Abstract
Patients with active bleeding complications who concomitantly develop overt pulmonary embolism (PE) present distinct therapeutic dilemmas, since they are perceived to be at substantial risk for the progression of the embolism in the absence of treatment and for aggravation of the hemorrhagic lesions if treated with anticoagulants. A 76-year-old patient with nephrotic syndrome, which is associated with an increased risk of thromboembolism, concurrently developed acute PE and intracranial bleeding because of traumatic brain injury. In this case, we prioritized the treatment for PE with the intravenous unfractionated heparin followed by warfarinization. Despite the transient hemorrhagic progression of the brain contusion after the institution of anticoagulation, our patient recovered favorably from the disease without any signs of neurological compromise. Several conundrums regarding anticoagulation that emerged in this case are also discussed.
Keywords: nephrotic syndrome, therapeutic anticoagulation, venous thromboembolism, intracranial bleeding, traumatic brain injury
Introduction
An association between excessive clotting disorders and nephrotic syndrome (NS), which is characterized by heavy proteinuria, hypoalbuminemia, hyperlipidemia, and edema, has been shown for years.1–3 The exact pathogenesis of such a relationship is poorly understood. However, it is likely to be precipitated by hypercoagulable states mediated by the increased synthesis of thrombosis-promoting factors as well as the preferential loss of proteins involved in the systemic hemostatic cascade resulting from the disease.3 Therefore, pharmacological control of blood coagulation has been an imperative therapeutic option among nephrotic patients with overt thromboembolism, although no optimal strategies including the therapeutic regimens have been established.3 In the current report, we describe our experience with a case of NS due to focal segmental glomerulosclerosis in a patient complicated by acute pulmonary embolism (PE) and intracranial bleeding because of traumatic brain injury (TBI). The association between the NS and PE has been a well-described entity4,5; however, there is a paucity of literature on nephrotic patients concurrently complicated by thromboembolism and such a hemorrhagic event. Several conundrums regarding the anticoagulation that emerged in this case are also discussed. The patient has given her consent for publication of this report.
Case Report
A 76-year-old female was admitted to our hospital complaining of progressive swelling of her lower extremities. Two months prior to this admission, the patient had recognized the leg swelling when she was found to have a reduced serum albumin (Alb) level of 3.4 g/dL and 2+ proteinuria at another hospital, despite having no apparent history of renal disease. Thereafter, the symptoms gradually worsened, and she was subsequently referred for a further workup. Other medical histories included hypertension and hyperlipidemia, which had been treated by her general practitioner with olmesartan medoxomil and rosuvastatin for more than a year.
At the time of admission (clinical day 0), she had gained 5 kg in the previous two months, bringing her weight to 63.3 kg. Laboratory examinations revealed the following results: white blood cell count, 5,300/µL; hemoglobin, 11.2 g/dL; platelet count, 23.0 × 104/µL; prothrombin time (PT), 10.9 seconds (control value: 11.5); active partial thromboplastin time (APTT), 27.0 seconds (control value: 29.9), blood urea nitrogen, 20 mg/dL; creatinine (Cr), 1.2 mg/dL; total protein 4.8 g/dL, Alb, 2.1 g/dL; C-reactive protein, 0.01 mg/dL; immunoglobulin (Ig) G, 375 mg/dL; IgA, 212 mg/dL; IgM, 282 mg/day; anti-double stranded deoxyribonucleic acid antibodies, 1.5 IU/mL; fibrinogen degradation product, 2.4 µg/mL; and d-dimer, 11.3 µg/mL. A 24-hour urine specimen contained 4.5 g of protein with a Cr clearance of 37.6 mL/minute. A diagnosis of NS was made, and the patient was scheduled for renal biopsy three days later. She was empirically subjected to intravenous unfractionated heparin at a dose of 10,000 U/day (7.2 U/kg/hour) as prophylactic anticoagulation, which was continued until 5 hours before the procedure.
The patient was in a good state during the 26-hour post-procedural bed rest without the use of any kind of sequential compression devices, while she suddenly fainted and collapsed just after the first ambulation, resulting in falling backward and striking the back of her head. A few minutes later, her consciousness fully recovered spontaneously with a blood pressure of 131/85 mmHg when she displayed tachypnea with an oxygen saturation below 90% while breathing ambient air. She was then started on a non-rebreather mask with an oxygen reservoir bag flowing at 10 L/minute. Urgent brain computed tomography (CT) showed subarachnoid hemorrhaging with a left frontal subdural hematoma (Fig. 1A), and subsequent contrast-enhanced diagnostic CT revealed bilateral thrombosis of the pulmonary arteries (Fig. 2A and B), despite the absence of venous filling defects in the abdominal veins and the lower limbs. An urgent laboratory analysis revealed a platelet count of 13.7 × 104/µL and d-dimer of 51.2 µg/mL, while echocardiogram revealed right ventricular enlargement with an increased estimated pulmonary artery systolic pressure of 55 mmHg. We then decided to prioritize the treatment of the PE under multidisciplinary collaboration involving cardiologists and neurosurgeons. Intravenous unfractionated heparin was resumed just after the radiological confirmation of the diseases, and the patient was transferred to the intensive care unit for close monitoring of her clinical status.
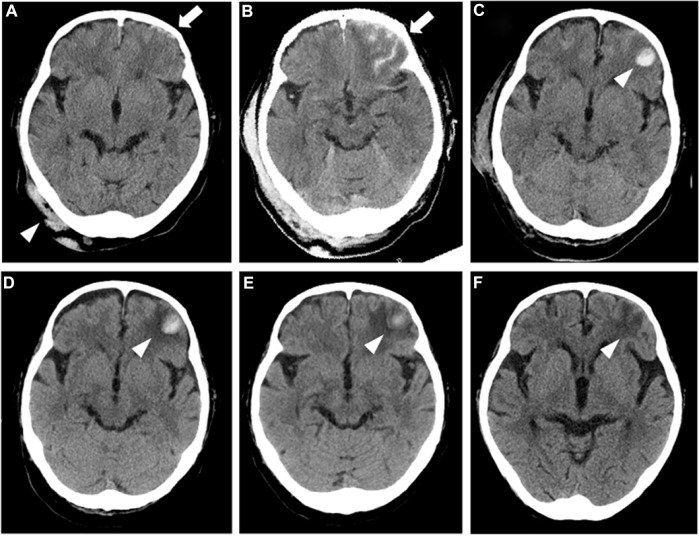
Figure 1.
The findings of the serial brain CT examinations. An urgent study (A) just after the blow to the right occipital area of her head (arrowhead) shows subarachnoid hemorrhaging with left frontal subdural hematoma (arrow). The following studies 6 hours (B) and 24 hours later (C) demonstrate exaggeration of the left frontal contusion (arrow) and further expansion of the contusion into the intraparenchymal hematoma (arrowhead), respectively. Thereafter, gradual improvements in the size of the hematoma (arrowhead) are noticed on clinical days 5 (D) and 11 (E). Disappearance of the hemorrhagic lesion marked by an area of hypodensity (arrowhead) is shown on clinical day 33 (F), indicating the resolution of the contusion.
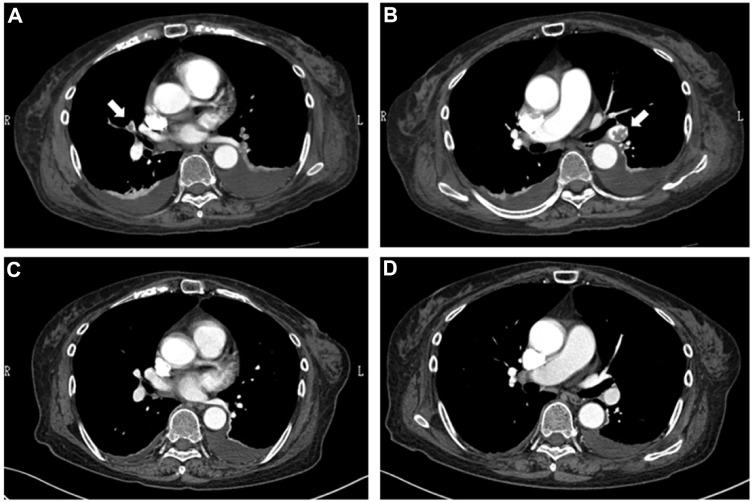
Figure 2.
The findings of chest CT angiogram. An initial study (A and B) shows intraluminal thrombi in the main pulmonary arteries (arrows), while no filling defects are demonstrated in the repeat study (C and D).
Despite the expansions of the left frontal contusion demonstrated by repeat brain CT examinations 6 and 24 hours later (Fig. 1B and C), the patient did not manifest any neurological deficits. We thus continued the heparin with a titrated dosage ranging from 10,000–13,000 U/day (7.2–9.3 U/kg/hour) with close monitoring of neurological signs under the supervision of experienced neurosurgeons, and her APTT was sequentially monitored on a regular basis, showing variations from 42.0 to 75.3 seconds, which corresponded to 1.4 to 2.5 times the control values. The heparinization was switched to warfarin (3 mg/day) on clinical day 8, delivering a PT-international normalized ratio of 1.7 to 2.5, and subsequent brain CT examinations performed on clinical days 11 (Fig. 1D) and 17 (Fig. 1E) showed gradual improvement in the intracranial lesion. Her NS was finally ascribed to pathologically confirmed focal segmental glomerulosclerosis. Another session of contrast-enhanced CT performed on clinical day 18 revealed the disappearance of the thromboses (Fig. 2C and D), and thus, oral prednisolone (PSL) at 40 mg/day was commenced 2 days later when she was no longer dependent on the oxygen administration. Repeat brain CT performed on clinical day 33 showed the resolution of the contusion (Fig. 1F), while at 1 month of follow-up, she was still being treated with oral PSL (30 mg/day) combined with the warfarinization, having reached partial remission with a 24-hour urine protein level of 1.3 g and marginal improvement in the serum Alb level to 2.2 g/dL. The patient was transferred to the regional geriatric hospital on clinical day 47.
Discussion
PE is a major phenotype of NS-associated thromboembolism.1–3 The prevalence of the disease in nephrotic patients has varied among studies, ranging from approximately 0.2% to 30%.2 A considerable number of nephrotic patients with PE are asymptomatic,4,5 while awareness of several characteristic manifestations including dyspnea, tachypnea, chest pain, palpitation, cough, and hemoptysis may reduce diagnostic delays.6,7 Occasionally, patients with the disease may present with syncope,8,9 which is defined as an abrupt transient loss of consciousness with loss of postural tone.10 Arrhythmias resulting from right ventricular overload and a vasovagal reflex as well as right ventricular failure and impaired left ventricular filling, leading to a reduction in cardiac output, arterial hypotension, and reduced cerebral blood flow, have been regarded as pathogenic bases of PE-mediated syncope.8,9 We feel it is still challenging to associate such a sign with PE,11 although some studies have revealed that 10%–20% of patients with the disease have significant hemodynamic instability that manifests as either shock or syncope,11,12 indicating that syncope as an initial sign of PE is not uncommon at all.11 In this regard, the clinical scenario characterized by a set of events including NS, syncope, and PE may not be surprising. However, the significance of the present case should be evaluated carefully in terms of the concurrent syncopal event-mediated intracranial hemorrhage requiring serial radiological monitoring during anticoagulation.
Patients with acute intracranial hemorrhage who concomitantly develop clinically apparent PE should present a distinctively difficult therapeutic dilemma, since they are perceived to be at substantial risk for the progression of the embolic disease in the absence of treatment and for aggravation of the hemorrhagic lesion if treated with anticoagulants.13 This obliged us to weigh the risks and benefits of several therapeutic options for the current patient. One may argue that the placement of an inferior vena cava filter might be a candidate, since it can be indicated in subjects contraindicated for anticoagulation.14 Such filters may be effective in reducing the likelihood of PE; however, they increase the subsequent incidence of deep vein thrombosis and have not been shown to increase overall survival.15 Furthermore, these kinds of devices do not address propagating the thromboembolic burden, and thus a considerable portion of patients may ultimately rely on pharmacological anticoagulation.16 In the current case, the persistence of a presumable hypercoagulable state due to NS as well as our failure to confirm deep venous thrombotic lesions, despite the limited sensitivity and specificity of the diagnostic imaging modality,6,17,18 discouraged us from adopting this procedure. A number of patients with intracranial hemorrhaging due to TBI require anticoagulation for various indications; as such, the use of anticoagulants in this setting may not necessarily be contraindicated.19–22 Low-dose heparins have been recommended as prophylactics for venous thromboembolism among some hemorrhagic stroke patients with limited mobility in the practical therapeutic guidance23; however, data on TBI patients who have received therapeutic anticoagulation in the postin-jury period are extremely scarce, and no consensus has been reached regarding the optimum regimen, including the appropriate timing, preferred agent, and dosage, for therapeutic anticoagulation in this population.19 Indeed, while a survey of deep venous thrombosis management among patients with TBI in acute trauma hospitals revealed a number of therapeutic strategies, neither the timing of starting anticoagulation nor the effect on bleeding was mentioned.20 Some patients with traumatic intracranial bleeding may be able to successfully receive therapeutic anticoagulation under close monitoring with serial brain CT scans, which help confirm the stability of the hemorrhagic focus,21 but some patients may still suffer from progression of hemorrhagic TBI due to therapeutic anticoagulation, despite the absence of any signs of neurologic deterioration.22
The validity of our therapeutic and operating policies for the PE complicated by hemorrhagic TBI applied in the current patient remains to be delineated, although the fact that our patient recovered favorably from the disease without any signs of neurological compromise despite the transient hemorrhagic progression of the brain contusion after the institution of anticoagulation may imply the feasibility of the warfarinization as well as the heparinization among select patients with traumatic intracranial hemorrhagic lesions. Our observation does not substantially clarify the safety profile of these kinds of therapeutics, and we are still dependent on anecdotal experiences or expert opinions.19–22 Needless to say, carefully weighing all of the options and potential outcomes on a case-by-case basis is necessary to balance the risks and benefits of the procedure. Obviously, the accumulation of experience from a larger number of patients similar to our case should be needed to establish appropriate strategies for the overall management of NS-mediated thromboembolism with various bleeding complications. At present, the use of target-specific oral anticoagulants such as rivaroxaban, apixaban, and dabigatran, in this situation, may not be appropriate because of the insufficient availability of their antidotes.24 However, considering their novel characteristics, which allow for a fixed-dose regimen without routine laboratory monitoring, we believe that the application of these agents will function as a tremendous step forward in our ability to pursue attractive anticoagulation therapy, even in nephrotic patients, with steady assessments.24,25
In the present patient, whether the original thrombus in the pulmonary arteries formed before or after the start of the initial heparinization as prophylactic anticoagulation remains unclear, although we believe that immobility due to bed rest during hospitalization, especially after the renal biopsy, might have predisposed our patient to the disease.12,26 Considering the significant bleeding risk with renal biopsy,27 no one would argue against our decision to stop the heparinization to facilitate the procedure. Instead, our policy may invite criticism because of the controversy regarding prophylactic anticoagulation among nephrotic patients with glomerulopathies other than membranous nephropathy.28,29 However, the risk of venous thromboembolism may be significant even in patients with focal segmental glomerulosclerosis,30–32 and the low rate of reported adverse events with therapeutic anticoagulation as well as our patient’s markedly decreased serum Alb levels encouraged us to pursue pharmacological prophylaxis as practiced in the ordinary clinical settings.28,29,32 While the clinical scenario of the current case prevents evaluation of the significance of prophylactic anticoagulation, which was done before the renal biopsy, this case may otherwise emphasize the need for a periprocedural strategy for thromboembolic prophylaxis, which remains an unresolved clinical issue in the field of nephrology.
Footnotes
ACADEMIC EDITOR: Athavale Nandkishor, Associate Editor
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totalled 1040 words, excluding any confidential comments to the academic editor.
FUNDING: This study was supported in part by a Grant-in-Aid for Research on Advanced Chronic Kidney Disease, Practical Research Project for Renal Diseases from the Japan Agency for Medical Research and Development, AMED. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Drafted the manuscript: TA. Made contributions to the acquisition of the clinical data: TY. Provided a detailed review of the contents and structure of the manuscript, resulting in significant changes to the original document: EK, DN. All the authors have read and approved the final manuscript.
REFERENCES
- 1.Llach F, Papper S, Massry SG. The clinical spectrum of renal vein thrombosis: acute and chronic. Am J Med. 1980;69(6):819–27. doi: 10.1016/s0002-9343(80)80006-4. [DOI] [PubMed] [Google Scholar]
- 2.Kayali F, Najjar R, Aswad F, Matta F, Stein PD. Venous thromboembolism in patients hospitalized with nephrotic syndrome. Am J Med. 2008;121(3):226–30. doi: 10.1016/j.amjmed.2007.08.042. [DOI] [PubMed] [Google Scholar]
- 3.Singhal R, Brimble KS. Thromboembolic complications in the nephrotic syndrome: pathophysiology and clinical management. Thromb Res. 2006;118(3):397–407. doi: 10.1016/j.thromres.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Zhang LJ, Zhang Z, Li SJ, et al. Pulmonary embolism and renal vein thrombosis in patients with nephrotic syndrome: prospective evaluation of prevalence and risk factors with CT. Radiology. 2014;273(3):897–906. doi: 10.1148/radiol.14140121. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y, Lv J, Zhou F, et al. Risk factors of pulmonary thrombosis/embolism in nephrotic syndrome. Am J Med Sci. 2014;348(5):394–8. doi: 10.1097/MAJ.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 6.Goldhaber SZ. Pulmonary embolism. N Engl J Med. 1998;339(2):93–104. doi: 10.1056/NEJM199807093390207. [DOI] [PubMed] [Google Scholar]
- 7.Tapson VF. Acute pulmonary embolism. N Engl J Med. 2008;358(10):1037–52. doi: 10.1056/NEJMra072753. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe TR, Allen TL. Syncope as an emergency department presentation of pulmonary embolism. J Emerg Med. 1998;16(1):27–31. doi: 10.1016/s0736-4679(97)00228-x. [DOI] [PubMed] [Google Scholar]
- 9.Demircan A, Aygencel G, Keles A, Ozsoylar O, Bildik F. Pulmonary embolism presenting as syncope: a case report. J Med Case Rep. 2009;3:7440. doi: 10.4076/1752-1947-3-7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manolis AS, Linzer M, Salem D, Estes NA., III Syncope: current diagnostic evaluation and management. Ann Intern Med. 1990;112(11):850–63. doi: 10.7326/0003-4819-112-11-850. [DOI] [PubMed] [Google Scholar]
- 11.Varon J, Fromm RE., Jr Syncope: the forgotten sign of pulmonary embolism. J Emerg Med. 1998;16(1):117–8. doi: 10.1016/s0736-4679(97)00249-7. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura M, Fujioka H, Yamada N, et al. Clinical characteristics of acute pulmonary thromboembolism in Japan: results of a multicenter registry in the Japanese Society of Pulmonary Embolism Research. Clin Cardiol. 2001;24(2):132–8. doi: 10.1002/clc.4960240207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly J, Hunt BJ, Lewis RR, Rudd A. Anticoagulation or inferior vena cava filter placement for patients with primary intracerebral hemorrhage developing venous thromboembolism? Stroke. 2003;34(12):2999–3005. doi: 10.1161/01.STR.0000102561.86835.17. [DOI] [PubMed] [Google Scholar]
- 14.Comerota AJ. Retrievable IVC filters: a decision matrix for appropriate utilization. Perspect Vasc Surg Endovasc Ther. 2006;18(1):11–7. doi: 10.1177/153100350601800105. [DOI] [PubMed] [Google Scholar]
- 15.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998;338(7):409–15. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 16.Tardy B, Mismetti P, Page Y, et al. Symptomatic inferior vena cava filter thrombosis: clinical study of 30 consecutive cases. Eur Respir J. 1996;9(10):2012–6. doi: 10.1183/09031936.96.09102012. [DOI] [PubMed] [Google Scholar]
- 17.Ghaye B, Dondelinger RF. Non-traumatic thoracic emergencies: CT venography in an integrated diagnostic strategy of acute pulmonary embolism and venous thrombosis. Eur Radiol. 2002;12(8):1906–21. doi: 10.1007/s00330-002-1505-0. [DOI] [PubMed] [Google Scholar]
- 18.Tovey C, Wyatt S. Diagnosis, investigation, and management of deep vein thrombosis. BMJ. 2003;326(7400):1180–4. doi: 10.1136/bmj.326.7400.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergouwen MD, Roos YB, Kamphuisen PW. Venous thromboembolism prophylaxis and treatment in patients with acute stroke and traumatic brain injury. Curr Opin Crit Care. 2008;14(2):149–55. doi: 10.1097/MCC.0b013e3282f57540. [DOI] [PubMed] [Google Scholar]
- 20.Carlile MC, Yablon SA, Mysiw WJ, Frol AB, Lo D, Diaz-Arrastia R. Deep venous thrombosis management following traumatic brain injury: a practice survey of the traumatic brain injury model systems. J Head Trauma Rehabil. 2006;21(6):483–90. doi: 10.1097/00001199-200611000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Byrnes MC, Irwin E, Roach R, James M, Horst PK, Reicks P. Therapeutic anticoagulation can be safely accomplished in selected patients with traumatic intracranial hemorrhage. World J Emerg Surg. 2012;7(1):25. doi: 10.1186/1749-7922-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsushima K, Inaba K, Cho J, et al. Therapeutic anticoagulation in patients with traumatic brain injury. J Surg Res. 2016;205(1):186–91. doi: 10.1016/j.jss.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Lansberg MG, O’Donnell MJ, Khatri P, et al. American College of Chest Physicians Antithrombotic and thrombolytic therapy for ischemic stroke: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):601S–36S. doi: 10.1378/chest.11-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connors JM. Antidote for factor Xa anticoagulants. N Engl J Med. 2015;373(25):2471–2. doi: 10.1056/NEJMe1513258. [DOI] [PubMed] [Google Scholar]
- 25.Dupree LH, Reddy P. Use of rivaroxaban in a patient with history of nephrotic syndrome and hypercoagulability. Ann Pharmacother. 2014;48(12):1655–8. doi: 10.1177/1060028014549349. [DOI] [PubMed] [Google Scholar]
- 26.Onishi A, Inoue M, Imai T, et al. Nephrotic syndrome complicated with deep venous thrombosis in the upper extremities. Case Rep Nephrol Dial. 2014;5(1):1–5. doi: 10.1159/000365567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook BW. Anticoagulation management. Semin Intervent Radiol. 2010;27(4):360–7. doi: 10.1055/s-0030-1267849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: a clinical conundrum. J Am Soc Nephrol. 2007;18(8):2221–5. doi: 10.1681/ASN.2006111300. [DOI] [PubMed] [Google Scholar]
- 29.Pincus KJ, Hynicka LM. Prophylaxis of thromboembolic events in patients with nephrotic syndrome. Ann Pharmacother. 2013;47(5):725–34. doi: 10.1345/aph.1R530. [DOI] [PubMed] [Google Scholar]
- 30.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7(3):513–20. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li SJ, Tu YM, Zhou CS, Zhang LH, Liu ZH. Risk factors of venous thromboembolism in focal segmental glomerulosclerosis with nephrotic syndrome. Clin Exp Nephrol. 2016;20(2):212–7. doi: 10.1007/s10157-015-1149-4. [DOI] [PubMed] [Google Scholar]
- 32.Medjeral-Thomas N, Ziaj S, Condon M, et al. Retrospective analysis of a novel regimen for the prevention of venous thromboembolism in nephrotic syndrome. Clin J Am Soc Nephrol. 2014;9(3):478–83. doi: 10.2215/CJN.07190713. [DOI] [PMC free article] [PubMed] [Google Scholar]