Abstract
The loss of lean muscle mass occurring with advancing age is termed sarcopenia. This condition often leads to a concomitant loss of strength, increased frailty and risk of falls and an overall loss of functional independence in the elderly. Muscle protein metabolism is a dynamic process characterized by the balance between the synthesis and breakdown of muscle proteins. A disturbance of this equilibrium can lead to the loss of muscle mass, and a perturbation of muscle protein turnover with aging has been proposed to play a role in the development of sarcopenia. However, basal muscle protein synthesis and breakdown rates do not differ between young and old adults, which has led to the hypothesis that older adults are resistant to anabolic stimuli. In support of this hypothesis, older adults have either no response or a blunted response to nutrients, insulin and resistance exercise, and this anabolic resistance is likely a key factor in the loss of skeletal muscle mass with aging. Recent studies have investigated potential interventions to overcome this anabolic resistance. In particular, combining resistance exercise with essential amino acid supplementation restores the muscle protein anabolic response in older men. The novel rehabilitation technique of performing light resistance exercise during blood flow restriction was also successful in overcoming the anabolic resistance to exercise. Future research is needed to determine whether these novel interventions will be successful in preventing sarcopenia and improving muscle strength and function in older adults.
Keywords: aging, FSR, mTOR, exercise, nutrition, sarcopenia
INTRODUCTION
With the worldwide population of individuals over 60 years of age expected to triple in the next fifty years, a greater emphasis has been placed on research concerning the aging process and the many physiological changes that occur with advancing age. The rising age of our global population will have a significant impact on health care systems and maintaining good health during aging is necessary not only to prevent many chronic diseases but also to remain independent. A pivotal factor in the ability to remain healthy and functionally independent is the capacity to preserve skeletal muscle mass and strength. A progressive loss of muscle mass often observed with aging is termed sarcopenia [1–4]. This loss of lean mass is accompanied by a concomitant loss of muscular strength [5] which can lead to a greater chance of disability and a loss of functionality in older individuals [6–12]. A recent study by Ruiz et al. demonstrated that all cause, as well as cancer based, mortality was lowest for men in the highest tertile of strength [13]. On a muscle-specific level, sarcopenia is characterized by muscle fiber necrosis, grouping of fiber types, atrophy of type II muscle fibers and a loss of satellite cell content in type II fibers [14–21]. Another consequence of aging on skeletal muscle is reduced muscle specific and whole body oxidative capacity [4,22]. These changes place older individuals at a greater risk of developing chronic diseases such as insulin resistance, hyperlipidemia and hypertension.
The physiological changes that occur with aging are fairly well characterized, yet a basic understanding of the underlying mechanisms driving these changes is still elusive. Greater knowledge of the mechanisms leading to sarcopenia is required to better establish interventions to prevent the onset of symptoms associated with sarcopenia and promote the independent living of older persons. This review examines changes that occur in the regulation of muscle protein metabolism with aging, including information on resistance exercise and nutritional countermeasures that may help attenuate sarcopenia.
AGING AND MUSCLE PROTEIN METABOLISM
The balance between muscle protein synthesis and breakdown is responsible for the quality and maintenance of lean mass. A disturbance of this relationship has been proposed to facilitate the loss of lean muscle mass observed with aging. Early research focused on assessing fasted rates of muscle protein synthesis and breakdown, looking for potential age-related differences [23–29], and several of these studies reported lower rates of muscle protein synthesis in the elderly [24–26,28,29]. Lower rates of muscle protein synthesis have been observed in other chronic wasting conditions, such as renal failure [30], chronic obstructive pulmonary disease [31,32], cancer cachexia [33–35], cirrhosis [36] and thyroid disease [37]. These disease states are often characterized by moderate to severe muscle atrophy, whereas sarcopenia is a much more gradual loss of lean mass, often occurring over several decades. The large discrepancy (~25%) observed in the fasted rates of muscle protein synthesis between young and old [24–26,28,29] would lead to severe muscle wasting in older subjects without an accompanying decrease in the rate of muscle protein breakdown. However, more recent studies have observed little to no difference in the fasting rates of muscle protein synthesis between the young and elderly [23,27,38–41]. It is generally accepted that the difference in fasted rates of muscle protein synthesis or breakdown are not altered in healthy older adults and this has led to the hypothesis that other factors that affect muscle protein turnover (e.g., feeding, insulin, physical activity) may be important in the etiology of sarcopenia.
AGING AND NUTRIENT INGESTION
Essential amino acids, leucine in particular, are potent stimulators of skeletal muscle with the rise in intracellular amino acid concentrations driving the increase in muscle protein synthesis following meal ingestion [42,43]. The ingestion or infusion of large quantities of amino acids/protein yield similar increases in muscle protein synthesis in both young and older individuals [40,43–48]. Each of these studies provided a large bolus of protein or amino acids, typically 30–40g, which was effective in stimulating muscle protein synthesis in adults regardless of age [40,43–48]. This would indicate that if sufficient amino acids or protein is ingested in the elderly, the ability of amino acids to stimulate muscle protein synthesis would not be compromised. However, it is well known that older adults often do not eat a sufficient amount of protein and a few recent studies have shown that older subjects are resistant to the ingestion of smaller amounts of essential amino acids (6–15g) [38,39]. This effect appears to be due to nutrient signaling deficits in the mammalian target of rapamycin (mTOR) signaling pathway [27,49]. It is the essential amino acids (EAA), leucine in particular, that are responsible for the post-prandial increase in muscle protein synthesis [48,50–53]. EAA, leucine in particular, can activate mTOR signaling through various associative proteins, allowing mTOR to directly stimulate protein synthesis through improved translation initiation and elongation [51,54–56]. Leucine’s ability to stimulate muscle protein synthesis has been demonstrated in recent studies where its addition to an EAA bolus or a balanced meal led to a greater stimulation of muscle protein synthesis than either intervention alone [39,57]. These studies provide some evidence that the addition of excess leucine to meals may be able to overcome the anabolic resistance to feeding with aging, which could have important clinical implications in the design of nutritional interventions. One countermeasure for this nutritional anabolic resistance that we have recently proposed is that older adults should ingest a sufficient amount of protein (25–30 grams) at each meal in order to generate a significant muscle protein anabolic response to feeding [58]. However, long-term clinical trials are required to determine whether this nutritional approach would be able to prevent sarcopenia.
The role of insulin in regulating muscle protein synthesis is less well understood. Insulin is thought to have a relatively permissive and/or smaller effect on the stimulation of muscle protein synthesis [41,59–64] than amino acids and protein. However, recent studies show a blunted anabolic response to insulin with aging [41,59], perhaps partly due to insulin’s inability to stimulate vasodilation, and therefore increase amino acid delivery and overall nutritive flow to skeletal muscle in older adults. In a study by Fujita et al., the anabolic effect of insulin on muscle protein synthesis was rescued in older subjects with a 45 minute bout of aerobic exercise the day prior [65]. This would suggest that physical activity is capable of improving insulin sensitivity for muscle protein metabolism, and perhaps that a reduction in physical activity in older adults contributes to the anabolic resistance of feeding [27,49].
As mentioned above, the less than optimal diet chosen by many older individuals may also contribute to the loss of lean muscle mass observed with aging [66–68]. Specifically, inadequate protein intake can facilitate the loss of muscle and strength through insufficient stimulation of muscle protein synthesis [66–68] and the digestion and absorption of amino acids and protein is impaired in older individuals [40,69,70]. The appearance of amino acids in the circulatory system is also affected by absorption of nutrients in the small intestine, and older individuals have been shown to have a greater splanchnic uptake of amino acids than young controls, which would imply fewer amino acids available for uptake by skeletal muscle to be used for protein synthesis [40,69,70]. The digestion rates of different proteins can also affect net protein retention following ingestion of a meal. In young subjects, the ingestion of a slowly-digested protein (casein) yields greater protein retention than the ingestion of a quickly-digested (whey) protein [69,71–73]. However, the opposite is true in older subjects, with the ingestion of a quickly-digested protein leading to greater retention of protein [72,73]. This may be attributed to a smaller rise in intracellular amino acids with a slowly-digested protein, whereas a quickly-digested protein can cause a much more rapid and larger rise in the intracellular concentration of amino acids, thereby overcoming the anabolic resistance to nutrients [39]. Similarly, the act of protein pulse feeding, giving up to 80% of the daily requirement of protein in one meal, yielded greater protein retention in older women compared to equal protein intake distributed over four meals [74,75]. There is some debate as to the timing and amount of protein intake throughout the day, especially in an older population [58,76], but it is apparent that ingestion of a sufficient amount of protein and/or increasing physical activity in older adults is capable of restoring the muscle protein anabolic response to amino acids and insulin.
MUSCLE PROTEIN METABOLISM AND RESISTANCE EXERCISE
It is well accepted that resistance exercise training over time yields a net accrual of muscle proteins and muscle hypertrophy. The basic principal of resistance exercise is to provide an overload stimulus, or a load heavier than that which the muscle typically contracts against. Numerous studies have shown that older individuals, like their younger counterparts, respond to resistance training with increases in strength and lean muscle mass [77–90] although the response is typically less in the older adults. This is important in an aging population, as muscle strength and power are inversely associated with risk of falls and fractures and overall functional independence [91–98] and reducing the risk or incidence of falls is highly significant, as falling often denotes a loss of independence and a sharp decline in overall quality of life.
Resistance exercise increases muscle strength through hypertrophy of muscle fibers, which is accomplished through the net accrual of myofibrillar proteins. A single bout of resistance exercise has been shown to stimulate muscle protein synthesis in as little as 1–4 hours in young adults [99,100], with protein synthesis remaining elevated for 24–48 hours following exercise [100–102]. The rate of muscle protein breakdown is also stimulated following a bout of resistance exercise [100,103], but to a lesser extent than the rate of synthesis. This yields an improvement in net muscle protein balance (rate of muscle protein synthesis – rate of muscle protein breakdown), but the overall net protein balance remains negative following exercise. It is only when nutrients, namely amino acids or protein, are ingested following resistance exercise that a positive net protein balance occurs [104–109]. It is well established that the ingestion of nutrients following exercise enhances muscle protein synthesis to a greater extent than resistance exercise or nutrients alone. For an in depth review of the effect of nutrient timing in relation to resistance exercise, see Drummond et al. [110].
A few groups have studied the ingestion of proteins/amino acids before or during a bout of resistance exercise to see if the post-exercise rate of protein synthesis is augmented. A study by Beelen et al. looked at protein co-ingestion during a bout of resistance exercise and observed increases in both whole body and mixed muscle protein synthesis during exercise [104]. However, a recent study by our research group provided young subjects with essential amino acids and carbohydrate 1 hour prior to exercise and observed no improvement in muscle protein synthesis following exercise as compared to a fasted exercise group [111]. It remains unclear whether nutrient ingestion prior to or during exercise is beneficial to the anabolic response of resistance exercise whereas nutrient ingestion following exercise clearly has an additive effect. The ingestion of carbohydrates following resistance exercise is known to stimulate the release of insulin, which aids in the reduction of muscle protein breakdown [112–114], but exerts only a modest effect on muscle protein synthesis without a concomitant increase in plasma amino acid availability [27,115–117]. Nutrient ingestion (EAA or EAA+Carbohydrates) following resistance exercise leads to a greater anabolic response through activation of the mTOR signaling pathway and subsequent activation of muscle protein synthesis [118–120].
As with essential amino acids, resistance exercise increases muscle protein synthesis through activation of the mTOR signaling pathway [99,119,121–123]. Recently, Drummond et al. showed the importance of mTOR in the anabolic response to exercise in young men, when the activation of several proteins in the mTOR signaling pathway was attenuated with the administration of rapamycin, an mTOR inhibitor [124]. This also led to a blunting of the post-exercise increase in muscle protein synthesis [124]. However, evidence also indicates that the extracellular-related kinase (ERK) 1/2 signaling pathway contributes to the contractile-induced increase in muscle protein synthesis following resistance exercise [122,125,126] in both an mTOR dependent [127] and independent manner [125,126,128]. The ERK1/2 signaling pathway signals downstream through map kinase-interacting kinase 1 (MNK1), which can activate the translation initiation complex through eukaryotic initiation factor 4E (eIF4E) [125,126,128]. While the phosphorylation status of signaling proteins is often difficult to correlate with changes in protein turnover [129], increases in the phosphorylation of ribosomal p70S6 Kinase 1 (S6K1) is a good marker for muscle hypertrophy following resistance exercise training [130,131].
RESISTANCE EXERCISE AND AGING MUSCLE
Resistance exercise training stimulates muscle hypertrophy and improves muscular strength in older individuals, albeit to a lesser extent than in their younger counterparts [81,82,88,132,133]. This difference may be attributed to a blunted anabolic response to resistance exercise in older adults. A few recent studies have observed a blunted muscle protein synthesis response following an acute bout of resistance exercise in older subjects [134–136]. Kumar et al. observed that signaling to 2 downstream targets of mTOR, eukaryotic initiation factor 4E binding protein 1 (4E-BP1) and S6K1, was depressed in older subjects in addition to the blunted muscle protein synthesis response [135]. The dysregulation of anabolic signaling in response to resistance exercise may be a driving cause of the diminished response to exercise that has been observed recently. This goes in line with the proposed anabolic resistance of aging noted with nutrient ingestion [27,129] and the response to insulin [65,137]. It has also been observed that the expression of proteolytic genes, such as MuRF1 and Atrogin-1, are upregulated in older muscle compared to younger controls at rest and following resistance exercise [138]. This implies that muscle protein breakdown through the ubiquitin proteasome pathway may be up-regulated in the elderly. Whether they experience an increase in protein breakdown following exercise or just a blunted synthesis response, older individuals clearly have a less robust anabolic response to resistance exercise or training as their younger counterparts.
However, a few recent studies have been able to rescue the effects of exercise in older subjects with the provision of nutrients in the form of protein/amino acids and carbohydrates following a bout of exercise. Koopman et al. provided older subjects with protein or protein with leucine following 30 minutes of physical activity similar to various activities of daily living [139]. Both groups saw similar increases in muscle protein synthesis following ingestion of nutrients, and the authors concluded that excess leucine did not further stimulate the response to nutrient ingestion following physical activity when ample protein was ingested [139]. Drummond et al. provided young and older subjects with essential amino acids 1 hour after performing a bout of resistance exercise [122]. Subjects were observed for 6 hours following exercise, and both young and old subjects had similar increases in muscle protein synthesis over the 6 hour post-exercise period [122]. Interestingly, younger subjects had a more rapid increase in muscle protein synthesis in the first 3 hours of post-exercise recovery, with older subjects displaying a delayed anabolic response through the first 3 hours of recovery [122]. This result may have been due to greater activation of anabolic signaling proteins, such as ERK 1/2 or its downstream target MNK1, in the younger group [122], and goes in line with other studies showing a blunted anabolic signaling response to resistance exercise in older subjects [135]. While age-related differences in activation of the mTOR and ERK 1/2 pathways following exercise have been noted, the exact mechanisms underlying the diminished response to exercise with aging remain to be elucidated.
We recently published a study of the effect of low-intensity resistance exercise in combination with a reduction in blood flow to the working muscles on muscle protein synthesis in older men [140]. Following a bout of exercise with blood flow restriction, subjects had an increased rate of muscle protein synthesis and a stimulation of both mTOR and ERK1/2 intracellular signaling [140]. This novel exercise method, capable of stimulating both anabolic signaling and muscle protein synthesis in older subjects, could serve as a potential muscle rehabilitation intervention to counteract sarcopenia [140]. We propose that, to achieve a maximal stimulation of muscle protein synthesis following exercise, concurrent activation of both the mTOR and ERK1/2 pathways is needed. In a previous study, younger subjects were able to activate both pathways and saw an increase in the rate of protein synthesis following resistance exercise and EAA ingestion, whereas older subjects only activated the mTOR pathway and saw a delayed increase in the rate of protein synthesis [122]. The delayed response may be due to an inability of older muscle to adequately stimulate both mTOR and ERK signaling pathways following resistance exercise [122], which may explain the blunted anabolic response to exercise observed in older individuals. More research is needed to further investigate blood flow restricted exercise as a novel rehabilitation exercise method in older subjects.
CONCLUSIONS
An associated loss of muscle mass and strength clearly occurs with the aging process. Sarcopenia, the age associated loss of lean muscle mass, is a debilitating process that can lead to an increased risk of falls, fractures and an overall loss of independence. Research has shown minimal differences in basal, fasted rates of muscle protein synthesis which most likely cannot account for the chronic loss of muscle seen with advancing age. On the other hand, anabolic resistance has been observed for three major anabolic stimuli: amino acid intake, insulin and resistance exercise. Fortunately, recently tested interventions can restore the anabolic response to these stimuli. Specifically, the following interventions are likely to counteract sarcopenia: 1) ingestion of a sufficient amount of protein (25–30 grams) at each meal and/or increase of overall physical activity of older adults to restore the anabolic effect of feeding; 2) essential amino acid supplementation following traditional resistance exercise; and 3) blood flow restriction exercise. However, more research (e.g., clinical trials) is needed to determine whether these three interventions will improve muscle strength and function to promote more independence in older adults at risk for sarcopenia.
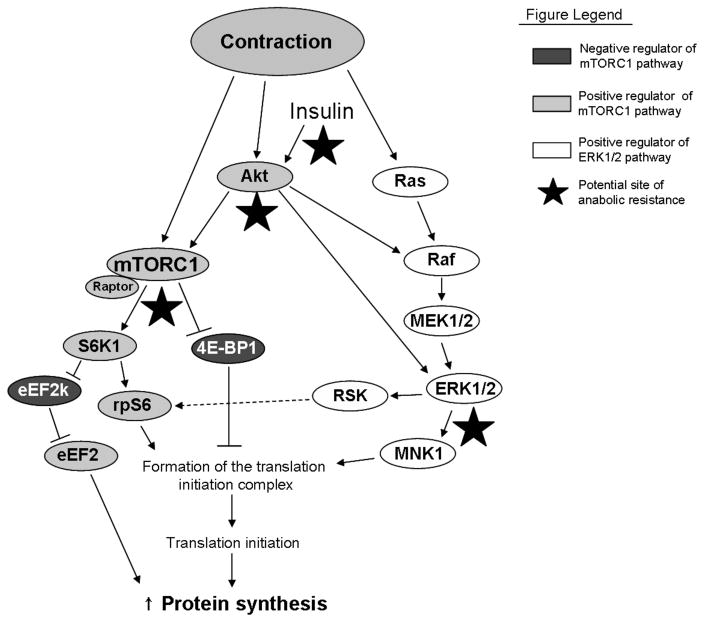
Figure 1.
A simplified diagram of mTORC1 and ERK1/2 signaling following muscle contraction. We hypothesize that dual activation of both pathways is required to maximally stimulate muscle protein synthesis following a bout of resistance exercise. The mTORC1 pathway is indicated in green and red and the ERK1/2 pathway is indicated in blue. Akt, protein kinase B; mTORC1, mammalian target of rapamycin complex 1; Raptor, regulatory associated protein of mTOR; S6K1, p70 ribosomal S6 kinase 1; rpS6, ribosomal protein S6; eEF2K, eukaryotic elongation factor 2 kinase; eEF2, eukaryotic elongation factor 2; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; MEK1/2, mitogen activated protein kinase kinase 1/2; ERK1/2, extracelullar signal-regulated kinase 1/2; MNK1, map kinase-interacting kinase 1; RSK, p90 ribosomal protein S6 kinase.
Table 1.
Age-associated differences in measures of muscle protein turnover.
| Parameter | Response of older subjects compared to young |
|---|---|
| Basal Protein Turnover | |
| Resting rate of MPS |

|
| Resting rate of MPB |

|
| Anabolic Resistance | |
| MPS response to insulin |

|
| MPS response to small dose of AA/protein |

|
| MPS response to mixed meal |

|
| MPS response to RE |

|
| mTOR signaling response to nutrition |

|
| mTOR signaling response to RE |

|
| ERK1/2 signaling response to RE |

|
| Digestion/absorption of AA/protein |

|
| Interventions that Overcome Anabolic Resistance | |
| MPS response to large dose of AA/protein |

|
| MPS response to BFR exercise |

|
| MPS response to RE and AA/protein |

|
MPS - muscle protein synthesis; MPB - muscle protein breakdown; AA - amino acids; RE - resistance exercise; mTOR - mammalian target of rapamycin; ERK1/2 - extracellular- related kinase 1/2; BFR - Blood Flow Restriction
Acknowledgments
Supported from NIAMS R01-AR049877 (BBR), NIH/NIA P30 AG024832, NIH T32-HD07539 and NIH 1UL1RR029876-01. We would also like to thank Dr. Sarah Toombs-Smith for editing the manuscript.
LIST OF ABBREVIATIONS
- EAA
Essential Amino Acids
- mTOR
Mammalian Target of Rapamycin
- ERK1/2
Extracellular Related Kinase 1/2
- MNK1
Map Kinase-Interacting Kinase 1
- eIF4E
Eukaryotic Initiation Factor 4E
- 4E-BP1
Eukaryotic Initiation Factor 4E Binding Protein 1
- S6K1
Ribosomal Protein S6 Kinase 1
- MuRF1
Muscle RING Factor 1
References
- 1.Forbes GB, Reina JC. Adult lean body mass declines with age - some longitudinal observations. Metabolism. 1970;19(9):653–56. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- 2.Melton LJ, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000;48(6):625–30. [PubMed] [Google Scholar]
- 3.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in new mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 4.Nair KS. Aging muscle. Am J Clin Nutr. 2005;81(5):953–63. doi: 10.1093/ajcn/81.5.953. [DOI] [PubMed] [Google Scholar]
- 5.Aniansson A, Grimby G, Rundgren A. Isometric and isokinetic quadriceps muscle strength in 70-year-old men and women. Scand J Rehabil Med. 1980;12(4):161–8. [PubMed] [Google Scholar]
- 6.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995;50:64–67. doi: 10.1093/gerona/50a.special_issue.64. [DOI] [PubMed] [Google Scholar]
- 7.Landers KA, Hunter GR, Wetzstein CJ, Bamman MM, Weinsier RL. The interrelationship among muscle mass, strength, and the ability to perform physical tasks of daily living in younger and older women. J Gerontol A Biol Sci Med Sci. 2001;56(10):B443–B48. doi: 10.1093/gerona/56.10.b443. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy E. The relationship between leg strength and physical function in older adult women. Gerontologist. 2003;43:6–6. [Google Scholar]
- 9.Bassey EJ, Fiatarone MA, Oneill EF, Kelly M, Evans WJ, Lipsitz LA. Leg extensor power and functional performance in very old men and women. Clin Sci. 1992;82(3):321–27. doi: 10.1042/cs0820321. [DOI] [PubMed] [Google Scholar]
- 10.Vandervoort AA, Symons TB. Functional and metabolic consequences of sarcopenia. Can J Appl Physiol. 2001;26(1):90–101. doi: 10.1139/h01-007. [DOI] [PubMed] [Google Scholar]
- 11.Brown M, Sinacore DR, Host HH. The relationship of strength to function in the older adult. J Gerontol A Biol Sci Med Sci. 1995;50:55–59. doi: 10.1093/gerona/50a.special_issue.55. [DOI] [PubMed] [Google Scholar]
- 12.Larsson L, Karlsson J. Isometric and dynamic endurance as a function of age and skeletal-muscle characteristics. Acta Physiol Scand. 1978;104(2):129–36. doi: 10.1111/j.1748-1716.1978.tb06259.x. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz JR, Sui X, Lobelo F, Morrow JR, Jackson AW, Sjostrom M, et al. Association between muscular strength and mortality in men: Prospective cohort study. Br Med J. 2008;337(7661) doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg H, van Loon LJC. Satellite cell content is specifically reduced in type ii skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007;292(1):E151–E57. doi: 10.1152/ajpendo.00278.2006. [DOI] [PubMed] [Google Scholar]
- 15.Verdijk LB, Gleeson BG, Jonkers RAM, Meijer K, Savelberg H, Dendale P, et al. Skeletal muscle hypertrophy following resistance training is accompanied by a fiber type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64(3):332–39. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadi F, Charifi N, Denis C, Lexell J. Satellite cells and myonuclei in young and elderly women and men. Muscle Nerve. 2004;29(1):120–27. doi: 10.1002/mus.10510. [DOI] [PubMed] [Google Scholar]
- 17.Larsson L, Sjodin B, Karlsson J. Histochemical and biochemical changes in human skeletal-muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 1978;103(1):31–39. doi: 10.1111/j.1748-1716.1978.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsson L. Morphological and functional characteristics of aging skeletal-muscle in man - cross-sectional study. Acta Physiol Scand. 1978:5–36. [PubMed] [Google Scholar]
- 19.Lexell J, Henrikssonlarsen K, Winblad B, Sjostrom M. Distribution of different fiber types in human skeletal-muscles - effects of aging studied in whole muscle cross-sections. Muscle Nerve. 1983;6(8):588–95. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 20.Lexell J, Henrikssonlarsen K, Sjostrom M. Distribution of different fiber types in human skeletal-muscles .2. A study of cross-sections of whole m-vastus lateralis. Acta Physiol Scand. 1983;117(1):115–22. doi: 10.1111/j.1748-1716.1983.tb07185.x. [DOI] [PubMed] [Google Scholar]
- 21.Lexell J. Human aging, muscle mass, and fiber-type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 22.Nair KS. Muscle protein-turnover - methodological issues and the effect of aging. J Gerontol A Biol Sci Med Sci. 1995;50:107–12. doi: 10.1093/gerona/50a.special_issue.107. [DOI] [PubMed] [Google Scholar]
- 23.Volpi E, Sheffield-Moore M, Rasmussen BB, Wolfe RR. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286(10):1206–12. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarasheski KE, Welle S, Nair KS. Muscle protein synthesis in younger and older men. JAMA. 2002;287(3):317–8. doi: 10.1001/jama.287.3.317. [DOI] [PubMed] [Google Scholar]
- 25.Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93(26):15364–69. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab. 1997;273(4):E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- 27.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19(3):422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 28.Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases mhc and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278(4):E620–6. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- 29.Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol. 1993;264(5 Pt 1):E693–8. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- 30.Adey D, Kumar R, McCarthy JT, Nair KS. Reduced synthesis of muscle proteins in chronic renal failure. Am J Physiol Endocrinol Metab. 2000;278(2):E219–E25. doi: 10.1152/ajpendo.2000.278.2.E219. [DOI] [PubMed] [Google Scholar]
- 31.Morrison WL, Gibson JNA, Johnston RN, Clark RA, Rennie MJ. Depressed muscle protein-synthesis is the predominant mechanism of muscle wasting in emphysema. Clin Sci. 1986;70:P63–P63. [Google Scholar]
- 32.Morrison WL, Gibson JN, Scrimgeour C, Rennie MJ. Muscle wasting in emphysema. Clin Sci. 1988;75(4):415–20. doi: 10.1042/cs0750415. [DOI] [PubMed] [Google Scholar]
- 33.Dworzak F, Ferrari P, Gavazzi C, Maiorana C, Bozzetti F. Effects of cachexia due to cancer on whole body and skeletal muscle protein turnover. Cancer. 1998;82(1):42–48. [PubMed] [Google Scholar]
- 34.Emery PW, Edwards RH, Rennie MJ, Souhami RL, Halliday D. Protein synthesis in muscle measured in vivo in cachectic patients with cancer. Br Med J (Clin Res Ed) 1984;289(6445):584–6. doi: 10.1136/bmj.289.6445.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rennie MJ, Edwards RH, Emery PW, Halliday D, Lundholm K, Millward DJ. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clin Physiol. 1983;3(5):387–98. doi: 10.1111/j.1475-097x.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 36.Morrison WL, Bouchier IA, Gibson JN, Rennie MJ. Skeletal muscle and whole-body protein turnover in cirrhosis. Clin Sci. 1990;78(6):613–9. doi: 10.1042/cs0780613. [DOI] [PubMed] [Google Scholar]
- 37.Morrison WL, Gibson JN, Jung RT, Rennie MJ. Skeletal muscle and whole body protein turnover in thyroid disease. Eur J Clin Invest. 1988;18(1):62–8. doi: 10.1111/j.1365-2362.1988.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 38.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82(5):1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 39.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 40.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277(3):E513–E20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 41.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85(12):4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: Studies of incorporation of [1–13c]leucine. Clin Sci. 1989;76(4):447–54. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- 43.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: The effects of feeding and fasting. Clin Sci. 1982;63(6):519–23. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 44.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6(6):358–62. [PMC free article] [PubMed] [Google Scholar]
- 45.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286(3):E321–E28. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 46.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, X-J Zhang, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41(2):215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101(9):2000–07. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78(2):250–58. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, et al. Impaired anabolic response of muscle protein synthesis is associated with s6k1 dysregulation in elderly humans. FASEB J. 2004;18(11):1586–88. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 50.Tipton KD, Gurkin BE, Matin S, Wolfe RR. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999;10(2):89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 51.Bolster DR, Vary TC, Kimball SR, Jefferson LS. Leucine regulates translation initiation in rat skeletal muscle via enhanced eif4g phosphorylation. J Nutr. 2004;134(7):1704–10. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- 52.Norton LE, Layman DK. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J Nutr. 2006;136(2):533S–37S. doi: 10.1093/jn/136.2.533S. [DOI] [PubMed] [Google Scholar]
- 53.Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with l-[1–13c]leucine stimulates human muscle protein incorporation of continuously infused l-[1–13c]valine. Am J Physiol. 1992;262(3 Pt 1):E372–6. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 54.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, et al. Nutrient signaling in the regulation of human muscle protein synthesis. FASEB J. 2007;21(5):A713–A13. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolster DR, Jefferson LS, Kimball SR. Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc. 2004;63(2):351–6. doi: 10.1079/PNS2004355. [DOI] [PubMed] [Google Scholar]
- 56.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136(1):227S–31S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 57.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575(1):305–15. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12(1):86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(6):768–9. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujita S, Volpi E. Amino acids and muscle loss with aging. J Nutr. 2006;136(1):277S–80S. doi: 10.1093/jn/136.1.277S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291(4):E745–E54. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guillet C, Zangarelli A, Gachon P, Morio B, Giraudet C, Rousset P, et al. Whole body protein breakdown is less inhibited by insulin, but still responsive to amino acid, in nondiabetic elderly subjects. J Clin Endocrinol Metab. 2004;89(12):6017–24. doi: 10.1210/jc.2003-031323. [DOI] [PubMed] [Google Scholar]
- 63.Phillips SM. Insulin and muscle protein turnover in humans: Stimulatory, permissive, inhibitory, or all of the above? Am J Physiol Endocrinol Metab. 2008;295(4):E731–E31. doi: 10.1152/ajpendo.90569.2008. [DOI] [PubMed] [Google Scholar]
- 64.Volpi E, Lucidi P, Cruciani G, Monacchia F, Reboldi G, Brunetti P, et al. Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes. 1996;45(9):1245–52. doi: 10.2337/diab.45.9.1245. [DOI] [PubMed] [Google Scholar]
- 65.Fujita S, Rasmussen BB, Cadenas JG, Sattler FR, Volpi E. An acute bout of aerobic exercise restores the physiological response of muscle protein synthesis to insulin in healthy older subjects. FASEB J. 2005;19(5):A1569–A69. [Google Scholar]
- 66.Campbell WW, Evans WJ. Protein requirements of elderly people. Eur J Clin Nutr. 1996;50:S180–S85. [PubMed] [Google Scholar]
- 67.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56(6):M373–80. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 68.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87(5):1562S–66S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 69.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94(26):14930–35. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65(2):489–95. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- 71.Dangin M, Boirie Y, Garcia-Rodenas C, Gachon P, Fauquant J, Callier P, et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280(2):E340–E48. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 72.Dangin M, Boirie Y, Guillet C, Beaufere B. Influence of the protein digestion rate on protein turnover in young and elderly subjects. J Nutr. 2002;132(10):3228S–33S. doi: 10.1093/jn/131.10.3228S. [DOI] [PubMed] [Google Scholar]
- 73.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, et al. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549(2):635–44. doi: 10.1113/jphysiol.2002.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, et al. Protein pulse feeding improves protein retention in elderly women. Am J Clin Nutr. 1999;69(6):1202–08. doi: 10.1093/ajcn/69.6.1202. [DOI] [PubMed] [Google Scholar]
- 75.Arnal MA, Mosoni L, Boirie Y, Houlier ML, Morin L, Verdier E, et al. Protein feeding pattern does not affect protein retention in young women. J Nutr. 2000;130(7):1700–04. doi: 10.1093/jn/130.7.1700. [DOI] [PubMed] [Google Scholar]
- 76.Dreyer HC, Volpi E. Role of protein and amino acids in the pathophysiology and treatment of sarcopenia. J Am Coll Nutr. 2005;24(2):140S–45S. doi: 10.1080/07315724.2005.10719455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians - effects on skeletal-muscle. JAMA. 1990;263(22):3029–34. [PubMed] [Google Scholar]
- 78.McCartney N, Hicks AL, Martin J, Webber CE. Long-term resistance training in the elderly - effects on dynamic strength, exercise capacity, muscle, and bone. J Gerontol A Biol Sci Med Sci. 1995;50(2):B97–B104. doi: 10.1093/gerona/50a.2.b97. [DOI] [PubMed] [Google Scholar]
- 79.McCartney N, Hicks AL, Martin J, Webber CE. A longitudinal trial of weight training in the elderly: Continued improvements in year 2. J Gerontol A Biol Sci Med Sci. 1996;51(6):B425–B33. doi: 10.1093/gerona/51a.6.b425. [DOI] [PubMed] [Google Scholar]
- 80.Taaffe DR, Pruitt L, Pyka G, Guido D, Marcus R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clin Physiol. 1996;16(4):381–92. doi: 10.1111/j.1475-097x.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- 81.Hakkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, et al. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci. 1998;53(6):B415–B23. doi: 10.1093/gerona/53a.6.b415. [DOI] [PubMed] [Google Scholar]
- 82.Ivey FM, Roth SM, Ferrell RE, Tracy BL, Lemmer JT, Hurlbut DE, et al. Effects of age, gender, and myostatin genotype on the hypertrophic response to heavy resistance strength training. J Gerontol A Biol Sci Med Sci. 2000;55(11):M641–M48. doi: 10.1093/gerona/55.11.m641. [DOI] [PubMed] [Google Scholar]
- 83.Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol. 2001;535(1):301–11. doi: 10.1111/j.1469-7793.2001.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003;177(1):69–78. doi: 10.1046/j.1365-201X.2003.01050.x. [DOI] [PubMed] [Google Scholar]
- 85.Kostek MC, Delmonico MJ, Reichel JB, Roth SM, Douglass L, Ferrell RE, et al. Muscle strength response to strength training is influenced by insulin-like growth factor 1 genotype in older adults. J Appl Physiol. 2005;98(6):2147–54. doi: 10.1152/japplphysiol.00817.2004. [DOI] [PubMed] [Google Scholar]
- 86.Valkeinen H, Hakkinen K, Pakarinen A, Hannonen P, Hakkinen A, Airaksinen O, et al. Muscle hypertrophy, strength development, and serum hormones during strength training in elderly women with fibromyalgia. Scand J Rheumatol. 2005;34(4):309–14. doi: 10.1080/03009740510018697. [DOI] [PubMed] [Google Scholar]
- 87.Adams KJ, Swank AM, Berning JM, Sevene-Adams PG, Barnard KL, Shimp-Bowerman J. Progressive strength training in sedentary, older african american women. Med Sci Sports Exerc. 2001;33(9):1567–76. doi: 10.1097/00005768-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 88.Izquierdo M, Hakkinen K, Ibanez J, Garrues M, Anton A, Zuniga A, et al. Effects of strength training on muscle power and serum hormones in middle-aged and older men. J Appl Physiol. 2001;90(4):1497–507. doi: 10.1152/jappl.2001.90.4.1497. [DOI] [PubMed] [Google Scholar]
- 89.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Singh MAF. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50(4):655–62. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 90.Newton RU, Hakkinen K, Hakkinen A, McCormick M, Volek J, Kraemer WJ. Mixed-methods resistance training increases power and strength of young and older men. Med Sci Sports Exerc. 2002;34(8):1367–75. doi: 10.1097/00005768-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 91.Chan BKS, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men - the osteoporotic fractures in men study. Am J Epidemiol. 2007;165(6):696–703. doi: 10.1093/aje/kwk050. [DOI] [PubMed] [Google Scholar]
- 92.Skelton DA, Young A, Greig CA, Malbut KE. Effects of resistance training on strength, power, and selected functional abilities of women aged 75 and older. J Am Geriatr Soc. 1995;43(10):1081–87. doi: 10.1111/j.1532-5415.1995.tb07004.x. [DOI] [PubMed] [Google Scholar]
- 93.Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing. 2002;31(2):119–25. doi: 10.1093/ageing/31.2.119. [DOI] [PubMed] [Google Scholar]
- 94.Shaw JM, Snow CM. Weighted vest exercise improves indices of fall risk in older women. J Gerontol A Biol Sci Med Sci. 1998;53(1):M53–M58. doi: 10.1093/gerona/53a.1.m53. [DOI] [PubMed] [Google Scholar]
- 95.Miszko TA, Cress ME, Slade JM, Covey CJ, Agrawal SK, Doerr CE. Effect of strength and power training on physical function in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2003;58(2):171–75. doi: 10.1093/gerona/58.2.m171. [DOI] [PubMed] [Google Scholar]
- 96.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: A systematic review and meta-analysis. J Am Geriatr Soc. 2004;52(7):1121–29. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 97.Volpato S, Leveille SG, Blaum C, Fried LP, Guralnik JM. Risk factors for falls in older disabled women with diabetes: The women’s health and aging study. J Gerontol A Biol Sci Med Sci. 2005;60(12):1539–45. doi: 10.1093/gerona/60.12.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orr R, de Vos NJ, Singh NA, Ross DA, Stavrinos TM, Fiatarone-Singh MA. Power training improves balance in healthy older adults. J Gerontol A Biol Sci Med Sci. 2006;61(1):78–85. doi: 10.1093/gerona/61.1.78. [DOI] [PubMed] [Google Scholar]
- 99.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases ampk activity and reduces 4e-bp1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576(2):613–24. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol Endocrinol Metab. 1997;36(1):E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 101.Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young man. Am J Physiol Regul Integr Comp Physiol. 2008;294(1):R172–R78. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- 102.Chesley A, Macdougall JD, Tarnopolsky MA, Atkinson SA, Smith K. Changes in human muscle protein-synthesis after resistance exercise. J Appl Physiol. 1992;73(4):1383–88. doi: 10.1152/jappl.1992.73.4.1383. [DOI] [PubMed] [Google Scholar]
- 103.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(3 Pt 1):E514–20. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 104.Beelen M, Koopman R, Gijsen AP, Vandereyt H, Kies AK, Kuipers H, et al. Protein coingestion stimulates muscle protein synthesis during resistance-type exercise. Am J Physiol Endocrinol Metab. 2008;295(1):E70–E77. doi: 10.1152/ajpendo.00774.2007. [DOI] [PubMed] [Google Scholar]
- 105.Wilkinson SB, Tarnopolsky MA, MacDonald MJ, MacDonald JR, Armstrong D, Philips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85(4):1031–40. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 106.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88(2):386–92. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 107.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276(4 Pt 1):E628–34. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- 108.Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects 24-h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab. 2003;284(1):E76–E89. doi: 10.1152/ajpendo.00234.2002. [DOI] [PubMed] [Google Scholar]
- 109.Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273(1 Pt 1):E122–9. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- 110.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mtorc1 signaling. J Appl Physiol. 2009;106(4):1374–84. doi: 10.1152/japplphysiol.91397.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol. 2009;106(5):1730–39. doi: 10.1152/japplphysiol.90395.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J Appl Physiol. 2004;96(2):674–78. doi: 10.1152/japplphysiol.00333.2003. [DOI] [PubMed] [Google Scholar]
- 113.Gelfand RA, Barrett EJ. Effect of physiological hyperinsulinemia on skeletal-muscle protein-synthesis and breakdown in man. J Clin Invest. 1987;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hillier TA, Fryburg DA, Jahn LA, Barrett EJ. Extreme hyperinsulinemia unmasks insulin’s effect to stimulate protein synthesis in the human forearm. Am J Physiol Endocrinol Metab. 1998;274(6):E1067–E74. doi: 10.1152/ajpendo.1998.274.6.E1067. [DOI] [PubMed] [Google Scholar]
- 115.Bell JA, Fujita S, Volpi E, Cadenas JG, Rasmussen BB. Short-term insulin and nutritional energy provision do not stimulate muscle protein synthesis if blood amino acid availability decreases. Am J Physiol Endocrinol Metab. 2005;289(6):E999–E1006. doi: 10.1152/ajpendo.00170.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Drummond MJ, Bell JA, Fujita S, Dreyer HC, Volpi E, Rasmussen BB. Nutrient signaling in insulin resistant human skeletal muscle during reduced amino acid availability. FASEB J. 2007;21(5):A714–A14. [Google Scholar]
- 117.Drummond MJ, Bell JA, Fujita S, Dreyer HC, Glynn EL, Volpi E, et al. Amino acids are necessary for the insulin-induced activation of mtor/s6k1 signaling and protein synthesis in healthy and insulin resistant human skeletal muscle. Clin Nutr. 2008;27(3):447–56. doi: 10.1016/j.clnu.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koopman R, Wagenmakers AJM, Manders RJF, Zorenc AHG, Senden JMG, Gorselink M, et al. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288(4):E645–53. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 119.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mtor signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294(2):E392–E400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Koopman R, Pennings B, Zorenc AHG, van Loon LJC. Protein ingestion further augments s6k1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr. 2007;137(8):1880–86. doi: 10.1093/jn/137.8.1880. [DOI] [PubMed] [Google Scholar]
- 121.Koopman R, Zorenc AHG, Gransier RJJ, Cameron-Smith D, van Loon LJC. Increase in S6k1 Phosphorylation in Human Skeletal Muscle Following Resistance Exercise Occurs Mainly in Type II Muscle Fibers. Am J Physiol Endocrinol Metab. 2006;290(6):E1245–52. doi: 10.1152/ajpendo.00530.2005. [DOI] [PubMed] [Google Scholar]
- 122.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104(5):1452–61. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Glover EI, Oates BR, Tang JE, Moore DR, Tarnopolsky MA, Phillips SM. Resistance exercise decreases eif2b epsilon phosphorylation and potentiates the feeding-induced stimulation of p70(s6k1) and rps6 in young men. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R604–R10. doi: 10.1152/ajpregu.00097.2008. [DOI] [PubMed] [Google Scholar]
- 124.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587(7):1535–46. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Williamson D, Gallagher P, Harber M, Hollon C, Trappe S. Mitogen-activated protein kinase (mapk) pathway activation: Effects of age and acute exercise on human skeletal muscle. J Physiol. 2003;547(Pt 3):977–87. doi: 10.1113/jphysiol.2002.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Williamson DL, Kubica N, Kimball SR, Jefferson LS. Exercise-induced alterations in extracellular signal-regulated kinase 1/2 and mammalian target of rapamycin (mtor) signalling to regulatory mechanisms of mrna translation in mouse muscle. J Physiol. 2006;573(Pt 2):497–510. doi: 10.1113/jphysiol.2005.103481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ma L, Chen ZB, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of tsc2 by erk: Implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121(2):179–93. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 128.Atherton PJ, Rennie MJ. Protein synthesis a low priority for exercising muscle. J Physiol. 2006;573(2):288–89. doi: 10.1113/jphysiol.2006.110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295(3):E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Terzis G, Georgiadis G, Stratakos G, Vogiatzis I, Kavouras S, Manta P, et al. Resistance exercise-induced increase in muscle mass correlates with p70s6 kinase phosphorylation in human subjects. Eur J Appl Physiol. 2008;102(2):145–52. doi: 10.1007/s00421-007-0564-y. [DOI] [PubMed] [Google Scholar]
- 131.Baar K, Esser K. Phosphorylation of p70(s6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol. 1999;276(1 Pt 1):C120–7. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 132.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. Older adults. J Appl Physiol. 2006;101(2):531–44. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 133.Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM. Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab. 2006;291(5):E937–E46. doi: 10.1152/ajpendo.00190.2006. [DOI] [PubMed] [Google Scholar]
- 134.Sheffield-Moore M, Paddon-Jones D, Sanford AP, Rosenblatt JI, Matlock AG, Cree MG, et al. Mixed muscle and hepatic derived plasma protein metabolism is differentially regulated in older and younger men following resistance exercise. Am J Physiol Endocrinol Metab. 2005;288(5):E922–9. doi: 10.1152/ajpendo.00358.2004. [DOI] [PubMed] [Google Scholar]
- 135.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(1):211–17. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mayhew D, Kim J, Cross J, Ferrando A, Bamman M. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. J Appl Physiol. 2009;107(5):1655–62. doi: 10.1152/japplphysiol.91234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20(2):768–71. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci. 2007;62(12):1407–12. doi: 10.1093/gerona/62.12.1407. [DOI] [PubMed] [Google Scholar]
- 139.Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJM, et al. Co-Ingestion of Leucine with Protein Does Not Further Augment Post-Exercise Muscle Protein Synthesis Rates in Elderly Men. Brit J Nutr. 2008;99(3):571–80. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- 140.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, et al. Blood Flow Restriction Exercise Stimulates Mtorc1 Signaling and Muscle Protein Synthesis in Older Men. J Appl Physiol. 2010;108:1199–1209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]