Abstract
This study investigated the adjunctive effects of Antrodia cinnamomea mycelial fermentation broth (AC-MFB), a Taiwanese medicinal fungus, in enhancing the radiosensitivity of esophageal cancer cells. Human CE81T/VGH squamous and BE3 adenocarcinoma esophageal cancer cells were used in this study. A colony formation assay showed that pretreatment with AC-MFB decreased the survival of irradiated esophageal cancer cells, with a maximum sensitizer enhancement ratio of 1.91% and 37% survival. A DNA histogram study showed that AC-MFB pretreatment enhanced cell cycle arrest at the G2/M phase, the most radiosensitive phase. An immunofluorescence assay and a Western blotting assay showed that AC-MFB delayed the abrogation of γ-H2AX, upregulated p21 expression, and attenuated the radiation-induced phosphorylation of ataxia telangiectasia-mutated kinase and checkpoint kinase 2. An in vivo validation study showed that AC-MFB treatment tended to have a synergistic effect with radiation on the tumor growth delay of CE81T/VGH cells in BALB/c mice. These data suggest that this edible fungus product could enhance the effect of radiotherapy against esophageal cancer.
Keywords: mycelial, G2/M, fermentation broth, radiosensitization, p21
Introduction
Esophageal cancer is associated with a high mortality rate, with average 5-year survival rates not exceeding 25%.1 Locally advanced esophageal carcinoma is often refractory to current therapeutic approaches, and its prognosis is poor.2 Concurrent chemoradiotherapy (CCRT) is now recommended as a standard treatment for locally advanced, unresectable, or inoperable disease. Although various treatment methods, including surgical intervention, CCRT, and chemotherapy regimens, are available, esophageal cancer carries a very poor prognosis, with a median survival time of 29 months and 3- and 5-year survival rates of 44.7% and 36.8%, respectively.3,4 Cisplatin is the major chemotherapeutic drug most widely used for CCRT for esophageal cancer, for which extensive clinical evidence is available. However, the associated renal toxicity and resistance to this drug remain major concerns. Therefore, novel and potent compounds that can control or ameliorate both local and distant tumor progression in patients with esophageal cancer are urgently needed.
Antrodia cinnamomea, also known as niu-chang-chih, Taiwanofungus camphorate, or Antrodia camphorata, is a medicinal fungus indigenous to Taiwan that grows on decayed Cinnamomum kanehirai.5,6 A. cinnamomea has been shown to exhibit anticancer properties,7–9 and many studies have attempted to determine its exact bioactive compounds.10–12 For example, Chen et al has reported that the oral administration of A. cinnamomea fruiting bodies significantly increases the lifespan of ATCC BNL IMEA.7R.1 hepatoma-bearing mice.13 It has also been reported that polysaccharides from A. cinnamomea mycelia extract can inhibit the angiogenic activities of endothelial cells.14 A. cinnamomea crude extract has been reported to be an antimetastatic agent with antiproliferative activity that acts by inducing accumulation of cells in the G2/M phase in bladder cancer T24 cells.15 In addition, it has been shown to inhibit the growth of androgen-independent PC-3 prostate cancer cells via G2/M phase arrest mediated by the regulation of p21 and cyclin B1/Cdc2.16 Because cells in the G2/M phase are the most sensitive to radiation, the use of agents that cause G2/M arrest, such as A. cinnamomea extract, may represent a design strategy for the development of a novel radiosensitizer. Though we have shown that A. cinnamomea mycelial fermentation broth (AC-MFB), a biotechnological product, could inhibit the growth of hepatocellular carcinoma cells both in vivo and in vitro,17 the radiation enhancement effect of AC-MFB has never been studied. In the present study, we will investigate the role and possible mechanism of AC-MFB in enhancing the radiosensitivity of human esophageal cancer cell lines.
Materials and methods
Cell cultures
The cell lines used in this study included CE81T/VGH, an esophageal squamous cell line, and BE3, an adenocarcinoma (ADC) cell line. CE81T/VGH was kindly provided by Professor Cheng-Po Hu (Department of Life Science, Tunghai University, Taichung, Taiwan). BE3 esophageal ADC cells were kindly provided by Dr Wen-Chien Huang (Department of Chest Surgery, Mackay Memorial Hospital, Taipei, Taiwan). The cell lines were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM/high glucose, Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Biological Industries, Cromwell, CT, USA), 1× nonessential amino acids (Corning Incorporated, Corning, NY, USA), 1 mM sodium pyruvate (Corning Incorporated), and 5 μg/mL G418 (Sigma-Aldrich Co., St Louis, MO, USA) at 37°C in a humidified incubator under 5% CO2 and 95% air. Luciferase genes were transfected into both cell lines for optical imaging. The cells were passaged every 2–3 days upon reaching 80% confluence and were maintained in an exponential growth phase.
Plant materials, fermentation, and characterization
A. cinnamomea strain B137, identified by the fungi specialist Dr TT Chang (Taiwan Forestry Research Institute, Taipei, Taiwan), was maintained on pasteurized potato dextrose agar in Petri dishes and transferred to fresh medium at 1-month intervals. Mycelial agar discs (eight pieces, 0.5 cm each) were obtained with a sterilized tip and used to inoculate a shaking flask preculture. The preculture medium (LM-B) consisted of the following components (mg/mL): 30 glucose, 20 sucrose, 15 yeast extract, 13 peptone, 0.3 MgSO4, 0.3 KH2PO4, and 0.3 K2HPO4, with an initial pH of 4.0. For the preculture, 200 mL of medium was prepared in a 500 mL flask and inoculated, followed by a 7-day incubation at 28°C on a rotary shaker (100 rpm). For a series of experiments, 100 mL of medium (LM-B) was prepared in a 500 mL flask and inoculated with a mycelium suspension (6%) from the preculture broth, followed by a 14-day incubation at 28°C on a rotary shaker (100 rpm) with a final mycelial dry cell weight of 3.055 g/L and a pH of 3.88. The fermentation product was then harvested and poured through nonwoven fabric on a 20-mesh sieve to separate the deep-red fermented culture broth and mycelia, followed by centrifugation at 3,000× g for 10 minutes and then by a passage through a 0.22 μm pore-size filter. Next, the culture broth (1.0 L) was concentrated into a colloid (47.6 mg) under vacuum and stored at −30°C before analysis.17 The AC-MFB colloid was redissolved and directly used in the study without further extraction. The polysaccharide components of AC-MFB were used in quantitation analysis for standardization in this study. A phenol–sulfuric acid assay, a colorimetric method, was employed to determine the total concentration of carbohydrates present in AC-MFB.18–20
Assessment of cell viability
Cells were seeded at a density of 5×104 cells per well onto a 24-well plate (Orange Scientific, Braine-l’Alleud, Belgium) and incubated for 24 hours prior to drug treatment. The cells were harvested at 24–72 hours after treatment with various concentrations of AC-MFB. The 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium bromide (MTT) assay was used to determine cell viability.21 All tests and analyses were performed in triplicate.
Morphology
Liu’s Stain technique is a modified version of the Romanowsky Stain technique (Baso, Taichung City, Taiwan).22 For morphological observation, the cells were seeded onto a slide after treatment with AC-MFB or vehicle alone and were then stained with Liu’s Stain to observe morphological changes under an Olympus light microscope at a magnification of ×400.
Drug treatment and delivery of radiation
For radiation sensitization, the cells were pretreated with 0.5 mg/mL AC-MFB for 24 hours. They were then washed, and 6 MeV of electron-beam energy was delivered by a linear accelerator (Clinac 1800; Varian Associates, Inc., Palo Alto, CA, USA). The dose rate was 2.4 Gy/min, with doses of 0.5, 1, 2, 4, or 6 Gy administered in a single fraction. Full electron equilibrium was ensured for each fraction using a parallel-plate ionization chamber (PR-60C; Capintel, Inc., Ramsey, NJ, USA). After irradiation (IR), the cells were plated for a colony formation assay.
Colony formation assay
Viable tumor cells (200 for CE81T/VGH and 500 for BE3) were plated onto a six-well cell culture plate and allowed to grow in DMEM/high-glucose medium containing 10% FBS at 37°C in a humidified incubator with 5% CO2. After 14 days, the dishes were stained with 3% crystal violet. Colonies containing 50 cells or more were counted. The surviving fraction is the mean number of colonies/(the cells inoculated × the plating efficiency). The control plating efficiencies for the BE3 and CE81T/VGH cells ranged from 40% to 60%. Survival curves were fitted using a linear-quadratic model.23 The sensitizer enhancement ratio was calculated as the dose of radiation needed for IR alone divided by that necessary for AC-MFB plus IR to yield a surviving fraction of 37%.
D0 is the final slope of the survival curves fitted using a linear-quadratic model.
Flow cytometric analysis for cell cycle distribution
The flow cytometry assay was performed for the analysis of the effect of AC-MFB on cell cycle distribution.24 For cell cycle analysis, after 24 hours of treatment with AC-MFB and 24, 48, and 72 hours after radiation, the cells were harvested and fixed with 70% ethanol at 4°C overnight. They were then stained for 30 minutes with a solution containing 0.5 mg/mL propidium iodide and 2 mg/mL RNase obtained from a reagent kit (CycleTest Plus DNA; Becton Dickinson, Lincoln Park, NJ, USA). The DNA content was determined using a flow cytometer (FACSCalibur; Becton Dickinson). Data from 105 cells were collected and analyzed using software (ModFit; Becton Dickinson).
Measurement of apoptosis by flow cytometry
After incubation with various concentrations of AC-MFB for 24 hours, the cells were harvested, washed with phosphate-buffered saline (PBS), and resuspended (1×106 cells/mL) in Annexin-V-FLUOS labeling solution (Annexin-V-FLUOS staining kit, Roche Applied Science, Penzerg, Germany) for 15 minutes in the dark at 37°C. The fluorescence was analyzed using a FACSCalibur flow cytometer (Epics Altra, Beckman Coulter, Tainan, Taiwan). Green fluorescence was measured to indicate the proportion of cells undergoing apoptosis (fluorescein isothiocyanate-conjugated Annexin-V), and red fluorescence (propidium iodine) was measured to indicate the proportion of necrotic cells.
Immunofluorescence analysis of γ-H2AX expression
Phosphorylated γ-H2AX forms microscopically visible foci, and the number of phosphorylated γ-H2AX foci correlates well with the number of double-strand breaks induced by low-LET radiation.25 For the immunofluorescence staining of γ-H2AX, CE81T/VGH human esophageal cancer cells were treated with 0.5 mg/mL AC-MFB for 24 hours, IR (2 Gy), or AC-MFB plus IR. The cells were harvested from 5 minutes to 6 hours after exposure to radiation, washed, fixed in 4% paraformaldehyde for 10 minutes and permeabilized in 1% Triton-X-100 in ddH2O for 5 minutes. After being washed with wash buffer (PBS and 1% FBS), the cells were exposed to 4% FBS in PBS before incubation with a primary rabbit antihuman monoclonal antibody against H2AX phosphorylated at serine 139 (1:400 dilution; Cell Signaling, Danvers, MA, USA) and a rhodamine red-conjugated goat antirabbit immunoglobulin G secondary antibody (1:200 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA). The cells were then incubated in Hoechst 33342 (Sigma-Aldrich Co.) to stain the cell nuclei. The slides were observed using an Axiophot fluorescence microscope (Carl Zeiss Meditec AG, Jena, Germany) equipped with a digital camera system (Carl Zeiss AxioCam HRm, v.2.0, black and white version, Carl Zeiss Meditec AG). The experiments were repeated at least three times.
Western blot analysis
Western blotting is a widely used analytical technique for the detection of specific proteins in a sample of tissue homogenate or extract.26 For Western blot analysis, the cells were cultured with 1.0 mg/mL AC-MFB for 24 hours, 3 Gy of IR, or AC-MFB plus IR for an additional 1 or 6 hours. The cellular proteins were extracted, quantified, and subjected to gel electrophoresis using 5%–10% (wt/vol) sodium dodecyl sulfate polyacrylamide gels. The protein samples were then blotted onto a nitrocellulose membrane (BioTrace). Primary antibodies were used following dilution and were detected with a goat antimouse antibody (Merck Millipore, Darmstadt, Germany) or a goat antirabbit antibody (Jackson ImmunoResearch Laboratories, Inc.), after which enhanced chemiluminescence kits were used (T-Pro Biotechnology, New Taipei City, Taiwan). An antibody against actin was used as an internal control.
In vivo tumorigenesis and toxicity assay in mice
A subcutaneous xenograft animal model was used for a tumorigenesis assay. Specific pathogen-free male BALB/c nude mice (4 weeks old, 25–28 g) were obtained from the National Laboratory Animal Center (Taipei, Taiwan) and maintained under pathogen-free conditions. The animals were kept at our animal facility for at least 2 weeks before use. All of the mice were between 6 and 8 weeks of age and were cared for following the Guide for the Care and Use of Laboratory Animals (NIH publication no 85-23, revised in 1985). The mice were subcutaneously injected with 1×107 CE81T/VGH cells into the flank, and eight mice were placed into each group. The animals were treated with different protocols, including oral feeding by feeding tube with 100 μL of 1.0 mg/mL AC-MFB or with 100 μL of a normal saline vehicle for 34 days. Local radiotherapy was delivered at 3 Gy in three consecutive fractions on the first, third, and fifth days of treatment. The tumor size for each mouse was measured with a caliper and calculated by the following formula, where W is the shortest dimension and L is the longest dimension in centimeters: L × W2/2. The tumor size was estimated every 2 to 3 days after the size increased to 0.1 mm3. The white blood cell (WBC) count and body weight were used as markers for the toxicity assay. Blood samples were collected every 3 days to obtain WBC counts. The study and the protocol used in this study were approved by the Institutional Animal Care and Use Committee, MacKay Memorial Hospital, Taiwan (IACUC approval no MMH-A-S-99051).
Statistical analysis
The data are expressed as the mean ± standard error of the mean. Statistical software (Statistical Package for the Social Sciences, version 10.0; SPSS Inc., Chicago, IL, USA) was used to analyze the data. Significant differences (P<0.05) between the mean of the two test groups were analyzed by analysis of variance.
Results
Effect of AC-MFB pretreatment on the viability and radiosensitivity of esophageal cancer cells
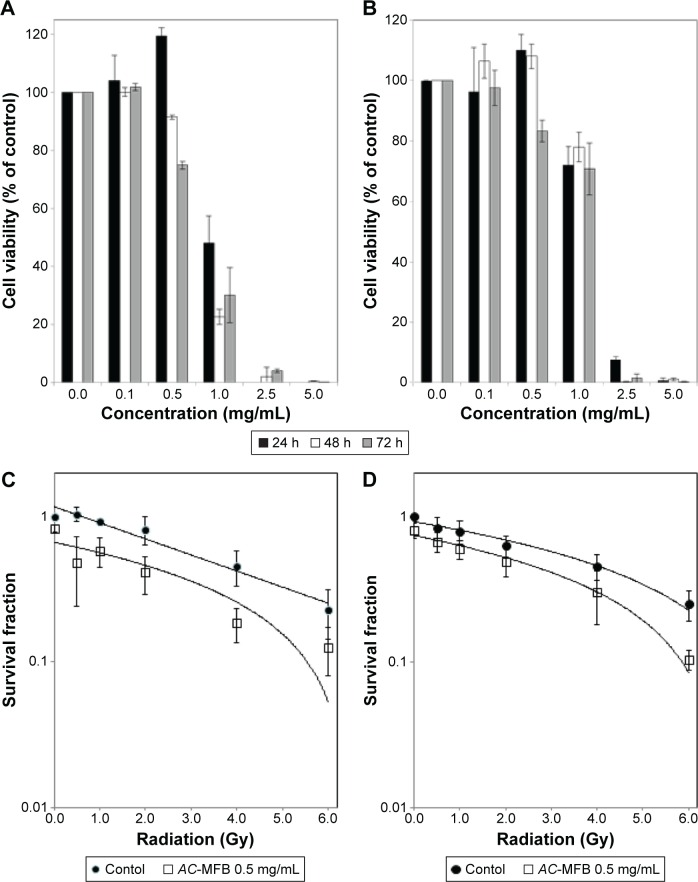
To evaluate the ability of AC-MFB to sensitize tumor cells to IR, nontoxic doses (resulting in cell viability of >75%) were estimated in vitro before the radiosensitization experiments. After 24 to 72 hours of exposure, AC-MFB inhibited the viability of both CE81T/VGH and BE3 cells in a time- and dose-dependent manner (P<0.001). The IC50 values of AC-MFB in CE81T/VGH and BE3 cells were 1.0 and 1.1 mg/mL, respectively. The highest nontoxic dose of AC-MFB for the CE81T/VGH and BE3 cells was 0.5 mg/mL at 24 hours (Figure 1A and B).
Figure 1.
Effects of AC-MFB on human esophageal cancer cells.
Notes: Pretreatment with AC-MFB inhibited cellular viability of CE81T/VGH SCC cells (A), BE3 ADC cells (B), and enhanced radiation effects on the CE81T/VGH SCC cells (C) and BE3 ADC cells (D). Both cells were treated with 0.1–5.0 mg/mL of AC-MFB for 24, 48, and 72 h, then determined using an MTT assay for cell viability assay. In radiation sensitivity study, cells were treated with 0.5 mg/mL AC-MFB for 24 h before irradiation. Radiation doses of 0.5, 1, 2, 4, or 6 Gy were given in a single fraction. Data are expressed as mean ± SD for three independent experiments.
Abbreviations: AC-MFB, Antrodia cinnamomea mycelial fermentation broth; ADC, adenocarcinoma; Gy, gray; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazo; h, hours; SCC, squamous cell carcinoma; SD, standard deviation.
IR of untreated CE81T/VGH and BE3 cells at doses ranging from 0 to 6 Gy reduced the surviving fractions to 23% and 25%, respectively. Pretreatment with 0.5 mg/mL AC-MFB markedly decreased the survival of irradiated CE81T/VGH tumor cells (Figure 1C and D). The sensitizer enhancement ratios were 1.91 and 1.54 for CE81T/VGH and BE3 cells, respectively, at an AC-MFB dose of 0.5 mg/mL.
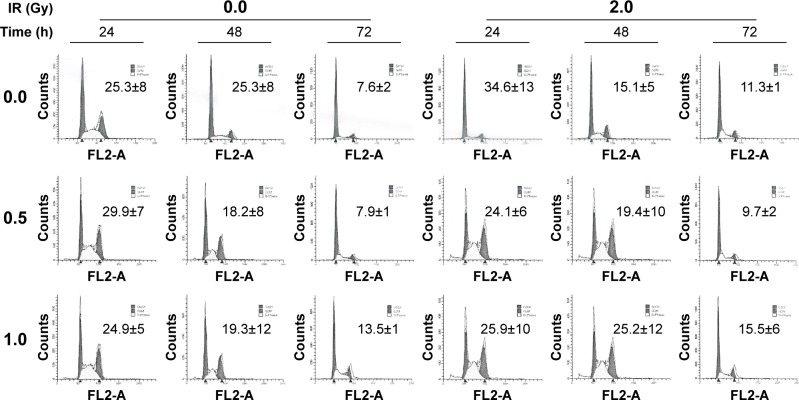
Effect of AC-MFB on the cell cycle distribution of CE81T/VGH cells
We evaluated the effect of AC-MFB on cell cycle distribution under the same conditions that affected radiosensitivity using DNA histograms. After 24 hours of treatment with 0.5 or 1.0 mg/mL AC-MFB, the proportion of cells in the G0/G1 phase was decreased compared with the proportion of G0/G1 cells in the untreated cells. In contrast, the percentage of cells in the S phase slightly increased (Figure 2). The proportion of cells in the G2/M phase was markedly sustained at 25.3%±8% to 10.9%±4% versus 24.9%±5% to 19.3%±12% without or with 1.0 mg/mL AC-MFB pretreatment, respectively. IR affected the cell cycle distribution by rapidly increasing the proportion of cells in the G2/M phase at 24 hours (from 25.3% to 34.6%), followed by marked decreases at 48 hours (15.1%) and 72 hours (11.3%), indicating the occurrence of a DNA damage response followed by repair. In contrast, pretreatment with AC-MFB maintained the proportion of G2/M cells at 25.9% at 24 hours and 25.2% at 48 hours, whereas the proportion decreased to 15.5% at 72 hours in the irradiated cells. These findings indicate that AC-MFB pretreatment may block the restoration of the cell cycle after radiation by affecting DNA damage repair.
Figure 2.
Effect of AC-MFB on cell cycle.
Notes: CE81T/VGH cells that were not treated or treated with IR with 2 Gy, 0.5 or 1.0 mg/mL AC-MFB plus IR. Representative flow cytometric analysis for cell cycle distribution 24, 48, and 72 h after radiation was demonstrated. Numeric data represented the percentage of G2/M phase. Data are expressed as mean ± SD for three independent experiments.
Abbreviations: AC-MFB, Antrodia cinnamomea mycelial fermentation broth; Gy, gray; h, hours; IR, irradiation; SD, standard deviation.
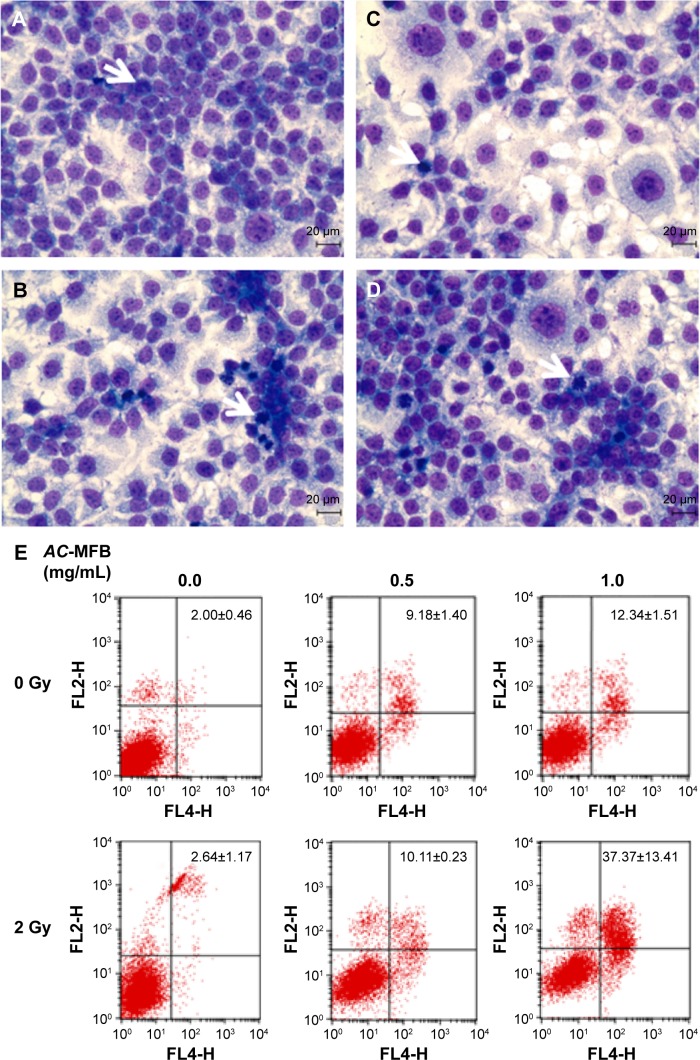
AC-MFB induces the apoptosis of CE-81T/VGH cells
In accordance with the changes in the DNA histogram, IR caused moderate mitotic arrest in CE-81T/VGH cells, and AC-MFB pretreatment further enhanced the accumulation of mitotically arrested cells at 24 hours (Figure 3A–D). The Annexin-V assay showed that AC-MFB increased the apoptosis rate from 2.00%±0.46% to 9.18%±1.40% and 13.34%±1.51% with 0.5 and 1.0 mg/mL AC-MFB, respectively (P=0.001 and P=0.0003) (Figure 3E). There was also a statistically significant synergistic increase in the apoptosis rate noted from 2.64%±1.17% to 10.11%±0.23% with IR and 0.5 mg/mL AC-MFB and to 37.37%±13.41% with IR and 1.0 mg/mL AC-MFB (P=0.0004 and P=0.011).
Figure 3.
AC-MFB induced mitotic arrest morphology change and apoptosis of CE81T/VGH cell.
Notes: Cells were not treated (A, control) or treated with 0.5 and 1.0 mg/mL AC-MFB for 24 h (B), irradiation of 2 Gy (C), or AC-MFB plus irradiation (D). Arrows indicate representative cells characteristic of mitotic arrest. (E) Cellular apoptotic percentages induced by AC-MFB. Cell image was obtained by Liu’s Stain and microscopy at magnification ×400. Cell apoptosis percentages were determined by flow cytometry with Annexin-V/PI staining. Data are expressed as mean ± SD for three independent experiments.
Abbreviations: AC-MFB, Antrodia cinnamomea mycelial fermentation broth; Gy, gray; h, hours; PI, propidium iodine; SD, standard deviation.
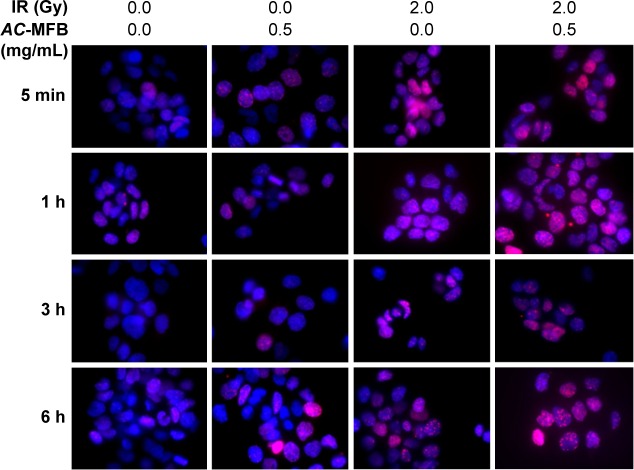
Effect of AC-MFB on the regulation of the DNAdamage-repair machinery
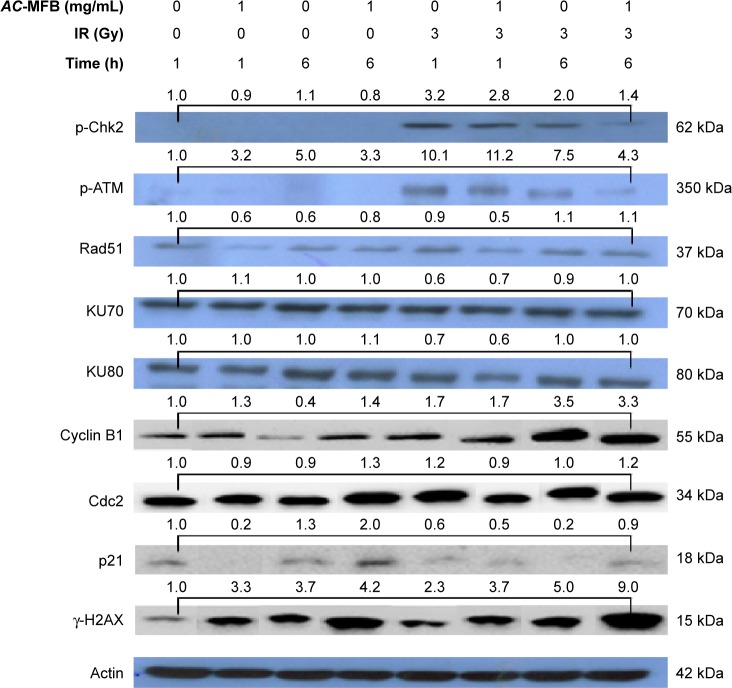
Pretreatment with AC-MFB delayed the abrogation of the IR-induced expression of γ-H2AX, an indicator of DNA double-strand breaks, as demonstrated by immunofluorescence (Figure 4). The Western blot assay also showed that AC-MFB could significantly increase γ-H2AX expression, especially at 1 hour in cells treated with AC-MFB alone; a synergistic effect was also observed with IR at 6 hours (P=0.013 and 0.008, respectively) (Figure 5). Using Western blotting, we also found that IR induced the extensive expression of phosphorylated ataxia telangiectasia-mutated (ATM) kinase at 1 hour (P=0.018). Pretreatment with 1.0 mg/mL AC-MFB for 24 hours markedly reduced this induction at 6 hours compared to IR alone (P=0.006) (Figure 5). IR also augmented and AC-MFB pretreatment reduced the phosphorylation of checkpoint kinase 2 (Chk2), a downstream DNA damage-repair molecule, although no significant difference was observed with AC-MFB pretreatment at 1 and 6 hours compared to IR alone (P=0.39 and P=0.29, respectively). AC-MFB had little inhibitory effect on the expression of Rad51 (P=0.25 and P=0.15, at 1 and 6 hours, respectively). There was no effect observed on the expression of Ku70 or Ku80 in irradiated cells pretreated with AC-MFB. The Western blot assay also showed that pretreatment with AC-MFB alone could not only increase p21 expression at 6 hours but also had a synergistic effect with IR at 6 hours (P<0.05). Pretreatment with AC-MFB could also increase cyclin B1 expression at 6 hours, but no enhancement was noted with IR. There was no effect on the expression of Cdc2 in irradiated cells treated with AC-MFB (Figure 5).
Figure 4.
AC-MFB delayed the abrogation of IR-induced expression of γ-H2AX on CE81T/VGH cell.
Notes: Cells were untreated (control) or treated with 0.5 mg/mL AC-MFB for 24 h, IR of 2 Gy, or AC-MFB plus IR. For immunofluorescent staining, cells were harvested 5 min, 1, 3, and 6 h after radiation, stained with γ-H2AX antibody, secondary Rhodamine red-conjugated antibody (red), and counter-stained with 4′,6-diamidino-2-phenylindole (blue). Original magnification ×400. The time showed represent the time points of cells harvested after radiation.
Abbreviations: AC-MFB, Antrodia cinnamomea mycelial fermentation broth; Gy, gray; h, hours; IR, irradiation; min, minutes.
Figure 5.
AC-MFB regulated p21 expression, phosphorylation of ATM kinase, and Chk2.
Notes: Cells were treated with vehicle (control), 1.0 mg/mL AC-MFB for 24 h, IR of 3 Gy, or AC-MFB plus IR and then were lysed for the determination of the indicated protein levels by immunoblotting. Actin was used as internal control. The apparent molecular weights for detected proteins are indicated. The numeric data above the bar means the mean folds of Western blot data in different conditions compared to cells that were harvested at 0 min.
Abbreviations: AC-MFB, Antrodia cinnamomea mycelial fermentation broth; ATM, ataxia telangiectasia-mutated kinase; Chk2, checkpoint kinase 2; Gy, gray; h, hours; IR, irradiation; p-ATM, phosphorylated ATM; p-ChK2, phosphorylated Chk2.
Effect of AC-MFB on tumor growth inhibition in mice
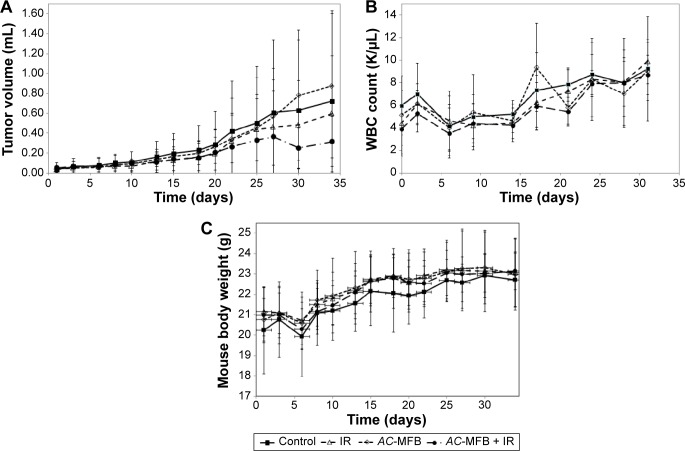
In nude BALB/c mice-bearing CE81T/VGH esophageal cancer cells, the oral intake of 100 μL AC-MFB (1.0 mg/mL) for 34 days had a synergistic effect on radiation therapy, as shown by a delay in tumor growth. For tumors that increased in volume by fivefold, the tumor growth delay was 19.0 days following treatment with AC-MFB alone, 19.6 days following radiation alone, and 22.5 days following combination treatment, compared with 15.2 days in the control group (Figure 6A). There were no significant differences in the WBC counts or body weights of the mice during the treatment (Figure 6B and C).
Figure 6.
AC-MFB effects in an animal model.
Notes: The results of AC-MFB on tumor growth (A), WBC count (B), and body weight (C) in an animal model. CE81T/VGH-bearing BALB/C nude mice were treated with vehicle (control), oral feeding by feeding tube with 100 μL of 1.0 mg/mL AC-MFB for 34 days, 3Gy ×3 fraction in first week, or AC-MFB plus IR. Data from eight mice in each group are presented as mean (SEM).
Abbreviations: AC-MFB, Antrodia cinnamomea mycelial fermentation broth; IR, irradiation; SEM, standard error of the mean; WBC, white blood cell.
Discussion
Our results demonstrated that AC-MFB sensitized human esophageal cancer CE81T/VGH (squamous cell carcinoma [SCC]) and BE3 (ADC) cells to IR. This effect may involve the induction of G2/M cell cycle arrest and DNA damage-repair machinery by IR. The modulation of the cyclin-dependent protein kinase inhibitor p21 and the IR-induced activation of ATM and Chk2 in CE81T/VGH cells were noted. This radiosensitization by AC-MFB was validated using an in vivo xenograft model of esophageal cancer.
The G2/M DNA damage checkpoint serves to prevent the cell from entering mitosis (M phase) with genomic DNA damage and, therefore, the G2/M phase is the most sensitive phase to radiation.27,28 Numerous proteins and kinases are involved in the cell cycle; cyclin B1 and Cdc2 are the main proteins that control the G2/M phase, which is essential for mitosis.29 p21 is a potent cyclin-dependent kinase inhibitor that can inhibit Cdc2 activity to cause G2 arrest.30 Our results showed that though AC-MFB upregulated cyclin B1 expression at 6 hours, p21 expression was markedly upregulated at 6 hours, which caused G2/M arrest.
Cancer radiotherapy and chemotherapy are designed to kill cancer cells, mainly by inducing DNA damage that must be repaired for tumors to continue growing. The inhibition of DNA repair may enhance therapeutic effects in combination with DNA-damaging therapy, leading to increased tumor cell death.31 Variations in the genes involved in DNA repair are important prognostic factors associated with the overall survival and progression-free survival of esophageal cancer patients treated with cisplatin.32 The capacity of cancer cells to repair IR-induced DNA damage has been demonstrated to be associated with treatment resistance, especially in esophageal cancer.33 According to the DNA histogram, IR-mediated cell cycle arrest at G2/M was sustained by AC-MFB priming, indicating a possible block in the progression through checkpoints. The results of our assessment of DNA double-strand breakage, as measured by the expression of γ-H2Ax, indicate that AC-MFB may increase this lethal type of DNA damage and impair DNA repair. Among the complicated processes involved in DNA damage repair, we found that the phosphorylation of Chk2 and ATM induced by IR was suppressed by pretreatment with AC-MFB. Unequal changes in the expression of p-Chk2 and p-ATM were noted, which might have occurred because ATM has more complicated functions than p-Chk2 and plays roles in processes other than the DNA damage response.34 Given that ATM and Chk2 act as sensors and transducers of DNA damage, AC-MFB may affect DNA damage repair during an earlier phase of the cell cycle. Although the expression of Rad51 is known to be cell cycle-dependent and is higher in the S and G2/M phases than in the G0/G1 phase,35 as noted in our cell cycle experiments, there was no significant inhibition of Rad51 observed in this study based on our Western blotting experiments.
Taken together, these results suggest that AC-MFB may sensitize esophageal cancer cells to radiation therapy, accompanied by both the upregulation of p21 expression-induced G2/M arrest and the modulation of DNA damage repair induced by IR.
Although surgical resection is the optimal treatment for patients with localized cancer of the esophagus, only 20% of patients are found to have truly localized esophageal cancer at diagnosis.36 In clinical practice, IR plays an important role in the management of the majority of esophageal cancer patients with unresectable or locally advanced disease. However, long-term survival is still rarely achieved. RTOG trial 85-01 and others have demonstrated the survival benefits of CCRT, but the high local regional relapse rates and mortality rates remain unsatisfactory.37 The incidence of esophageal ADC has risen rapidly over the past 25 years in the US as well as in several Western European countries. In contrast, the major histological type of esophageal cancer in Asian countries is SCC. To address this discrepancy, we examined the inhibitory effects of AC-MFB against esophageal cancer using both an SCC-derived cell line (CE81T/VGH) and an ADC-derived cell line (BE3). Our in vitro results showed that AC-MFB could inhibit cell viability and enhance the effects of IR in both SCC and ADC. Pretreatment with 0.5 mg/mL AC-MFB had more robust inhibitory effects on radiation survival in SCC compared with ADC cells, with a higher sensitizer enhancement ratio (1.91 vs 1.54, respectively). In the cell cycle distribution assay, pretreatment with 1.0 mg/mL AC-MFB maintained the proportion of G2/M cells at 25.9% at 24 hours and 25.2% at 48 hours, whereas the proportion decreased to 15.5% at 72 hours in CE81T/VGH cells after IR. Therefore, we will focus on the synergistic effects of IR and AC-MFB on esophageal SCC in a future animal study.
A. cinnamomea is a medicinal fungus commonly used in traditional Chinese medicine in Taiwan. A mixture containing an A. cinnamomea extract prepared as an herbal formulation in traditional Chinese medicine may have the advantage of multiple target regulation, in contrast with single-target antagonists. Several problems, including unknown multiple constituent mixtures, an unknown mechanism of action, variable raw material quality, and difficult analytical methods, limit its further research and clinical use. The limitation of our study is that although AC-MFB is considered to be a biotechnological product with stable quality in terms of harvesting, drying, storage, transportation, and processing, it contains multiple constituents and thus may act through multiple mechanisms.
The arrest of cell cycle progression in G2/M, which is the most radiosensitive phase, is one strategy to sensitize tumor cells to radiation. AC-MFB augmented radiation-induced cell cycle arrest at the G2/M phase at 48 hours, thus making the cells more sensitive to the subsequent dose of radiation during fractionated radiotherapy.
Our results showed that AC-MFB may sensitize CE81T/VGH and BE3 human esophageal cancer cells to IR. This effect may involve the modulation of p21-mediated G2/M arrest and IR-induced ATM and Chk2 activation during the repair of DNA double-strand breaks.
Conclusion
AC-MFB, a medicinal fungus from Taiwan, synergistically enhanced the radiosensitivity of esophageal cancer cell lines. In both human CE81T/VGH squamous and BE3 ADC esophageal cancer cells, our results showed that AC-MFB pretreatment could enhance cell cycle arrest at the G2/M phase, delay the abrogation of γ-H2AX, and attenuate the radiation-induced phosphorylation of ATM kinase and Chk2. In addition, an in vivo study showed that AC-MFB treatment tended to have a synergistic effect with radiation on the tumor growth delay of CE81T/VGH cells in BALB/c mice. Subsequent clinical trials evaluating the radiosensitization effect and the toxicity of AC-MFB in esophageal cancer are warranted.
Acknowledgments
This work was supported by grants V104C-093 from Taipei Veterans General Hospital, CMRPD3E0281 from Chang Gung Memorial Hospital, and MOST103-2314-B-195-013-MY3 from the Ministry of Science and Technology, Taiwan. We declare that this work has not been published or submitted elsewhere for review. The manuscript has been checked by language editing services (American Journal Experts 80B4-2758-1158-93F0-D4E3).
Footnotes
Author contribution
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25(10):1160–1168. doi: 10.1200/JCO.2005.04.7118. [DOI] [PubMed] [Google Scholar]
- 3.Lin CC, Hsu CH, Cheng JC, et al. Concurrent chemoradiotherapy with twice weekly paclitaxel and cisplatin followed by esophagectomy for locally advanced esophageal cancer. Ann Oncol. 2007;18(1):93–98. doi: 10.1093/annonc/mdl339. [DOI] [PubMed] [Google Scholar]
- 4.Kato K, Muro K, Minashi K, et al. Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group (JCOG) Phase II study of chemoradiotherapy with 5-Fluorouracil and cisplatin for stage II–III esophageal squamous cell carcinoma: JCOG Trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81(3):684–690. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Chang TT, Chou WN. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol Res. 1995;99(6):756–758. [Google Scholar]
- 6.Sheng-Hua W, Zhi-He Y, Yu-Cheng D, et al. Taiwanofungus, a polypore new genus. Fung Sci. 2004;19(3–4):109–116. [Google Scholar]
- 7.Hsu YL, Kuo YC, Kuo PL, Ng LT, Kuo YH, Lin CC. Apoptotic effects of extract from Antrodia camphorata fruiting bodies in human hepatocellular carcinoma cell lines. Cancer Lett. 2005;221(1):77–89. doi: 10.1016/j.canlet.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Hsu YL, Kuo PL, Cho CY, et al. Antrodia cinnamomea fruiting bodies extract suppresses the invasive potential of human liver cancer cell line PLC/PRF/5 through inhibition of nuclear factor kappa B pathway. Food Chem Toxicol. 2007;45(7):1249–1257. doi: 10.1016/j.fct.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Cheng PC, Huang CC, Chiang PF, et al. Radioprotective effects of Antrodia cinnamomea are enhanced on immune cells and inhibited on cancer cells. Int J Radiat Biol. 2014;90(10):841–852. doi: 10.3109/09553002.2014.911989. [DOI] [PubMed] [Google Scholar]
- 10.Yang SW, Shen YC, Chung-Siung C. Steroids and triterpenoids of Antrodia cinnamomea – a fungus parasitic on Cinnamomum micranthum. Phytochemistry. 1996;41(5):1389–1392. [Google Scholar]
- 11.Hsiao G, Shen ML, Lin KH, et al. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J Agric Food Chem. 2003;51(11):3302–3308. doi: 10.1021/jf021159t. [DOI] [PubMed] [Google Scholar]
- 12.Geethangili MT, Tzeng YM. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds. Evid Based Complement Alternat Med. 2009;2011:212641. doi: 10.1093/ecam/nep108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YY, Chang HH, Lee HY, Sheu F. Immunomodulatory and anti-tumor effects of oral administration with Antrodia cinnamomea fruiting bodies in BALB/c Mice. Taiwan J Agric Chem Food Sci. 2008;46:87–95. [Google Scholar]
- 14.Cheng JJ, Huang NK, Chang TT, Wang DL, Lua MK. Study for anti-angiogenic activities of polysaccharides isolated from Antrodia cinnamomea in endothelial cells. Life Sci. 2005;76(26):3029–3042. doi: 10.1016/j.lfs.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Peng CC, Chen KC, Peng RY, Su CH, Hsieh-Li HM. Human urinary bladder cancer T24 cells are susceptible to the Antrodia camphorata extracts. Cancer Lett. 2006;243(1):109–119. doi: 10.1016/j.canlet.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Chen KC, Peng CC, Peng RY, et al. Unique formosan mushroom Antrodia camphorata differentially inhibits androgen-responsive LNCaP and -independent PC-3 prostate cancer cells. Nutr Cancer. 2007;57(1):111–121. doi: 10.1080/01635580701268360. [DOI] [PubMed] [Google Scholar]
- 17.Liu YM, Liu YK, Lan KL, Lee YW, Tsai TH, Chen YJ. Medicinal fungus Antrodia cinnamomea inhibits growth and cancer stem cell characteristics of hepatocellular carcinoma. Evid Based Complement Alternat Med. 2013;2013:569737. doi: 10.1155/2013/569737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 19.Liu CJ, Chiang CC, Chiang BH. The elicited two-stage submerged cultivation of Antrodia cinnamomea for enhancing triterpenoids production and antitumor activity. Biochem Eng J. 2012;64:48–54. [Google Scholar]
- 20.Ma TW, Lai Y, Chen LT, Yang FC. The cultivation strategy of enhancing triterpenoid production in submerged cultures of Antrodia cinnamomea by adding monoterpenes. J Taiwan Inst Chem Eng. 2016;58:210–218. [Google Scholar]
- 21.Plumb JA, Milroy R, Kaye SB. Effects of the pH dependence of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide-formazan absorption on chemosensitivity determined by a novel tetrazolium-based assay. Cancer Res. 1989;49(16):4435–4440. [PubMed] [Google Scholar]
- 22.Liu C. A new rapid method of staining thin blood film: first report. J Formasa Med Assoc. 1953;52:348–352. [Google Scholar]
- 23.Brenner DJ, Hlatky LR, Hahnfeldt PJ, Huang Y, Sachs RK. The linear-quadratic model and most other common radiobiological models result in similar predictions of time-dose relationships. Radiat Res. 1998;150(1):83–91. [PubMed] [Google Scholar]
- 24.Juan G, Traganos F, Darzynkiewicz Z. Methods to identify mitotic cells by flow cytometry. Meth Cell Biol. 2001;63:343–354. doi: 10.1016/s0091-679x(01)63019-x. [DOI] [PubMed] [Google Scholar]
- 25.Ismail IH, Wadhra TI, Hammarsten O. An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res. 2007;35(5):e36. doi: 10.1093/nar/gkl1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terasima T, Tolmach LJ. X-ray sensitivity and DNA synthesis in synchronous populations of HeLa cells. Science. 1963;140(3566):490–492. doi: 10.1126/science.140.3566.490. [DOI] [PubMed] [Google Scholar]
- 28.Sinclair WK, Morton RA. X-ray sensitivity during the cell generation cycle of cultured Chinese hamster cells. Radiat Res. 1966;29(3):450–474. [PubMed] [Google Scholar]
- 29.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes, and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11(2):211–219. [PubMed] [Google Scholar]
- 30.Smits VA, Klompmaker R, Vallenius T, Rijksen G, Mäkela TP, Medema RH. p21 inhibits Thr161 phosphorylation of Cdc2 to enforce the G2 DNA damage checkpoint. J Biol Chem. 2000;275(39):30638–30643. doi: 10.1074/jbc.M005437200. [DOI] [PubMed] [Google Scholar]
- 31.Helleday T, Lo J, van Gent DC, Engelward BP. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair. 2007;6(7):923–935. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Bradbury PA, Kulke MH, Heist RS, et al. Cisplatin pharmacogenetics, DNA repair polymorphisms, and esophageal cancer outcomes. Pharmacogenet Genomics. 2009;19(8):613–625. doi: 10.1097/FPC.0b013e32832f3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121(8):1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 34.Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37(1):15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen F, Nastasi A, Shen Z, Brenneman M, Crissman H, Chen DJ. Cell cycle-dependent protein expression of mammalian homologs of yeast DNA double-strand break repair genes Rad51 and Rad52. Mutat Res. 1997;384(3):205–211. doi: 10.1016/s0921-8777(97)00020-7. [DOI] [PubMed] [Google Scholar]
- 36.Pearson JG. The present status and future potential of radiotherapy in the management of esophageal cancer. Cancer. 1977;39(2 Suppl):882–890. doi: 10.1002/1097-0142(197702)39:2+<882::aid-cncr2820390726>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 37.al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: an intergroup study. J Clin Oncol. 1997;15(1):277–284. doi: 10.1200/JCO.1997.15.1.277. [DOI] [PubMed] [Google Scholar]