Abstract
OBJECTIVES
To assess the kinetics of procalcitonin (PCT) and C-reactive protein (CRP) in newborns after cardiothoracic surgery (CS), with and without cardiopulmonary bypass, and to assess whether PCT was better than CRP in identifying sepsis in the first 72 hours after CS.
PATIENTS AND METHODS
This is a prospective study of newborns admitted to the neonatal intensive care unit after CS.
INTERVENTIONS
PCT and CRP were sequentially drawn 2 hours before surgery and at 0, 12, 24, 48, and 72 hours after surgery.
RESULTS
A total of 65 patients were recruited, of which 14 were excluded because of complications. We compared the kinetics of PCT and CRP after CS in bypass and non-bypass groups without sepsis; there were no differences in the PCT values at any time (24 hours, P = 0.564; 48 hours, P = 0.117; 72 hours, P = 0.076). Thirty-five patients needed bypass, of whom four were septic (11.4%). Significant differences were detected in the PCT values on comparing the septic group to the nonseptic group at 48 hours after cardiopulmonary bypass (P = 0.018). No differences were detected in the CRP values in these groups. A suitable cutoff for sepsis diagnosis at 48 hours following bypass would be 5 ng/mL, with optimal area under the curve of 0.867 (confidence interval 0.709–0.958), P < 0.0001, and sensitivity and specificity of 87.5% (29.6–99.7) and 72.6% (53.5–86.4), respectively.
CONCLUSION
This is a preliminary study but PCT seems to be a good biomarker in newborns after CS. Values over 5 ng/mL at 48 hours after CS should alert physicians to the high risk of sepsis in these patients.
Keywords: procalcitonin, C-reactive protein, newborn, sepsis, inflammatory syndrome, postcardiopulmonary bypass
Introduction
Cardiothoracic surgery (CS), especially with cardiopulmonary bypass (CPB), is a potent inducer of systemic inflammatory response syndrome (SIRS),1 which includes fever, high white blood cell count, and increased C-reactive protein (CRP). This is especially remarkable in neonates in whom this phenomenon leads to an immediate postoperative period of low cardiac output and transient organ dysfunction, with an increase in microvascular permeability and the formation of interstitial edema, leading to a third space leak.
Although the outcomes for neonatal CS have improved dramatically in recent decades, patients who undergo CS are at high risk of infection, creating the challenge for the medical team of having to differentiate the nonspecific response related to CPB from sepsis. Clinical signs and currently available biomarkers (white blood cell count and CRP) are not useful in differentiating between these infection types,2–4 leading to the use of broad-spectrum antibiotics that may not always be needed and exposing these premature infants to their side effects.5,6 The antibiotic protocol may be different depending on the local microorganism, but it usually includes third-generation cephalosporin, carbapenem, glycopeptides, and aminoglycosides. These treatments may induce microorganism resistance and side effects such as fungal superinfection and clostridium colitis.
Procalcitonin (PCT) is a 116-amino-acid peptide and precursor of calcitonin, which is normally secreted by neuroendocrine cells in thyroid C cells and in pulmonary and pancreatic tissue.7,8 In baseline conditions, PCT concentrations in blood are negligible, with normal PCT levels in adult and children of less than <0.1 ng/mL. In healthy neonates, values change in the first 48 hours of life: <0.08 ng/mL soon after birth; <0.6 ng/mL, from 21 to 24 hours after birth; and then a return to initial values of <0.08 ng/mL from 24 to 48 hours of life.9 However, after contact with specific antigens such as bacterial endotoxins, PCT increases rapidly within 3–4 hours in peripheral blood, peaking at between 18 and 24 hours and with a plateau curve for at least 24–48 hours (unlike CRP).10,11 Therefore, if the process is controlled, PCT shows a progressive decline, allowing monitoring of the medical treatment and the progression of the infection and sepsis.
Recent studies have shown that PCT may be useful for screening of bacterial sepsis after CS in children,1,3,4,12,13 but none of these studies focused on the newborn population. In contrast to older children, uninfected and healthy newborns can present higher PCT values during the first days of life, making it difficult to establish a cutoff value for PCT.14–16
The aims of the present study were to assess the kinetics of PCT and CRP after CS in newborns and to learn whether there were differences between the kinds of CS (CPB or non-CPB). We also aimed to determine whether PCT was better than CRP in identifying sepsis in the first 72 hours after CPB.
Materials and Methods
We carried out a prospective observational study from January 2013 to December 2015. The setting was the Neonatal Intensive Care Unit (NICU) of Hospital Sant Joan de Déu (Esplugues de Llobregat, Barcelona, Spain).
Inclusion criteria
All newborns admitted to the NICU after CS were included. Exclusion criteria were the presence of other noninfectious morbidity with organ dysfunction that could increase the PCT levels, such as extracorporeal membrane oxygenation circulation, intestinal ischemia within three days of surgery, delayed chest closure, presurgery infection, and absence of parental consent.
Patient classification
Patients without any infection were divided into two groups according to surgery type, namely, CPB and non-CPB (NCPB) cases, in order to determine whether the existence of bypass affects PCT kinetics.
Afterward, only CPB patients were subclassified according to the diagnosis of sepsis or not, as either CPB and sepsis (CPB-S) or CPB without sepsis (CPB-NS).
PCT and CRP determination
PCT and CRP were sequentially drawn 2 hours before surgery and at 0, 12, 24, 48, and 72 hours after surgery time (ST). PCT < 0.5 ng/mL was considered normal, so the PCT level before surgery needed to be under this cutoff in all patients. PCT values were determined in duplicate by means of the LUMITest PCT immunoluminometric assay (ATOM SA; Brahms Diagnostica), which uses two monoclonal antibodies and requires 20 μL of serum or plasma; the assay sensitivity was 0.02 ng/mL. CRP was obtained using the immunoturbidimetric assay (COBAS INTEGRA; Roche).
Definitions
According to local protocol, a diagnosis of sepsis was made as positive culture within three days after surgery and serious systemic and life-threatening organ dysfunction caused by a dysregulated host response to infection, including fever, increased heart rate, low blood pressure, low urine ouput, and confusion.16,17
In accordance with local protocol, prophylactic cefazolin was administered less than 60 minutes before the surgery and redosed every 8 hours until chest drain was removed in all the patients. It has not been demonstrated that prophylactic antibiotic could modify PCT levels. Even though all patients in the study (independently on the study group) received prophylactic treatment with cefazolin, we hypothesize that prophylactic treatment would not influence the study results. Neonates undergoing CS did not receive presurgery corticoid therapy.
Cardiac heart diseases (CHDs) were classified as CHD with left–right shunt (septal defects, patent ductus arteriosus), cyanotic CHD (transposition of the great arteries, Tetralogy of Fallot, anomalous pulmonary venous return, truncus, double outlet right ventricle), and obstructive CHD (coarctation of the aorta, hypoplastic left heart syndrome).
Variables
Baseline demographic and surgical data were recorded, including gender, gestational age at delivery, age and weight at the ST, CHD diagnosis, CPB time, cross clamp time (CCT), and deep hypothermic circulatory arrest (DHCA).
Hemodynamic instability (assessed according to the vasoactive-inotropic score II)18,19 was noted, and data regarding respiratory support were collected. We recorded changes in antibiotic treatment during and after surgery.
Statistical analysis
Windows SPSS program 22.0® was used to analyze the data. Non-normally distributed quantitative variables were documented as median and percentile 25–75. All data were analyzed with nonparametric tests: chi-square test for nonparametric qualitative variables and Mann–Whitney U test for quantitative variables. Biomarker cutoffs, area under the curve (AUC), sensitivity, specificity, positive and negative predicted values, and likelihood ratios were analyzed using MedCalc Program 11.3®. Differences in P-value <0.05 were considered statistically significant.
The study was carried out in accordance with the Declaration of Helsinki and was approved by the Sant Joan de Déu Ethical Assistance Committee. Before the study, written informed consent was obtained from parents.
Results
General results
A total of 65 patients were recruited; 14 patients were excluded because of complications. Fifty-one patients were finally included. Thirty-two patients were male patients (62.7%), and the median age was 14 days (8–26 days) and the median weight was 3.200 g (2.800–3.670 g) at ST. CPB surgery had a median time of 113 minutes (78–133 minutes), CCT of 68 minutes (41–82 minutes), and a DHCA of 0 minutes (0–30 minutes).
A total of 35 (68.6%) CPB patients and 16 (31.4%) NCPB patients were analyzed. The main characteristics of our sample in terms of CPB vs NCPB are summarized in Table 1. There were no statistically significant differences between the two groups, except for weight at ST (0.015). CPB patients required more intensive hemodynamic support after 24, 48, and 72 hours of CS (0.006, 0.032, and 0.017, respectively). There were no differences in terms of duration of mechanical ventilation (0.295).
Table 1.
Characteristics of patients included in the study cohort.*
| GROUP | MALES | PRETERM (<37GA) | GESTATIONAL AGE (WEEKS) | LOW WEIGHT | BIRTH WEIGHT (GRAMS) | WEIGHT AT ST (GRAMS) | AGE AT ST (DAYS) | MV BEFORE ST (DAYS) | HOURS OF MV AFTER ST | HOSPITAL DISCHARGE (DAYS) |
|---|---|---|---|---|---|---|---|---|---|---|
| NCPB (n = 16) |
11 (68.80%) |
2 (12.50%) |
39 (37.5–40) |
3 (18.80%) |
3035 (2500–3262) |
3050 (2525–3345) |
14 (8.25–20.50) |
3 (18.80%) |
60 (48–157.5) |
32 (22.75–44.75) |
| CPB (n = 35) |
21 (60%) |
2 (5.70%) |
39 (38–40) |
4 (11.40%) |
3200 (2795–3580) |
3365 (3000–4000) |
14 (8–29) |
10 (28.60%) |
72 (48–168) |
37 (25–62) |
| P- value | 0.549 (1) |
0.403 (1) |
0.803 (2) |
0.481 (1) |
0.062 (2) |
0.015 (2) |
0.562 (2) |
0.455 (1) |
0.295 (2) |
0.399 (2) |
Notes:
Values expressed by n (percentages) and median (IQR). Chi-square test (1), Mann–Whitney U test (2).
Abbreviations: CPB, cardiopulmonary bypass; GA, gestational age; ST, surgery time; MV, mechanical ventilation; NCPB, non-cardiopulmonary bypass.
The main type of CHD was cyanotic CHD (43%), with 14 patients affected with transposition of great vessels, associated with septal defect or not, followed by 4 patients with anomalous pulmonary venous return. The second most frequent type of CHD was the obstructive one (41%), with 11 cases of aortic coarctation and 8 of aortic hypoplasia.
Regarding the presence of sepsis, there were four patients in the CPB group (11.4%) with positive cultures for Enterobacter cloacae,2 Serratia marcescens, or Staphylococcus epidermidis.
CPB group and sepsis incidence
Concerning the sepsis diagnosis in the CPB group, there were no differences related to the ST (0.745), CCT (0.437), or DHCA (0.531). There were no statistically significant differences for respiratory or hemodynamic support after surgery. The CPB-NS group had a median of 72 hours of mechanical ventilation (48–168 hours), compared to 108 hours for the CPB-S group (96–180 hours), 0.301. Inotropic median scores at 24, 48, and 72 hours for CPB-NS were 14 points (10–18), 12 (7–14), and 5.7 (3.7–8), respectively, compared to 17.85 (12.4–23.5), 17 (12.3–21.9), and 7.8 (4.5–14.2) for the CPB-S group, 0.213, 0.064, and 0.301.
Results of PCT and CRP for NCPB and CPB groups
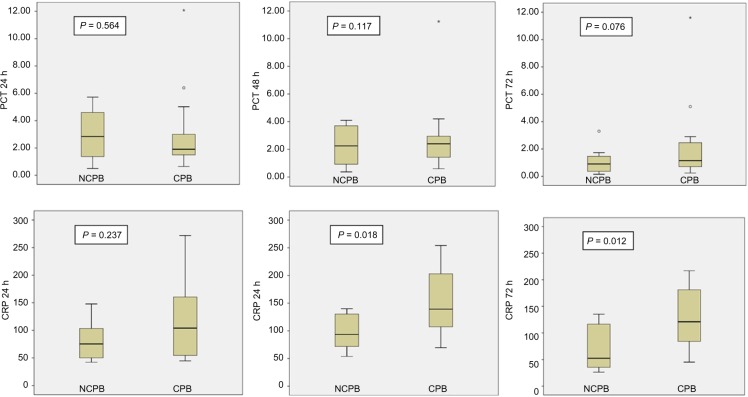
When patients from the NCPB and CPB groups were compared (excluding patients with infection), there were no statistically significant differences in PCT between them at any time. All values are presented in Table 2. Nevertheless, CRP values were different between NCPB and CPB patients at 48 and 72 hours: 99.2 mg/L (75.1–138.2 mg/L) and 60.55 mg/L (36–132.1 mg/L) in NCPB cases with respect to 143.6 mg/L (92.4–204.9 mg/L), and 125.6 mg/L (70.6–179.6 mg/L), in CPB patients, with P-value < 0.05. Figure 1 shows the results for each time.
Table 2.
Procalcitonin and C-reactive protein values in NCPB and CPB patients without sepsis.
| PCT | NCPB | CPB | P-VALUE* | CRP | NCPB | CPB | P-VALUE |
|---|---|---|---|---|---|---|---|
| Pre ST | 0.08 (0.02–0.35) | 0.07 (0.04–0.22) | 0.929 | Pre ST | 2.1 (0.3–10.45) | 3.25 (0.9–11.4) | 0.726 |
| PCT 0 h | 0.11 (0.09–0.39) | 0.06 (0.05–0.20) | 0.066 | CRP 0 h | 1.15 (0.3–7.15) | 1.5 (0.8–4.6) | 0.484 |
| PCT 12 h | 1.14 (0.48–1.28) | 0.56 (0.21–1.6) | 0.119 | CRP 12 h | 25.2 (17.8–72.1) | 35.25 (16.4–53.7) | 0.792 |
| PCT 24 h | 2.3 (1.01–5.00) | 2.5 (1.5–6.4) | 0.564 | CRP 24 h | 85.6 (50.1–114.9) | 104.1 (62.4–144.1) | 0.237 |
| PCT 48 h | 2.2 (0.8–4.1) | 2.95 (1.4–9.8) | 0.117 | CRP 48 h | 99.2 (75.1–138.2) | 143.6 (92.4–204.9) | 0.018 |
| PCT 72 h | 0.9 (0.44–2.02) | 1.73 (0.78–3.21) | 0.076 | CRP 72 h | 60.55 (36–132.1) | 125.6 (70.6–179–6) | 0.012 |
Notes:
Mann–Whitney U test. Values expressed by median (p 25–75).
Abbreviations: CRP, C-reactive protein (mg/L); CPB, cardiopulmonary bypass; NCPB, non-cardiopulmonary bypass; PCT, procalcitonin (ng/mL); pre ST, presurgery time.
Figure 1.
Comparison of procalcitonin and C-reactive protein levels according to NCPB and CPB groups without sepsis*. Figures show box representing interquartile range, line-subdividing box representing median, and the whisker span extending from the lowest to the highest values, excluding outliers. Outliers are shown ○ as and *.
Abbreviations: CRP, C-reactive protein (mg/L); PCT, procalcitonin (ng/mL).
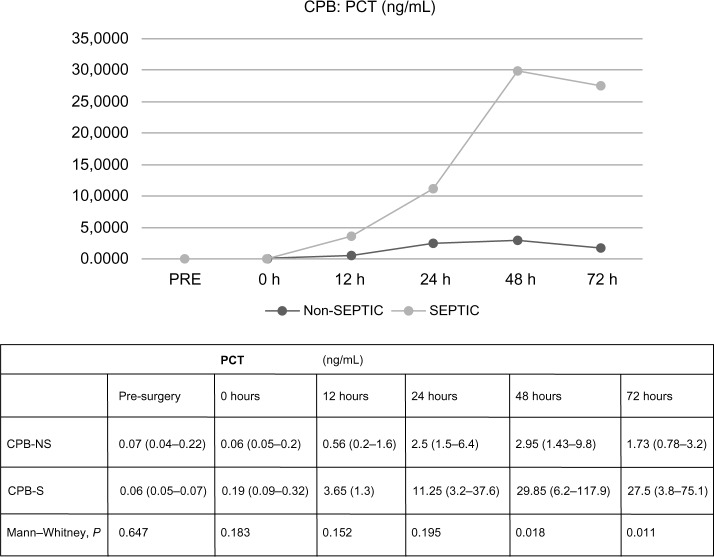
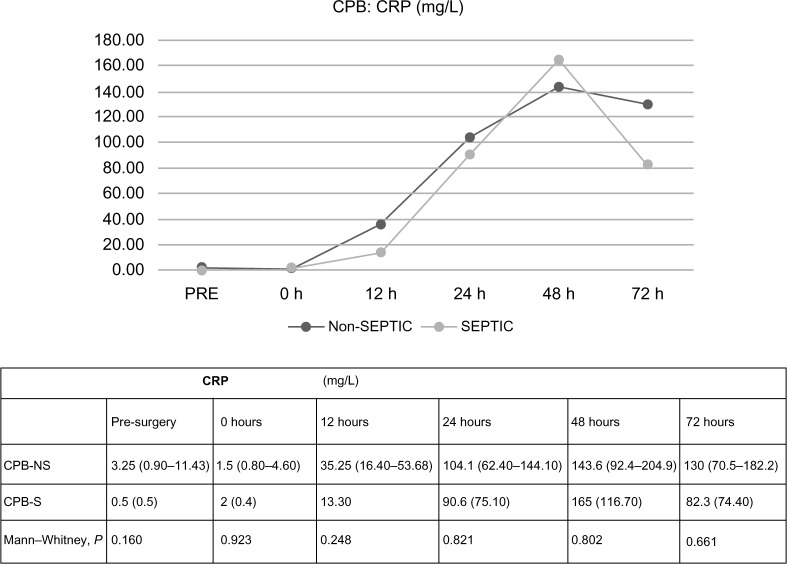
In CPB patients, there were no differences regarding PCT values in terms of the presence or absence of sepsis, statistically significant differences between PCT values were not found at 0 or 12 hours after ST (0.1831 and 0.1515, respectively). At 24 hours, PCT median values were higher in septic patients, but the difference did not achieve significance, 2.5 ng/mL (1.5–6.4 ng/mL) versus 11.25 ng/mL (3.2–37.6 ng/mL), 0.1948. At 48 and 72 hours, PCT results were significantly higher in septic patients, 0.0183 and 0.0111. The analysis of CRP data did not demonstrate significant differences at any time in terms of the presence of sepsis. All data are shown in Figures 2 and 3. There were no differences found in leukocytosis after surgery (at 24, 48, and 72 hours, 0.550, 0.140, and 0.389, respectively).
Figure 2.
Differences in procalcitonin values in terms of the diagnosis of sepsis, related to time.
Note: Values expressed by median (p 25–75).
Abbreviations: CPB-NS, nonseptic cardiopulmonary bypass; CPB-S, septic cardiopulmonary bypass; PCT, procalcitonin (ng/mL).
Figure 3.
Differences in C-reactive protein values in terms of the diagnosis of sepsis, related to time.
Note: Values expressed by median (p 25–75).
Abbreviations: CPB-NS, nonseptic cardiopulmonary bypass; CPB-S, septic cardiopulmonary bypass; CRP, C-reactive protein (mg/L).
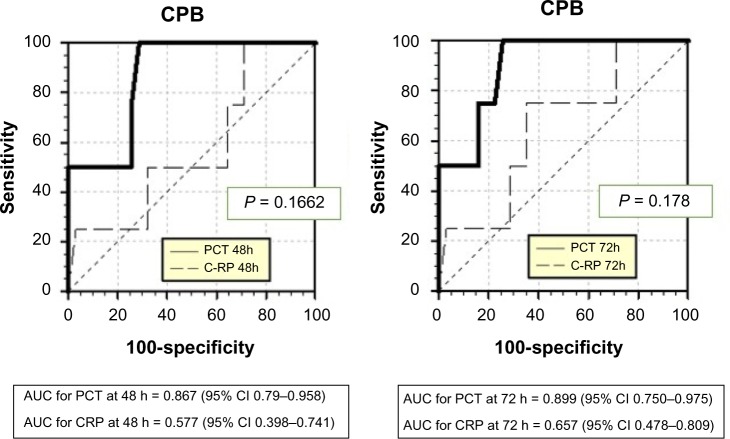
PCT and CRP cutoffs
Comparison of the AUC demonstrated greater sensitivity and specificity for PCT than for CRP in detecting sepsis after 48 hours of CPB with P-value < 0.0001 (Fig. 4 and Table 3). The best cutoff for PCT with respect to the sepsis diagnosis depended on post-ST. Approximations of the best cutoffs for PCT with maximum sensitivity and specificity, and the negative and positive predictive values for each time, are defined in Table 4.
Figure 4.
Sensitivity and specificity for procalcitonin (PCT) and C-reactive protein (CRP) with respect to the sepsis diagnosis in CPB patients at 48 and 72 hours after surgery, respectively.
Abbreviations: AUC, area under the curve; CI, confidence interval.
Table 3.
Discriminatory accuracy of procalcitonin and C-reactive protein for detection of sepsis after cardiopulmonary bypass.
| PCT | AUC | 95% CI | P VALUE | CRP | AUC | 95% CI | P VALUE |
|---|---|---|---|---|---|---|---|
| 24 h | 0.702 | 0.524–0.844 | 0.3101 | – | 0.621 | 0.442–0.779 | 0.3944 |
| 48 h | 0.867 | 0.709–0.958 | <0.0001 | – | 0.577 | 0.398–0.741 | 0.6512 |
| 72 h | 0.899 | 0.750–0.975 | <0.0001 | – | 0.657 | 0.478–0.809 | 0.3037 |
Abbreviations: AUC, area under the curve; CRP, C-reactive protein (mg/L); CI, confidence interval; PCT, procalcitonin (ng/mL).
Table 4.
Discriminatory performance of procalcitonin for detection of sepsis after cardiopulmonary bypass.
| CUTTOFF | SENSITIVITY (95% CI) | SPECIFICITY (95% CI) | POSITIVE PV, % | NEGATIVE PV, % | POSITIVE LIKELIHOOD RATIO, % (95% CI) | NEGATIVE LIKELIHOOD RATIO, % (95% CI) | |
|---|---|---|---|---|---|---|---|
| PCT 24 h | >4 ng/mL | 75.00 (19.4–99.4) | 75.6 (53.7–86.9) | 28.34 | 95.92 | 2.75 (1.3–6.1) | 0.34 (0.06–1.9) |
| PCT 48 h | >5 ng/mL | 87.5 (29.6–99.7) | 72.6 (53.5–86.4) | 29.12 | 97.83 | 3.17 (1.6–6.3) | 0.34 (0.06–1.9) |
| PCT 72 h | >5.5 ng/mL | 62.5 (13.1–96.3) | 83.9 (66.3–94.5) | 32.64 | 94.53 | 3.9 (1.3–11.7) | 0.45 (0.1–1.6) |
Abbreviations: CI, confidence interval; PCT, procalcitonin; PV, predictive value.
Discussion
This is the first published study to assess PCT after cardiac surgery in an exclusively neonatal population. There were no differences in the kinetics of PCT regarding cardiac surgery type, or CPB and NCPB patients, suggesting that PCT does not increase to significant levels due to cardiac bypass as do other biomarkers such as CRP. Moreover, it was documented that patients with confirmed sepsis infection after CPB had higher median PCT levels when compared with patients without infection.
CPB is a great inductor of SIRS because of exposure in the pump circuit to artificial surfaces, hemolysis, and reperfusion injury, which generates activation of the pro-inflamatory and anti-inflammatory cascades.9 As a result, conventional biomarkers of infection such as leukocytosis and CRP are useless to discriminate between infected and noninfected patients, as has been reported in previous studies in older children.1,3,11,20 In this sense, PCT could be a superior biomarker to CRP in discriminating between SIRS and sepsis.
In newborns, PCT particularities are well known at birth. This population has higher PCT levels in the first 30 hours of life, which implies a limitation on its usefulness.13 The PROKINECA study only included patients with CS after 48 hours of life, when PCT shows stability.21 Therefore, the study hypothesis was that PCT could be a useful marker for neonates suffering from CS.
As was the case in previous studies, we did not find differences in CRP between septic and nonseptic patients after CS.22,23 CRP probably increases after CS as a response to the SIRS, in an unspecific way with a nonoptimum AUC for the sepsis diagnosis. In our sample, PCT seems not to be altered by the SIRS, with values for the CPB and NCPB groups being similar.
Regarding the usefulness of PCT, mean PCT results increased to nearly 5, 10, and 30 ng/mL, from 24 hours post-CS in septic cases, with significant differences compared to the noninfected patients. The proposed PCT cutoff values in our study are above 4 ng/mL at 24 hours post-CPB, 5 ng/mL at 48 hours, or greater than 5.5 ng/mL at 72 hours post-CPB, for the best sensitivity, specificity, and positive and negative predicted values. These cutoffs are similar to those proposed in previous studies focused on children1,11,24 but much higher than those proposed for adult patients.25 In pediatric reports, a PCT result up to 2 ng/mL at 24 hours, or more than 4 ng/mL at 48 hours post-ST, was a good predictor of bacterial infection, with lower PCT values for localized infection and higher values in septic patients. Our higher values may be due to the increased susceptibility of newborns to generalized rather than localized infection, in contrast to older children and adults.26,27 Unlike other studies, in our sample, we did not find a subpopulation of patients with higher PCT values in the immediate postoperative period.1,21 A possible explanation for this is that in our sample we excluded patients with major complications such as those who required extracorporeal membrane oxygenation circulation or delayed chest closure, in order to obtain a more homogeneous sample.
Another interesting point of the present study is that all included patients had normal PCT levels prior to surgery. This could be useful in presurgery infection screening. The present study also suggests that PCT may help to identify those newborns with high risk of sepsis after CS. Although we agree with recent PCT studies28 that have pointed out the risk of substantial morbidity and mortality by withholding or discontinuing antibiotics in suspected infection, it is extremely important to optimize antibiotic treatment to prevent resistance29 and subsequent antibiotic complications.6,30,31 No change in the local antibiotic protocol is envisioned from the results of our study, but future studies should be designed to assess whether this threshold can reduce the total duration of antibiotics with a favorable response. Also, PCT cutoff values are not merely helpful in and of themselves; as previously noted in other studies,32,33 we found that PCT values in nonsepsis had a peak level at 48 hours and then decreased, but in sepsis the level remained higher. it should be considered that PCT could have other limitations, especially in organ dysfunction, postcardiac arrest, rheumatologic cases, and other diseases that could increase the PCT level.
There are several limitations to our study. First, this was a single-center study, so results may not be applicable to other units because of the local characteristics of the surgery and unit protocol. The other major limitation is the reduced number of infected patients, which reduces the quality of our results.
Conclusions
The main point of interest of our study is the absence of differences in PCT values in patients undergoing cardiac surgery with CPB and NCPB. PCT also seems to be a good biomarker in newborns to determine the absence of sepsis at 24 hours following CS, and it may serve to alert physicians to possible sepsis rather than simple SIRS after CS. This is a preliminary analysis; we believe that further, rigorous studies should be performed along these investigative lines.
Footnotes
ACADEMIC EDITOR: Karen Pulford, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1113 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Experiment conception and design: IJG, JR-F, SBP, MIS, JMH. Analysis of the data: IJG, JR-F, SBP. Writing of the first draft of the manuscript: SBP, JR-F. Contribution to the writing of the manuscript: IJG, MIS, JMH. Agreement with manuscript results and conclusions: all authors. Joint development of the structure and arguments for the paper: all authors. All the authors reviewed and approved the final manuscript.
REFERENCES
- 1.Davidson J, Tong S, Hauck A, Lawson DS, da Cruz E, Kaufman J. Kinetic of procalcitonin and C-reactive protein and the relationship to postoperative infection in young infants undergoing cardiovascular surgery. Pediatr Res. 2013;74:413–9. doi: 10.1038/pr.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arkader R, Troster EJ, Lopes MR, et al. Procalcitonin does discriminate between sepsis and systemic inflammatory response syndrome. Arch Dis Child. 2006;91:117–20. doi: 10.1136/adc.2005.077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMaster P, Park DY, Shann F, et al. Procalcitonin versus C-reactive protein and immature-to-total neutrophil ratio as markers of infection after cardiopulmonary bypass in children. Pediatr Crit Care Med. 2009;10:217–21. doi: 10.1097/PCC.0b013e31819369f3. [DOI] [PubMed] [Google Scholar]
- 4.Sponholz C, Sakr Y, Reinhart K, Brunkhorst F. Diagnostic value and prognostic implications of serum procalcitonin after cardiac surgery: a systematic review of the literature. Crit Care. 2006;10:R145. doi: 10.1186/cc5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015;135:617–26. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 6.Arboleya S, Sánchez B, Milani C, et al. Intestinal microbiotia development in preterm neonates and effect of perinatal antibiotics. J Pediatr. 2015;166:538–44. doi: 10.1016/j.jpeds.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Benitz WE. Adjunct laboratory tests in the diagnosis of early-onset neonatal sepsis. Clin Perinatol. 2010;37:421–38. doi: 10.1016/j.clp.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–8. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiesa C, Pacifico L, Osborn JF, Bonci E, Hofer N, Resch B. Early-onset neonatal sepsis: still room for improvement in procalcitonin diagnostic accuracy studies. Medicine (Baltimore) 2015;94(30):e1230. doi: 10.1097/MD.0000000000001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D, Su L, Han G, Yan P, Xie L. Prognostic value of procalcitonin in adults patients with sepsis: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0129450. doi: 10.1371/journal.pone.0129450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launes C, Esteban E, Balaguer M, Alsina M, Cambra FJ, Jordan I. Procalcitonin-guidance reduces antibiotic exposure in children with nosocomial infection (PRORANI) J Infect. 2016;72:250–3. doi: 10.1016/j.jinf.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Garcia IJ, Gargallo MB, Torné EE, et al. Procalcitonin: a useful biomarker to discriminate infection after cardiopulmonary bypass in children. Pediatr Crit Care Med. 2012;13:441–5. doi: 10.1097/PCC.0b013e31823890de. [DOI] [PubMed] [Google Scholar]
- 13.Crespo-Marcos D, Rey-Galán C, López-Herce J, et al. Kinetics of C-reactive protein and procalcitonin after paediatric cardiac surgery. An Pediatr (Barc) 2010;73:162–8. doi: 10.1016/j.anpedi.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 14.López Sastre JB, Solís DP, Serradilla VR, Colomer BF, Cotallo GD, Grupo de Hospitales Castrillo Evaluation of procalcitonin for diagnosis of neonatal sepsis of vertical transmission. BMC Pediatr. 2007;7:9. doi: 10.1186/1471-2431-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochi F, Higaki T, Ohta M, et al. Procalcitonin as a marker of respiratory disorder in neonates. Pediatr Int. 2015;57:263–8. doi: 10.1111/ped.12505. [DOI] [PubMed] [Google Scholar]
- 16.Auriti C, Fiscarelli E, Ronchetti MP, et al. Procalcitonin in detecting neonatal nosocomial sepsis. Arch Dis Child Fetal Neonatal Ed. 2012;97:368–70. doi: 10.1136/fetalneonatal-2010-194100. [DOI] [PubMed] [Google Scholar]
- 17.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;23(315):801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 19.Kleiber N, de Wildt SN, Cortina G, et al. Clonidine as a first-line sedative agent after neonatal cardiac surgery: retrospective cohort study. Pediatr Crit Care Med. 2016;17(4):332–41. doi: 10.1097/PCC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 20.Whitlock RP, Devereaux PJ, Teoh KH, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;26:1243–53. doi: 10.1016/S0140-6736(15)00273-1. [DOI] [PubMed] [Google Scholar]
- 21.Arkader R, Troster EJ, Abellan DM, et al. Procalcitonin and C-reactive protein kinetics in postoperative pediatric cardiac surgical patients. J Cardiothorac Vasc Anesth. 2004;18:160–5. doi: 10.1053/j.jvca.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 22.Costello JM, Pasquali SK, Jacobs JP, et al. Gestational age at birth and outcomes after neonatal cardiac surgery: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. Circulation. 2014;129:2511–7. doi: 10.1161/CIRCULATIONAHA.113.005864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeken U, Feindt P, Micek M, Petzold T, Schulte HD, Gams E. Procalcitonin (PCT) in cardiac surgery: diagnostic value in systemic inflammatory response syndrome (SIRS), sepsis and after heart transplantation (HTX) Cardiovasc Surg. 2000;8:550–4. doi: 10.1016/s0967-2109(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 24.Beghetti M, Rimensberger PC, Kalangos A, Habre W, Gervaix A. Kinetics of procalcitonin, interleukin 6 and C-reactive protein after cardiopulmonary-bypass in children. Cardiol Young. 2003;13:161–7. doi: 10.1017/s1047951103000301. [DOI] [PubMed] [Google Scholar]
- 25.Zhao D, Zhou J, Haraguchi G, et al. Procalcitonin for the differential diagnosis of infectious and non-infectious systemic inflammatory response syndrome after cardiac surgery. J Intensive Care. 2014;3:35. doi: 10.1186/2052-0492-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aoui A, Piriou V, Bastien O, et al. Usefulness of procalcitonin for diagnosis of infection in cardiac surgical patients. Crit Care Med. 2000;28:3171–6. doi: 10.1097/00003246-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Badurdeen S, Mulongo M, Berkley JA. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr Res. 2015;77:290–7. doi: 10.1038/pr.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shin AY, Jin B, Hao S, et al. Utility of clinical biomarkers to predict central line-associated bloodstream infections after congenital heart surgery. Pediatr Infect Dis J. 2015;34:251–4. doi: 10.1097/INF.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 29.Mandell IM, Aghamohammadi S, Deakers T, Khemani RG. Procalcitonin to detect suspected bacterial infections in the PICU. Pediatr Crit Care Med. 2016;17:4–12. doi: 10.1097/PCC.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 30.Cailes B, Vergnano S, Kortsalioudaki C, et al. The current and future roles of neonatal infection surveillance programs in combating antimicrobial resistance. Early Human Dev. 2015;91:613–8. doi: 10.1016/j.earlhumdev.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Alm B, Erdes L, Möllborg P, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics. 2008;121:697–702. doi: 10.1542/peds.2007-1232. [DOI] [PubMed] [Google Scholar]
- 32.Michalik DE, Duncan BW, Mee RB, et al. Quantitative analysis of procalcitonin after pediatric cardiothoracic surgery. Cardiol Young. 2006;16:48–53. doi: 10.1017/S1047951105002088. [DOI] [PubMed] [Google Scholar]
- 33.Zant R, Stocker C, Schlapbach LJ, et al. Procalcitonin in the early course post-pediatric cardiac surgery. Pediatr Crit Care Med. 2016;17:624–9. doi: 10.1097/PCC.0000000000000751. [DOI] [PubMed] [Google Scholar]