Abstract
Background
Various risk scoring systems have been recently developed to predict clinical outcomes in patients with upper gastrointestinal bleeding (UGIB). The two commonly used scoring systems include full Rockall score (RS) and the Glasgow-Blatchford score (GBS). Bleeding scores were assessed in terms of prediction of clinical outcomes in patients with UGIB.
Patients and methods
Two hundred patients (age >18 years) with obvious symptoms of UGIB in the emergency department of Rasoul Akram Hospital were enrolled. Full RS and GBS were calculated. We followed the patients for records of rebleeding and 1-month mortality. A receiver operating characteristic curve by using areas under the curve (AUCs) was used to statistically identify the best cutoff point.
Results
Eighteen patients were excluded from the study due to failure to follow-up. Rebleeding and mortality rate were 9.34% (n=17) and 11.53% (n=21), respectively. Regarding 1-month mortality, full RS was better than GBS (AUC, 0.648 versus 0.582; P=0.021). GBS was more accurate in terms of detecting transfusion need (AUC, 0.757 versus 0.528; P=0.001), rebleeding rate (AUC, 0.722 versus 0.520; P=0.002), intensive care unit admission rate (AUC, 0.648 versus 0.582; P=0.021), and endoscopic intervention rate (AUC, 0.771 versus 0.650; P<0.001).
Conclusion
We found the full RS system is better for 1-month mortality prediction while GBS system is better for prediction of other outcomes.
Keywords: full Rockall score, Glasgow-Blatchford score, gastrointestinal bleeding, mortality, prognosis
Introduction
Upper gastrointestinal bleeding (UGIB) is a life-threatening event with an overall incidence of 50 to 172 per 100,000 people leading a mortality rate of 2%–15% and a rebleeding rate of 10%–30%.1,2 Thus, proper management of this event with the purpose of lowering adverse consequences is clinically vital specially in UGIB based on higher mortality than lower gastrointestinal bleeding (GIB).3 Maintaining the hemodynamic stability is the first main approach to prevent adverse consequences in UGIB patients. Then diagnostic endoscopy and if needed intervention must be performed.4 The prognosis of UGIB ranges from mild self-limited symptoms to severe hemodynamic instability needs emergent resuscitation and also even leads to death.5 Moreover, urgent proper triage of the patients in emergency departments and also risk stratification according to risk scoring systems can help to identify patients for urgent diagnostic and therapeutic procedures such as endoscopy.6
Various risk scoring systems have been recently developed to categorize patients with UGIB to high-risk and low-risk subgroups. The two commonly used scoring systems include the full Rockall scoring (RS) system (with preendoscopic and endoscopic components which predict mortality) and the Glasgow-Blatchford scoring (GBS) system (with basic clinical and laboratory data and it assesses low-risk GIB that does not require intervention).7,8 In fact, these two systems are now recommended in the present guidelines to predict the needs for proper clinical interventions in patients with UGIB. However, the use of these scoring systems may be confounded by some subjective parameters opening potential interpretation.9 GBS system can predict outcomes of patients with UGIB based on subjective signs of UGIB such as syncope and melena, while the full RS system may use endoscopic criteria.10 Pulse rate, systolic blood pressure, blood urea nitrogen, hemoglobin, presentation of melena, hepatic disease, and cardiac failure are considered in GBS system. A score of 0 identifies low-risk patients who might be suitable for outpatient management, while scores of 6 or more are associated with a >50% risk of needing an intervention.6 Patient’s age, systolic blood pressure, pulse rate, presence of comorbid diseases, endoscopic finding as diagnosis, and stigmata of recent bleeding are considered in full RS system. Summing up the different levels of a point grading system assigned to each of the components yields a subject’s risk score bounded on a scale of 0 to 11, with 11 representing the highest risk.11 Therefore, the prognostic value of these tools may be affected by various eliminative factors. The present study aimed to assess how full RS and GBS systems can predict clinical outcomes of patients’ UGIB.
Methods
Two hundred patients with the age of >18 years who presented with obvious symptoms of UGIB including hematemesis (fresh blood or coffee-ground emesis), or melena in the emergency department of Rasoul Akram Hospital, enrolled in the study with written informed consent. This study was approved by the Ethics Committee of Iran University of Medical Sciences and observed the Declaration of Helsinki. Diagnostic endoscopy was performed for all assigned patients on admission day or during the hospital stay. Known-case of variceal bleeding patients and UGIB patients who received any treatment before admission were excluded. Demographic information, vital signs, physical exam findings, laboratory values, history of comorbid disease (e.g., renal, liver, cardiac, pulmonary disease, and cancer), and anticoagulant and/or antiplatelet drug users were recorded on admission. Endoscopic findings, rebleeding, need for endoscopic and/or surgical intervention, blood transfusion, and intensive care unit (ICU) admission were also recorded. In addition, we followed the cases for records of rebleeding and 1-month mortality.
Full RS and GBS systems were calculated for each patient. Pulse rate, systolic blood pressure, blood urea nitrogen, hemoglobin, presentation of melena, hepatic disease, and cardiac failure were recorded as variables of GBS system. Patient’s age, systolic blood pressure, pulse rate, presence of comorbid diseases, and endoscopic findings (diagnosis and stigmata of recent bleeding) were recorded as variables of full RS system.
Results were presented as mean ± standard deviation for quantitative parameters. Categorical variables were compared using chi-square test or Fisher’s exact test when more than 20% of cells with expected count of <5 were observed. Independent sample t-test was also used to compare the mean values of continuous independent variables between two groups. A receiver operating characteristic (ROC) curve was used to identify the best cutoff point in order to maximize the sensitivity and specificity of the two scoring systems to predict clinical outcomes (mortality, rebleeding, need for endoscopic intervention, blood transfusion, and ICU admission) of patients with UGIB. We used SPSS (SPSS Inc., Chicago, IL, USA) for statistical analysis. P-values of 0.05 or less were considered statistically significant.
Results
From 200 patients, 18 patients were excluded due to failure in their 1-month follow-up. Thus the data of 182 patients were analyzed (Figure 1). The baseline demographic, clinical, and laboratory data, endoscopic findings, and clinical outcomes are shown in Tables 1–3. Also, specificity and sensitivity of the two scoring systems in predicting clinical outcomes are shown in Table 4.
Figure 1.
Flowchart shows the process of enrolling cases.
Table 1.
Baseline demographic, clinical, and laboratory data of the cases
| Item | N (%) |
|---|---|
| Age, years (mean ± SD) | 62.04±18.56 |
| Sex (male) | 127 (63.5) |
| Smoking | 55 (27.5) |
| Chronic kidney disease | 3 (1.5) |
| Cardiovascular disease | 9 (4.5) |
| Liver disease | 5 (2.5) |
| Cancer | 1 (0.5) |
| Hb (mean ± SD) | 9.60±2.86 |
| Creatinine, mg/dL (mean ± SD) | 1.37±0.63 |
| SYS BP, mmHg (mean ± SD) | 115.64±19.44 |
| PR, bpm (mean ± SD) | 90.39±15.00 |
| Anticoagulant | 10 (5.5) |
| Antiplatelet | 92 (50.5) |
| GIB type | |
| Hematemesis | 53 (26.5) |
| Hematemesis-melena | 54 (27.0) |
| Hematochezia-melena | 3 (1.5) |
| Melena | 67 (33.5) |
Abbreviations: GIB, gastrointestinal bleeding; SD, standard deviation; SYS BP, systolic blood pressure; Hb, hemoglobin; PR, pulse rate.
Table 2.
Endoscopic findings and recent bleeding stigmata of cases
| Item | n=200 (%) |
|---|---|
| Endoscopic finding | |
| Normal condition | 27 (13.5) |
| Duodenal ulcer | 69 (34.5) |
| Gastric ulcer | 23 (21.5) |
| Gastric erosion | 39 (19.5) |
| Esophageal varices | 3 (1.5) |
| Endoscopic stigmata | |
| Active bleeding | 5 (3.6) |
| Blood oozing | 15 (7.5) |
| Clot | 21 (10.5) |
| Clear | 154 (77) |
Table 3.
Clinical outcomes of the patients
| Item | n=182 (%) |
|---|---|
| Mortality rate | 21 (11.53) |
| Rebleeding rate | 17 (9.34) |
| Need to transfusion | 32 (17.58) |
| ICU admission rate | 24 (12) |
| Endoscopy intervention | 29 (14.5) |
| APC and diluted adrenalin injection | 10 (5) |
| Band ligation | 3 (1.5) |
| Clips and diluted adrenalin injection | 5 (2.5) |
| Epinephrine | 8 (4.3) |
| Surgical intervention | 3 (1.5) |
Abbreviations: APC, argon plasma coagulation; ICU, intensive care unit.
Table 4.
Sensitivity and specificity of GBS and full RS in predicting outcomes.
| Outcomes | GBS
|
Full RS
|
||
|---|---|---|---|---|
| Sensitivity (%) |
Specificity (%) |
Sensitivity (%) |
Specificity (%) |
|
| One-month mortality | 71.4 | 43.6 | 5 | 44 |
| ICU admission | 87.5 | 48.9 | 62.5 | 54.9 |
| Rebleeding | 88.2 | 47.5 | 70 | 54.9 |
| Need to transfusion | 93.8 | 51.8 | 71.8 | 51.8 |
| Need to endoscopic intervention | 82.4 | 49.1 | 77.8 | 53.1 |
Abbreviations: ICU, intensive care unit; GBS, Glasgow-Blatchford score; RS, Rockall score systems.
The mean full RS was 3.85±1.55 and the mean GBS was 5.96±3.60. The most and the least frequent full RS scores were 4 (29.5%) and 0 (0.5%), respectively. The mean full RS score was significantly higher in nonsurvived patients in comparison with survived ones (4.52±1.66 versus 3.77±1.52, P=0.047). However, there is no significant difference between mean of GBS in nonsurvived and survived cases (7.14±3.39 versus 5.82±3.61, P=0.100). Also, the mean GBS was significantly higher in those who were admitted to ICU than those who were not (8.83±2.49 versus 5.56±3.55, P<0.001), whereas this was not true for full RS (4.04±1.71 versus 3.82±1.53, P=0.556). The mean GBS was significantly higher in the patients with rebleeding than other cases (8.41±1.66 versus 5.73±3.65, P<0.001) but regarding full RS, this was not significant (3.88±1.79 versus 3.85±1.53, P=0.992). Similarly, the mean GBS was significantly higher in the patients who were needing transfusion than the other cases (8.66±2.14 versus 5.44±3.59, P<0.001), while this was not significant in full RS (3.91±1.67 versus 3.84±1.53, P=0.930).
According to ROC curve analysis, the value of full RS to predict 1-month mortality was higher compared to the GBS system with area under the curve (AUC): 0.648 (P=0.026) versus 0.582 (P=0.217) (Figure 2).
Figure 2.
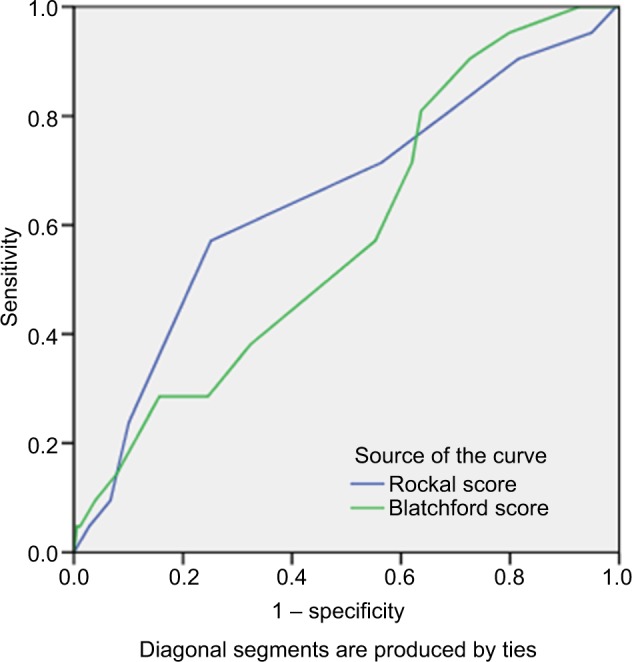
The area under the ROC curve to determine the value of full RS (0.648, cutoff =3.5, sensitivity: 71.4% and specificity: 43.6%) and GBS (0.582, cutoff =6.5, sensitivity: 5%, and specificity: 44%) for predicting 1-month mortality.
Abbreviations: GBS, Glasgow-Blatchford score; ROC, receiver operating characteristic; RS, Rockall score systems.
As shown in Figure 3, the value of GBS was higher than full RS in predicting ICU admission with AUC: 0.648 (P=0.026) versus 0.582 (P=0.217). The GBS system was also more valuable to predict the risk of rebleeding than the full RS with AUC: 0.722 (P=0.002) versus 0.520 (P=0.789) (Figure 4). Similarly, the GBS system was more valuable to predict those requiring transfusion compared to the full RS with AUC: 0.757 (P=0.001) versus 0.528 (P=0.620) (Figure 5). The GBS system was more valuable to predict those requiring endoscopic intervention compared to the full RS with AUC: 0.771 (P<0.001) versus 0.650 (P=0.008) (Figure 6).
Figure 3.
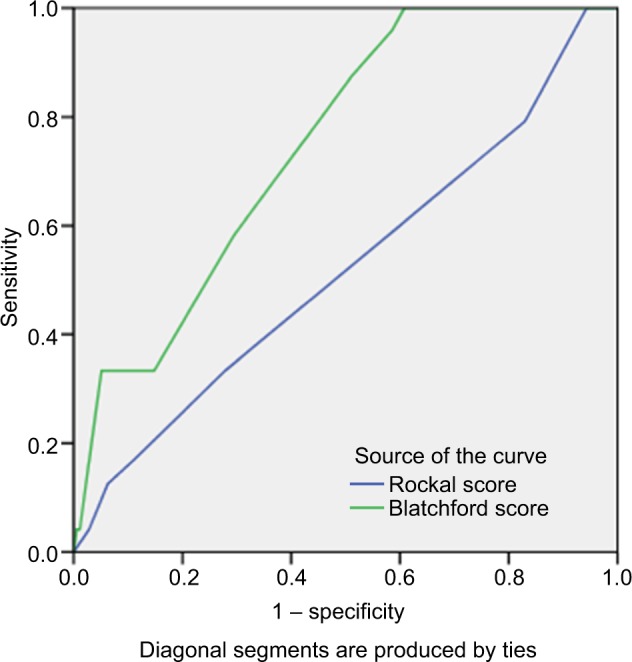
The area under the ROC curve to determine the value of GBS (0.745, cutoff =6.5, Sensitivity: 87.5%, specificity: 48.9%) and full RS (0.582 cutoff =2.5, sensitivity: 62.5%, specificity: 54.9%) for predicting ICU admission.
Abbreviations: ICU, intensive care unit; GBS, Glasgow-Blatchford score; ROC, receiver operating characteristic; RS, Rockall score systems.
Figure 4.
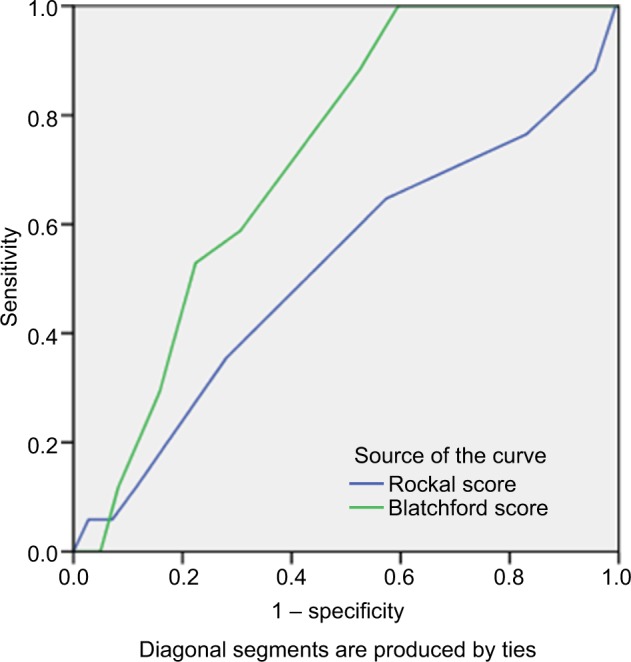
The area under the ROC curve to determine the value of GBS (0.72, cutoff =6.5, sensitivity: 88.2%, specificity: 47.5%) and full RS (0.520, cutoff =2.5, sensitivity: 70%, specificity: 54.9%) for predicting rebleeding.
Abbreviations: GBS, Glasgow-Blatchford score; ROC, receiver operating characteristic; RS, Rockall score systems.
Figure 5.
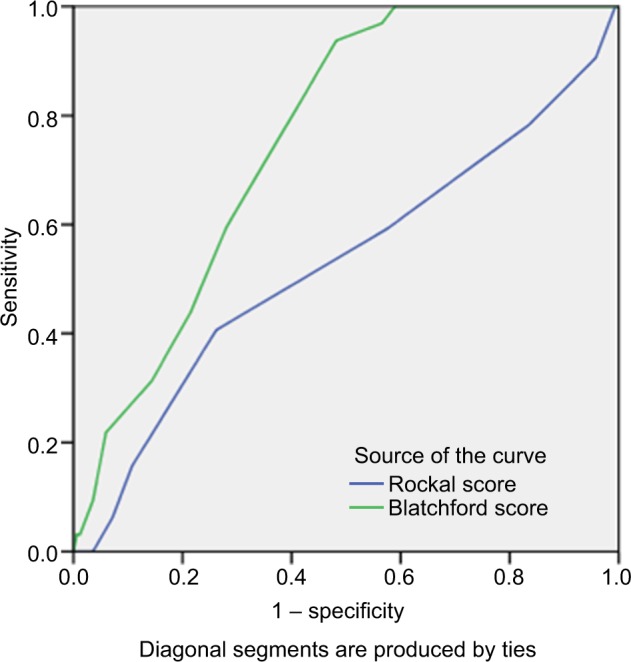
The area under the ROC curve to determine the value of GBS (0.757, cutoff =6.5, sensitivity: 93.8%, specificity: 51.8%) and full RS (0.528, cutoff =2.5, sensitivity: 71.8%, specificity: 51.8%) for predicting need for transfusion.
Abbreviations: GBS, Glasgow-Blatchford score; ROC, receiver operating characteristic; RS, Rockall score systems.
Figure 6.
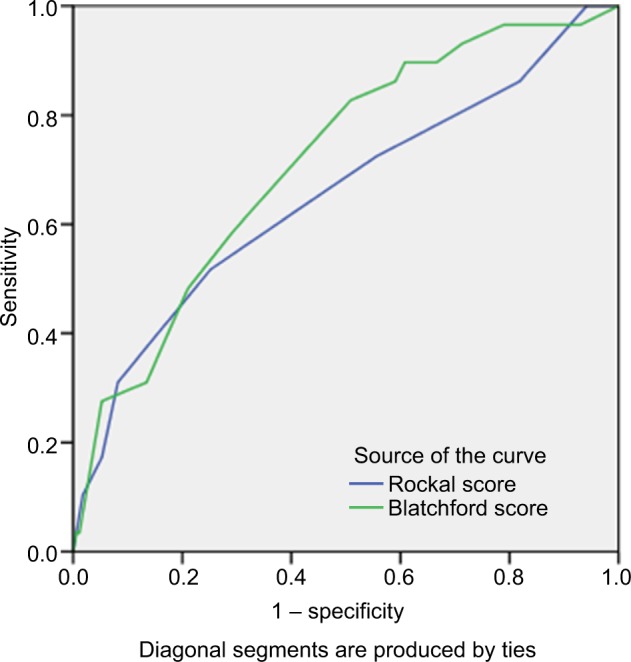
The area under the ROC curve to determine the value of GBS (0.771, cutoff =6.5, sensitivity: 82.4%, specificity: 49.1%) and full RS (0.650, cutoff =2.5, sensitivity: 77.8%, specificity: 53.1%) for predicting need for endoscopic intervention.
Abbreviations: GBS, Glasgow Blatchford score; ROC, receiver operating characteristic; RS, Rockall score systems.
Discussion
The present study attempted to assess and compare the value of two common applicable risk scoring systems including the full RS and the GBS systems to predict outcomes of patients with UGIB. The outcomes of UGIB were categorized as 1-month mortality, rebleeding, need for blood transfusion, endoscopic intervention, and ICU admission. Reviewing the literature achieved conflicting results as well as a variety of cutoff points for the two scoring system to predict clinical outcomes of UGIB. Bozkurt et al showed that the GBS score’s sensitivity for predicting rebleeding and need for endoscopic therapy was greater than those of other scoring systems, similar to our study. Also, they showed high sensitivity of two systems for predicting transfusion need, against our study.12 Both GBS and full RS systems were assessed in UGIB patients in Vietnam with similar results to predict clinical intervention need. None of them effectively excluded the need for endoscopic intervention.13 More interestingly, Yang et al in 2016 showed that the GBS was similar to the full RS in predicting the need for hospital-based intervention.14 In predicting mortality, the full RS was superior to the GBS that is same as our study. In terms of predicting rebleeding, the full RS system was superior to the GBS system, which is not the same as our study. As shown by Aquarius et al, the GBS system had the optimal combination of sensitivity (99.4%) and specificity (42.4%) at a cutoff value of up to 2 as the best determinative point for mortality prediction.15 Lee et al found that the full RS system was more accurate than the GBS system for predicting mortality, same as our study. However, neither the full RS system nor the GBS system could accurately predict rebleeding.16 Bryant et al showed that GBS and post endoscopy full RS scores were superior to preendoscopy full RS scores in predicting the need for endoscopic therapy and also rebleeding, however, the GBS was superior to full RS scores in predicting the need for blood transfusion.17 Similar to our study, Chandra et al showed that the prognostic accuracy of GBS and post endoscopy full RS was similarly high, but the specificity of both scores was suboptimal at all potential decision thresholds.18 Almost all similar studies could confirm high sensitivity but low specificity of two scoring systems to predict clinical outcome of UGIB. The GBS appears more accurate at identifying patients with low risk of requiring intervention or death than full RS score and therefore may be more accurate for use in clinical practice, allowing outpatient management in low-risk patients. However, there has been some debate as to the optimal GBS cutoff score for safely identifying this low-risk group. Many guidelines suggest that patients with a GBS of 0 can be safely managed as outpatients, but more recent studies have suggested that this threshold could potentially be safely increased to ≤1.19 In fact, systematically reviewed the literature may not achieve a global agreement to introduce a unique threshold for this scoring system to predict the outcome of UGIB. Although, the full RS remains important for risk assessment following endoscopy particularly as it includes the endoscopic diagnosis, introducing a unique cutoff threshold for this system could not be achieved yet.20 Clinical outcome predictions of the present study and other similar studies are shown in Table 5.
Table 5.
Clinical outcomes prediction of the present study and other similar studies
| Outcomes | The present study | Martinez-cara21 | Daniela Dicu RN22 | Stanley23 |
|---|---|---|---|---|
| Mortality rate | 10.5% AUC of RS =0.64 |
9.4% AUC of RS = GBS =0.71 |
18.7% AUC of RS = 0.82 |
4.8% AUC of GBS = RS =0.80 |
| Rebleeding rate | 8% AUC of GBS =0.72 |
4% AUC of GBS = RS =0.70 |
40% AUC of GBS = RS =0.73 |
– |
| Need for intervention | 14% AUC of GBS =0.71 |
40% AUC of GBS = 0.62 |
78% AUC of GBS = 0.86 |
14% AUC of GBS =0.85 |
| Needed transfusion | 16% AUC of GBS =0.75 |
62% AUC of GBS =0.85 |
52% AUC of GBS =0.88 |
23% AUC of GBS =0.93 |
Abbreviations: AUC, area under curve; GBS, Glasgow-Blatchford score; RS, Rockall score systems.
Limitations
Our study had some limitations. At the first stage of the study, six gastroenterologists performed 200 endoscopies. Thus, this procedure was not performed by only one gastroenterologist and this may have affected the full RS estimation. The other was failure to follow-up 1-month mortality rate and rebleeding detection in 18 cases (9%). Furthermore, it should be considered that GBS is more used for low-risk UGIB whereas full RS is more used for predicting mortality. However they were excluded from the study.
Conclusion
In conclusion, the full RS system seems to be better in 1-month mortality prediction. Furthermore, GBS system is better in predicting rebleeding, the need for ICU admission, blood transfusion, and endoscopic intervention in emergency departments. The cutoff points were considered for each system yielding high sensitivity but low specificity to predict these outcomes. More studies are needed to find an ultimate cutoff point for risk assessment of patients with UGIB.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Camellini L, Merighi A, Pagnini C, et al. Comparison of three different risk scoring systems in non-variceal upper gastrointestinal bleeding. Dig Liver Dis. 2004;36(4):271–277. doi: 10.1016/j.dld.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Köksal Ö, Özeren G, Özdemır F, Armağan E, Aydin Ş, Ayyildiz T. Prospective validation of the Glasgow Blatchford scoring system in patients with upper gastrointestinal bleeding in the emergency department. Turk J Gastroenterol. 23(5):448–455. doi: 10.4318/tjg.2012.0385. [DOI] [PubMed] [Google Scholar]
- 3.Göksu E, Erken Ö, Erçetin Y, Kılıçaslan I, Çete Y. Factors effecting mortality and demographic properties of patients presenting to the Emergency Department of Akdeniz University Hospital with upper gastrointestinal bleeding. Turk J Emerg Med. 2004;4:121–126. [Google Scholar]
- 4.Wang CH, Chen YW, Young YR, Yang CJ, Chen IC. A prospective comparison of 3 scoring systems in upper gastrointestinal bleeding. Am J Emerg Med. 2013;31(5):775–778. doi: 10.1016/j.ajem.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Lim JK, Ahmed A. Endoscopic approach to the treatment of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7(3):123–129. doi: 10.1053/j.tvir.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–1321. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 7.Farooq FT, Lee MH, Das A, Dixit R, Wong RCK. Clinical triage decision vs risk scores in predicting the need for endotherapy in upper gastrointestinal bleeding. Am J Emerg Med. 2012;30(1):129–134. doi: 10.1016/j.ajem.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Pang SH, Ching JYL, Lau JYW, Sung JJY, Graham DY, Chan FKL. Comparing the Blatchford and pre-endoscopic Rockall score in predicting the need for endoscopic therapy in patients with upper GI hemorrhage. Gastrointest Endosc. 2010;71(7):1134–1140. doi: 10.1016/j.gie.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Chen IC, Hung MS, Chiu TF, Chen JC, Hsiao CT. Risk scoring systems to predict need for clinical intervention for patients with non-variceal upper gastrointestinal tract bleeding. Am J Emerg Med. 2007;25(7):774–779. doi: 10.1016/j.ajem.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Cheng DW, Lu YW, Teller T, Sekhon HK, Wu BU. A modified Glasgow Blatchford score improves risk stratification in upper gastrointestinal bleed: a prospective comparison of scoring systems. Alimen Pharmacol Ther. 36(8):782–789. doi: 10.1111/apt.12029. [DOI] [PubMed] [Google Scholar]
- 11.Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38(3):316–321. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozkurt S, Köse A, Arslan ED, et al. Validity of modified early warning, Glasgow Blatchford, and pre-endoscopic Rockall scores in predicting prognosis of patients presenting to emergency department with upper gastrointestinal bleeding. Scand J Trauma Resusc Emerg Med. 2015;23(1):1–9. doi: 10.1186/s13049-015-0194-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quach DT, Dao NH, Dinh MC, et al. The performance of a modified Glasgow Blatchford score in predicting clinical interventions in patients with acute nonvariceal upper gastrointestinal bleeding: a Vietnamese prospective multicenter cohort study. Gut Liver. 2016;10(3):375–381. doi: 10.5009/gnl15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HM, Jeon SW, Jung JT, et al. Comparison of scoring systems for nonvariceal upper gastrointestinal bleeding: a multicenter prospective cohort study. J Gastroenterol Hepatol. 2016;31(1):119–125. doi: 10.1111/jgh.13057. [DOI] [PubMed] [Google Scholar]
- 15.Aquarius M, Smeets FG, Konijn HW, et al. Prospective multicenter validation of the Glasgow Blatchford bleeding score in the management of patients with upper gastrointestinal hemorrhage presenting at an emergency department. Eur J Gastroenterol Hepatol. 2015;27(9):1011–1016. doi: 10.1097/MEG.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Cheng CL, Liu NJ, et al. Comparison of Rockall and Blatchford scores to assess outcome of patients with bleeding peptic ulcers after endoscopic therapy. Hepatogastroenterology. 2013;60(128):1990–1997. [PubMed] [Google Scholar]
- 17.Bryant RV, Kuo P, Williamson K, Yam C, Schoeman MN, Holloway RH, Nguyen NQ. Performance of the Glasgow-Blatchford score in predicting clinical outcomes and intervention in hospitalized patients with upper GI bleeding. Gastrointest Endosc. 2013;78(4):576–583. doi: 10.1016/j.gie.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Chandra S, Hess EP, Agarwal D, et al. External validation of the Glasgow-Blatchford bleeding score and the Rockall score in the US setting. Am J Emerg Med. 2012;30(5):673–679. doi: 10.1016/j.ajem.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Waddell KM, Stanley AJ. Risk assessment scores for patients with upper gastrointestinal bleeding and their use in clinical practice. Hosp Pract. 2015;43(5):290–298. doi: 10.1080/21548331.2015.1103636. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro S, Gonçalves TC, Magalhães J, Cotter J. Upper gastrointestinal bleeding risk scores: who, when and why? World J Gastroint Pathophysiol. 2016;7(1):86–96. doi: 10.4291/wjgp.v7.i1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez-Cara JG, Jiménez-Rosales R, Úbeda-Muñoz M, de Hierro ML, de Teresa J, Redondo-Cerezo E. Comparison of AIMS65, Glasgow–Blatchford score, and Rockall score in a European series of patients with upper gastrointestinal bleeding: performance when predicting in-hospital and delayed mortality. United Eur Gastroenterol J. 2016;4(3):371–379. doi: 10.1177/2050640615604779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dicu D, Pop F, Ionescu D, Dicu T. Comparison of risk scoring systems in predicting clinical outcome at upper gastrointestinal bleeding patients in an emergency unit. Am J Emerg Med. 2013;31(1):94–99. doi: 10.1016/j.ajem.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Stanley AJ, Dalton HR, Blatchford O, et al. Multicentre comparison of the Glasgow Blatchford and Rockall scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther. 2011;34(4):470–475. doi: 10.1111/j.1365-2036.2011.04747.x. [DOI] [PubMed] [Google Scholar]


