.Okubara PA, Dickman MB, Blechl AE. Molecular and genetic aspects of controlling the soilborne necrotrophic pathogens Rhizoctonia and Pythium. Plant Sci. 2014; 228:61–70. 10.1016/j.plantsci.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 3.García VG, Onco MP, Susan VR. Review Biology and systematics of the form genus Rhizoctonia. Spanish J Agricult Res. 2006; 4:55–79. [Google Scholar]
- 4.Anees M, Edel-Hermann V, Steinberg C. Build up of patches caused by Rhizoctonia solani. Soil Biol Biochem. 2010; 42:1661–1672. [Google Scholar]
- 5.Sneh B, Ichielevich-Auster M, Plaut Z. Mechanism of seedling protection induced by a hypovirulent isolate of Rhizoctonia solani. Can J Bot.1989; 67:2135–2141. [Google Scholar]
- 6.Bandy BR, Tavantzis SM. Effect of hypovirulent Rhizoctonia solani on Rhizoctonia disease growth and development of potato plants. Am Potato J 1990; 67:189–199. [Google Scholar]
- 7.Ghabrial SA, Castón JR, Jiang D, Nibert ML, Suzuki N. 50-plus years of fungal viruses. Virology. 2015; 479:356–368. 10.1016/j.virol.2015.02.034 [DOI] [PubMed] [Google Scholar]
- 8.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus taxonomy: Ninth report of the International Committee on Taxonomy of Viruses Elsevier/Academic Press, London, United Kingdom; 2011. [Google Scholar]
- 9.Yu X, Li B, Fu Y, Xie J, Cheng J, Ghabrial SA, et al. Extracellular transmission of a DNA mycovirus and its use as a natural fungicide. Proc natl Acad Sci. 2013; 110:1452–1457. 10.1073/pnas.1213755110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nuss DL. Hypovirulence: Mycoviruses at the fungal–plant interface. Nat Rev Microbiol. 2005; 3:632–642. 10.1038/nrmicro1206 [DOI] [PubMed] [Google Scholar]
- 11.Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009; 10:115–128 10.1111/j.1364-3703.2008.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roossinck MJ. Metagenomics of plant and fungal viruses reveals an abundance of persistent lifestyles. Front Microbiol. 2015; 5:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. A virus in a fungus in a plant: Three-way symbiosis required for thermal tolerance. Science. 2007; 315:513–515. 10.1126/science.1136237 [DOI] [PubMed] [Google Scholar]
- 14.Son M, Yu J, Kim KH. Five questions about mycoviruses. PLoS Pathog. 2015; 11:e1005172 10.1371/journal.ppat.1005172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie J, Jiang D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu Rev Phytopathol. 2014; 52:45–68. 10.1146/annurev-phyto-102313-050222 [DOI] [PubMed] [Google Scholar]
- 16.Dawe AL, Nuss DL. Hypoviruses and chestnut blight: Exploiting viruses to understand and modulate fungal pathogenesis. Annu Rev Genet. 2001; 35:1–29. 10.1146/annurev.genet.35.102401.085929 [DOI] [PubMed] [Google Scholar]
- 17.Bharathan N, Tavantzis SM. Genetic diversity of double-stranded RNA from Rhizoctonia solani. Phytopathology. 1990; 80:631–635. [Google Scholar]
- 18.Bharathan N, Saso H, Gudipati L, Bharathan S, Whited K. Double‐stranded RNA: Distribution and analysis among isolates of Rhizoctonia solani AG‐2 to‐13. Plant Pathol. 2005; 54:196–203. [Google Scholar]
- 19.Kim SO. Chung HS, Lee YH. Double-stranded RNAs in Korean isolates of Rhizoctonia solani AG4. FEMS Microbiol Lett. 1996; 141:203–206. [DOI] [PubMed] [Google Scholar]
- 20.Zanzinger DH, Bandy BP, Tavantzis SM. High frequency of finding double-stranded RNA in naturally occurring isolates of Rhizoctonia solani. J Gen Virol. 1984; 65:1601–1605. [Google Scholar]
- 21.Jian J, Lakshman DK, Tavantzis SM. A virulence-associated 64-kb double-stranded RNA from Rhizoctonia solani is phylogenetically related to plant bromoviruses and electron transport enzymes. Mol Plant-Microbe Interact. 1998; 11:601–609. 10.1094/MPMI.1998.11.7.601 [DOI] [PubMed] [Google Scholar]
- 22.Strauss EE. Lakshman DK, Tavantzis SM. Molecular characterization of the genome of a partitivirus from the basidiomycete Rhizoctonia solani. J Gen Virol. 2000; 81:549–555. 10.1099/0022-1317-81-2-549 [DOI] [PubMed] [Google Scholar]
- 23.Zheng L, Liu H, Zhang M, Cao X, Zhou E. The complete genomic sequence of a novel mycovirus from Rhizoctonia solani AG-1 IA strain B275. Arch Virol. 2013; 158:1609–1612. 10.1007/s00705-013-1637-3 [DOI] [PubMed] [Google Scholar]
- 24.Zheng L, Zhang M, Chen Q, Zhu M, Zhou E. A novel mycovirus closely related to viruses in the genus Alphapartitivirus confers hypovirulence in the phytopathogenic fungus Rhizoctonia solani. Virology. 2014; 456:220–226. 10.1016/j.virol.2014.03.029 [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Xu P, Zhang L, Xia Z, Qin X, Yang G, et al. Molecular characterization of a novel mycovirus from Rhizoctonia fumigata AG-Ba isolate C-314 Baishi. Arch Virol. 2015; 160:2371–2374. 10.1007/s00705-015-2483-2 [DOI] [PubMed] [Google Scholar]
- 26.Zhong J, Chen CY, Gao BD. Genome sequence of a novel mycovirus of Rhizoctonia solani a plant pathogenic fungus. Virus Genes. 2015; 51:167–170. 10.1007/s11262-015-1219-4 [DOI] [PubMed] [Google Scholar]
- 27.Zhang T, Li W, Chen H, Yu H. Full genome sequence of a putative novel mitovirus isolated from Rhizoctonia cerealis. Arch Virol. 2015; 160:1815–1818. 10.1007/s00705-015-2431-1 [DOI] [PubMed] [Google Scholar]
- 28.Das S, Falloon RE, Stewart A, Pitman AR. Molecular characterisation of an endornavirus from Rhizoctonia solani AG-3PT infecting potato. Fungal Biol. 2014; 118:924–934. 10.1016/j.funbio.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 29.Rosario K, Breitbart M. Exploring the viral world through metagenomics. Curr Opin Virol. 2011; 1:289–297. 10.1016/j.coviro.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 30.Koonin EV, Dolja VV, Krupovic M. Origins and evolution of viruses of eukaryotes: The ultimate modularity. Virology. 2015, 479:2–25. 10.1016/j.virol.2015.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruenn JA. A structural and primary sequence comparison of the viral RNA‐dependent RNA polymerases. Nucleic Acids Res. 2003; 31:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koonin EV. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J Gen Virol. 1991; 72:2197–2206. 10.1099/0022-1317-72-9-2197 [DOI] [PubMed] [Google Scholar]
- 33.te Velthuis AJ. Common and unique features of viral RNA-dependent polymerases. Cell Mol Life Sci. 2014; 71:4403–4420. 10.1007/s00018-014-1695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanotto PD, Gibbs MJ, Gould EA, Holmes EC. A reevaluation of the higher taxonomy of viruses based on RNA polymerases. J Virol. 1996; 70:6083–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koonin EV, Dolja VV, Morris TJ. Evolution and taxonomy of positive-strand RNA viruses: Implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993; 28:375–430. 10.3109/10409239309078440 [DOI] [PubMed] [Google Scholar]
- 36.White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. PCR Protocols: A Guide to Methods and Applications New York: Academic Press Inc; 1990. pp 315–322. [Google Scholar]
- 37.Valverde RA, Nameth ST, Jordan RL. Analysis of double-stranded RNA for plant virus diagnosis. Plant Dis. 1990; 74:255–258. [Google Scholar]
- 38.Froussard P. A random-PCR method (rPCR) to construct whole cDNA library from low amounts of RNA. Nucleic Acids Res. 1992; 20:2900–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wibberg D, Andersson L, Tzelepis G, Rupp O, Blom J, et al. Genome analysis of the sugar beet pathogen Rhizoctonia solani AG2-2IIIB revealed high numbers in secreted proteins and cell wall degrading enzymes. BMC Genomics. 2016; 10.1186/s12864-016-2561-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wibberg D, Blom J, Jaenicke S, Kollin F, Rupp O, et al. Complete genome sequencing of Agrobacterium sp. H13-3, the former Rhizobium lupini H13-3, reveals a tripartite genome consisting of a circular and a linear chromosome and an accessory plasmid but lacking a tumor-inducing Ti-plasmid. J Biotechnol. 2011; 155:50–62. 10.1016/j.jbiotec.2011.01.010 [DOI] [PubMed] [Google Scholar]
- 41.Meyer F, Goesmann A, McHardy AC, Bartels D Bekel T, et al. GenDB—an open source genome annotation system for prokaryote genomes. Nucleic Acids Res. 2003; 31:2187–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Černý J, Bolfíková BČ, Valdes JJ, Grubhoffer L, Růžek D. Evolution of tertiary structure of viral RNA dependent polymerases. PloS one. 2014; 9:e96070 10.1371/journal.pone.0096070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boehr DD, Arnold JJ, Moustafa IM, Cameron CE. Structure dynamics and fidelity of RNA-dependent RNA polymerases. Nucleic Acid Polymerases. Springer Berlin Heidelberg; 2014; pp 309–333. [Google Scholar]
- 44.Xu X, Liu Y, Weiss S, Arnold E, Sarafianos SG, Ding J. Molecular model of SARS coronavirus polymerase: Implications for biochemical functions and drug design. Nucleic Acids Res. 2003; 31;7117–7130. 10.1093/nar/gkg916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6. Mol Biol Evol 30: 2725–2729. 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hillman BI, Cai G. The family Narnaviridae: Simplest of RNA viruses. Adv Virus Res. 2013; 86:149–176. 10.1016/B978-0-12-394315-6.00006-4 [DOI] [PubMed] [Google Scholar]
- 47.Koonin EV, Dolja VV. Virus world as an evolutionary network of viruses and capsidless selfish elements. Microbiol Mol Biol Rev. 2014; 78:278–303. 10.1128/MMBR.00049-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitahara R, Ikeda Y, Shimura H, Masuta C, Ezawa T. A unique mitovirus from Glomeromycota the phylum of arbuscular mycorrhizal fungi. Arch Virol. 2014; 159:2157–2160 10.1007/s00705-014-1999-1 [DOI] [PubMed] [Google Scholar]
- 49.Lakshman DK, Jian J, Tavantzis SM. A double-stranded RNA element from a hypovirulent strain of Rhizoctonia solani occurs in DNA form and is genetically related to the pentafunctional AROM protein of the shikimate pathway. PNAS. 1998; 95:6425–6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marzano SYL, Nelson BD, Ajayi-Oyetunde O, Bradley CA, Hughes TJ, Hartman GL, Eastburn DM, Domier LL. Identification of diverse mycoviruses through metatranscriptomics characterization of the viromes of five major fungal plant pathogens. J Virol; 2016, 90:6846–6863. 10.1128/JVI.00357-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roossinck MJ, Sabanadzovic S, Okada R, Valverde RA. The remarkable evolutionary history of endornaviruses. J Gen Virol. 2011; 92:2674–2678. 10.1099/vir.0.034702-0 [DOI] [PubMed] [Google Scholar]
- 52.Marzano SYL, Domier L. Novel mycoviruses discovered from metatranscriptomics survey of soybean phyllosphere phytobiomes. Virus Res. 2015; 219:11–21. [DOI] [PubMed] [Google Scholar]
- 53.Li W, Zhang T, Sun H, Deng Y, Zhang A, et al. Complete genome sequence of a novel endornavirus in the wheat sharp eyespot pathogen Rhizoctonia cerealis. Arch Virol. 2014; 159:1213–1216. 10.1007/s00705-013-1893-2 [DOI] [PubMed] [Google Scholar]
- 54.Howitt RL, Beever RE, Pearson MN, Forster RL. Genome characterization of Botrytis virus F a flexuous rod-shaped mycovirus resembling plant ‘potex-like’viruses. J Gen Virol. 2001; 82:67–78. 10.1099/0022-1317-82-1-67 [DOI] [PubMed] [Google Scholar]
- 55.Howitt RLJ, Beever RE, Pearson MN, Forster RLS. Genome characterization of a flexuous rod-shaped mycovirus Botrytis virus X reveals high amino acid identity to genes from plant ‘potex-like’viruses. Arch Virol. 2006. 151:563–579. 10.1007/s00705-005-0621-y [DOI] [PubMed] [Google Scholar]
- 56.Xie J, Wei D, Jiang D, Fu Y, Li G, et al. Characterization of debilitation-associated mycovirus infecting the plant-pathogenic fungus Sclerotinia sclerotiorum. J Gen Virol. 2006; 87:241–249. 10.1099/vir.0.81522-0 [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Fu Y, Jiang D, Li G Xie J, et al. A novel mycovirus that is related to the human pathogen Hepatitis E virus and rubi-like viruses. J Virol. 2009; 83:1981–1991. 10.1128/JVI.01897-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R, Hisano S, Tani A, Kondo H, Kanematsu S, et al. A capsidless ssRNA virus hosted by an unrelated dsRNA virus. Nat Microbiol. 2016; 10.1038/nmicrobiol.2015.1 [DOI] [PubMed] [Google Scholar]
- 59.Kozlakidis Z, Herrero N, Ozkan S, Bhatti MF, Coutts RH. A novel dsRNA element isolated from the Aspergillus foetidus mycovirus complex. Arch Virol. 2013; 158:2625–2628. 10.1007/s00705-013-1779-3 [DOI] [PubMed] [Google Scholar]
- 60.Chiba S, Salaipeth L, Lin YH, Sasaki A, Kanematsu S, et al. A novel bipartite double-stranded RNA mycovirus from the white root rot fungus Rosellinia necatrix: Molecular and biological characterization taxonomic considerations and potential for biological control. J Virol. 2009; 83:12801–12812. 10.1128/JVI.01830-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kanematsu S, Shimizu T, Salaipeth L, Yaegashi H, Sasaki A, et al. Genome rearrangement of a mycovirus Rosellinia necatrix megabirnavirus 1 affecting its ability to attenuate virulence of the host fungus. Virology. 2014; 450:308–315. 10.1016/j.virol.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 62.Vainio EJ, Jurvansuu J, Streng J, Rajamäki ML, Hantula J, et al. Diagnosis and discovery of fungal viruses using deep sequencing of small RNAs. J Gen Virol. 2015; 96:714–725. 10.1099/jgv.0.000003 [DOI] [PubMed] [Google Scholar]
- 63.Roossinck MJ, Martin DP, Roumagnac P. Plant virus metagenomics: Advances in virus discovery. Phytopathol. 2015; 105:716–727. [DOI] [PubMed] [Google Scholar]
- 64.Soueidan H, Schmitt LA, Candresse T, Nikolski M. Finding and identifying the viral needle in the metagenomic haystack: Trends and challenges. Front. Microbiol. 2014; 5:739 10.3389/fmicb.2014.00739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Diepeningen AD, Debets AJ, Hoekstra RF. Dynamics of dsRNA mycoviruses in black Aspergillus populations. Fungal Gen Biol. 2006; 43:446–452. [DOI] [PubMed] [Google Scholar]
- 66.Darissa O, Adam G, Schäfer WA. dsRNA mycovirus causes hypovirulence of Fusarium graminearum to wheat and maize. Europ J Plant Pathol. 2012; 134:181–189. [Google Scholar]
- 67.Simmonds P Methods for virus classification and the challenge of incorporating metagenomic sequence data. J Gen Virol 2015; 96:1193–1206. 10.1099/jgv.0.000016 [DOI] [PubMed] [Google Scholar]
- 68.Jian J, Lakshman DK, Tavantzis SM. Association of distinct double-stranded RNAs with enhanced or diminished virulence in Rhizoctonia solani infecting potato. Mol Plant-Microbe Interact. 1997; 10:1002–1009. [Google Scholar]
- 69.Sasaki A, Nakamura H, Suzuki N, Kanematsu S. Characterization of a new megabirnavirus that confers hypovirulence with the aid of a co-infecting partitivirus to the host fungus Rosellinia necatrix. Virus Res. 2015; 219:73–82. 10.1016/j.virusres.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 70.Wang M, Wang Y, Sun X, Cheng J Fu, et al. Characterization of a novel megabirnavirus from Sclerotinia sclerotiorum reveals horizontal gene transfer from single-stranded RNA virus to double-stranded RNA virus. J Virol. 2015; 89:8567–8579. 10.1128/JVI.00243-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nerva L, Ciuffo M, Vallino M, Margaria P, Varese GC, et al. Multiple approaches for the detection and characterization of viral and plasmid symbionts from a collection of marine fungi. Virus Res. 2016; 219:22–38. 10.1016/j.virusres.2015.10.028 [DOI] [PubMed] [Google Scholar]
- 72.Choi GH, Nuss DL. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992; 257:800–803. [DOI] [PubMed] [Google Scholar]
- 73.Urayama S, Kato S, Suzuki Y, Aoki N, Le MT, et al. Mycoviruses related to chrysovirus affect vegetative growth in the rice blast fungus Magnaporthe oryzae. J Gen Virol. 2010; 91:3085–3094 10.1099/vir.0.025411-0 [DOI] [PubMed] [Google Scholar]
- 74.Kanematsu S, Sasaki A, Onoue M, Oikawa Y, Ito T. Extending the fungal host range of a partitivirus and a mycoreovirus from Rosellinia necatrix by inoculation of protoplasts with virus particles. Phytopathol. 2010; 100:922–930. [DOI] [PubMed] [Google Scholar]
- 75.Kim JM, Song HY, Choi HJ, Yun SH, So KK, et al. Changes in the mycovirus (LeV) titer and viral effect on the vegetative growth of the edible mushroom fr. Virus Res. 2015; 197:8–12. 10.1016/j.virusres.2014.11.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All viral sequences used for analysis are available from the NCBI database; accession numbers are listed in the manuscript. Raw data is deposited at ENA under the Acc PRJEB15275.