Abstract
Epithelial cadherin (encoded by the CDH1 gene) is a tumor suppressor glycoprotein that plays a role in the invasion and metastasis of human cancers. As previous studies regarding the association between CDH1 promoter methylation and head and neck squamous cell carcinoma (HNSCC) have yielded inconsistent conclusions, a meta-analysis was performed. A systematic literature review was undertaken from four databases: PubMed, Embase, Google Scholar, and Web of Science. Finally, a total of 23 studies (including 1,727 cases of HNSCC and 555 normal controls) were included in the present study. Our results showed that the frequency of CDH1 promoter methylation in HNSCC was statistically greater than in controls (odds ratio [OR] =5.94, 95% confidence interval [CI]: 3.36–10.51, P<0.001). In reported cases of HNSCC, CDH1 promoter methylation was statistically associated with tumor stage (OR =0.46, 95% CI: 0.27–0.78, P=0.004) and a history of alcohol consumption (OR =6.04, 95% CI: 2.41–15.14, P<0.001). Moreover, the sensitivity, specificity, and area under the curve of the summary receiver operator characteristic for the included studies were 0.50 (95% CI: 0.4–0.61), 0.89 (95% CI: 0.79–0.95), and 0.74 (95% CI: 0.70–0.78), respectively. In conclusion, our meta-analyses indicated that CDH1 promoter methylation was associated with HNSCC risk, and may be utilized as a valuable diagnostic biomarker for HNSCC.
Keywords: CDH1, methylation, diagnosis, head and neck squamous cell carcinoma, HNSCC
Introduction
Head and neck cancer is the sixth most common cancer worldwide and the main histological type is head and neck squamous cell carcinoma (HNSCC).1,2 In the US alone, 48,330 new cases of HNSCC and 9,570 deaths from HNSCC are projected to occur in 2016.3 Although there have been some developments in the diagnosis and treatment of HNSCC during the recent decades,4–6 there has been limited improvement in patient survival and mortality rates,7–9 especially for advanced-stage disease and elderly patients.10 Therefore, the development of biomarkers that allow for early detection of HNSCC would be of value at this time.
There are many unknown mechanisms in the etiology and pathogenesis of HNSCC, but the role of alcohol consumption and smoking, as well as infection with high-risk subtypes of human papillomavirus are now known risk factors for HNSCC.11,12 Genetic and epigenetic factors are also involved in the initiation and progression of HNSCC.13,14 More recently, there has been increasing evidence in the published literature that aberrant methylation of cytosine -guanosine dinucleotide (CpG) islands of tumor suppressor gene (TSG) promoter regions is one of the most common epigenetic alterations that has a role in the pathogenesis of HNSCC.15,16 There are now more accurate and easily performed detection methods for DNA methylation, which have provided increasing evidence that abnormal DNA methylation patterns may be potential diagnostic biomarkers for the early detection of HNSCC.17
The CDH1 gene is located on chromosome 16 (16q22.1), encodes a transmembrane 120 kDa glycoprotein, epithelial cadherin (E-cadherin), and is a TSG that plays a role in the invasion and metastasis of human cancers.18,19 Cadherins belong to the family of cell–cell adhesion molecules, which are involved in maintaining intercellular connections and establishing the normal architecture of epithelial tissues.18,19 There has been increasing evidence showing that loss of CDH1 expression is involved in tumor cell invasion and metastasis in cancer, including HNSCC.20–22 Several studies have found that promoter methylation of CDH1 may lead to transcriptional inactivation of CDH1 and that this mechanism is involved in several types of malignancy, including breast,23 gastric,24 and colorectal cancers,25 and HNSCC.26,27
However, among the increasing number of studies on the role of CDH1 promoter methylation and HNSCC, some of the findings of these studies have been contradictory. Some studies have concluded that CDH1 methylation was related to the development of HNSCC.15,28 However, there have been other studies that the association between CDH1 methylation and HNSCC did not reach statistical significance.16,29
Therefore, in the current study, we performed a meta-analysis to quantitatively evaluate the association between CDH1 promoter methylation and HNSCC. Furthermore, we estimated the relationship between CDH1 promoter methylation and clinicopathological parameters in HNSCC. We also assessed the diagnostic value of CDH1 methylation for HNSCC, in order to provide evidence for the future application of CDH1 in the diagnosis of HNSCC.
Materials and methods
Study search strategy
A comprehensive literature search was performed from the following electronic databases: PubMed, Embase, Google Scholar, and Web of Science, without language restrictions. The last search was updated on March 3, 2016. The following key words were used in the database literature search: “methylation” or “DNA methylation” or “promoter methylation” or “demethylation” or “hypermethylation”; “squamous cell carcinoma” or “cancer”; “oral” or “oropharyngeal” or “oropharynx” or “head and neck” or “tonsil”; “CDH1” or “E-cadherin” or “epithelial cadherin” or “cadherin-1” or “uvomorulin”. Additionally, a manual search was conducted to find potentially relevant articles.
Literature selection criteria
The following criteria were used to evaluate the eligibility of included studies: 1) the study focused on the association between CDH1 promoter methylation and HNSCC; 2) all patients had a histologically confirmed diagnosis of HNSCC; and 3) the study provided sufficient information about the frequency of CDH1 promoter methylation. The study was excluded if it could not meet the required inclusion criteria. If the authors had published several studies using the same study population, only the most recent or the study with the largest sample size was included in the meta-analysis.
Data quality assessment
The quality of the studies was assessed according to the Newcastle–Ottawa Scale (NOS) criteria.30 The NOS study quality evaluation system includes three considerations: 1) the subject selection: 0–4 points; 2) comparability of the subject: 0–2 points; and 3) clinical outcome: 0–3 points. The NOS scores range from 0 to 9; a score ≥7 indicates a good quality study.
Data extraction
The data were independently extracted from the eligible studies by two authors using a standard data extraction form, including the first author’s name, country, year of publication, patient ethnicity, sample size, sample type in the case and the control group, clinicopathological characteristics, detection method of methylation and methylation frequency of CDH1 promoter, both in HNSCC cases and controls. Clinicopathological characteristics of the subjects – including age, gender (male vs female), smoking behaviors (cigarette smoking history vs no cigarette smoking history), alcohol consumption (alcohol consumption history vs no alcohol consumption history), differentiation grade (well vs moderate or poor), tumor stage (T1+2 vs T3+4), clinical stage (I + II vs III + IV), lymph node metastasis (yes vs no) – were noted. If there were any disagreements, a third reviewer and consensus were used.
Statistical analysis
In the current study, STATA-12.0 software (Stata Corporation, College Station, TX, USA) was used to analyze the data. The summary odds ratios (ORs) with its corresponding 95% confidence intervals (CIs) were calculated to determine the correlation between CDH1 promoter methylation and HNSCC, as well as the clinicopathological characteristics. Between-study heterogeneity was assessed and visually represented using χ2-based Cochran Q statistic test and I2 test.31,32 If the Q-test showed a P<0.05 or I2>50%, indicating significant heterogeneity, the random effect model (DerSimonian–Laird method)33 was conducted; otherwise, the fixed effect model (Mantel–Haenszel method)34 was used. The sources of heterogeneity were analyzed by meta-regression and subgroup analyses. Subgroup analysis was performed by control types (autogenous vs heterogeneous), ethnicity (African vs Caucasian vs Asian), sample size (≥60 vs <60), methylation detection method (with methylation-specific polymerase chain reaction [MSP] vs without MSP) and publication year (before 2010 vs during or after 2010). To evaluate the effect of single study on the pooled ORs, a sensitivity analysis was performed. The publication bias was exhibited by the funnel plot and assessed by Begg’s linear regression test.35 The Fail safe number (Nfs) was calculated to estimate the influence of publication bias to our conclusion by the Meta package in R (version 3.22, http://www.r-project.org/). The pooled sensitivity, specificity, and area under curve (AUC) of the summary receiver operator characteristic (ROC) with their 95% CIs were analyzed to determine the diagnostic value of CDH1 promoter methylation for HNSCC.36 All the tests were two-sided and a P-value of <0.05 was of statistical significance. All data were computed separately by two investigators and a final consensus was reached.
Results
Baseline characteristics of included studies
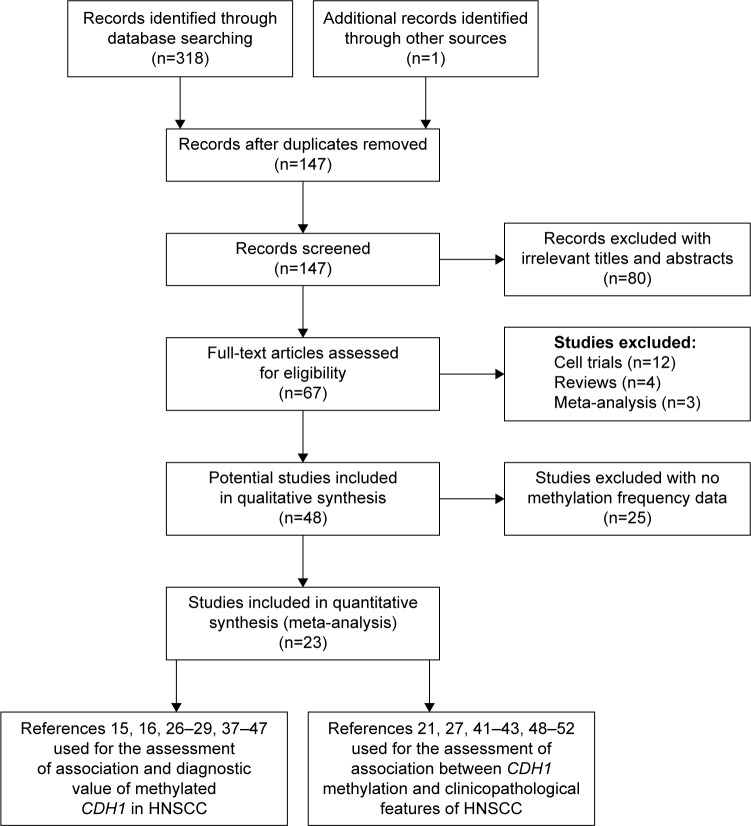
An initial total of 319 publications were selected with 318 publications from database searches and one publication from manual searching. Of these initial 319 publications, 172 studies were excluded due to study duplication and 130 studies due to lack of relevance. In final, there were 23 studies included in our systematic quantitative analysis, including 1,727 cases of HNSCC and 555 control cases. Among these 23 studies, 17 case–control studies assessed the association between CDH1 promoter methylation and HNSCC.15,16,26–29,37–47 Four of these 17 studies also evaluated the relationship of CDH1 promoter methylation and the clinicopathological characteristics of HNSCC.27,41–43 Eventually, a further six studies21,48–52 combined with these four studies were used to quantitatively assess the association between methylated CDH1 and the clinicopathological characteristics of the HNSCC cases. Figure 1 shows the selection procedure of our analysis. The NOS criteria30 scores of all included studies were more than 6. The individual characteristics of the included studies are summarized in Table 1.
Figure 1.
Flow diagram of the study selection process in this meta-analysis.
Abbreviation: HNSCC, head and neck squamous cell carcinoma.
Table 1.
The main characteristics of included studies in this meta-analysis
| References | Year | Country | Ethnicity | Method | Sample type | Case
|
Control
|
Control source | ||
|---|---|---|---|---|---|---|---|---|---|---|
| M | Total | M | Total | |||||||
| Saito et al27 | 1998 | Japan | Asian | MSRE | Tissue | 9 | 52 | 0 | 52 | Autologous |
| Yeh et al38 | 2002 | People’s Republic of China | Asian | MSP | Tissue | 41 | 48 | 16 | 48 | Autologous |
| Chang et al26 | 2002 | People’s Republic of China | Asian | MSRE | Tissue | 45 | 70 | 0 | 11 | Heterogeneous |
| Viswanathan et al37 | 2003 | India | African | MSRE | Tissue | 35 | 99 | 0 | 25 | Autologous |
| Shaw et al29 | 2006 | UK | Caucasian | Pyrosequencing | Tissue | 30 | 71 | 6 | 18 | Autologous |
| de Moraes et al39 | 2008 | Brazil | Caucasian | MSP | Tissue | 27 | 46 | 0 | 5 | Heterogeneous |
| Righini et al40 | 2007 | France | Caucasian | MSP | Tissue | 32 | 90 | 0 | 30 | Autologous |
| Steinmann et al41 | 2009 | Germany | Caucasian | MSP | Tissue | 23 | 54 | 2 | 23 | Autologous |
| Su et al42 | 2010 | People’s Republic of China | Asian | MSP | Tissue | 21 | 31 | 12 | 31 | Autologous |
| Kordi-Tamandani et al43 | 2010 | Iran | Caucasian | MSP | Tissue | 47 | 76 | 31 | 57 | Heterogeneous |
| Weiss et al16 | 2011 | Germany | Caucasian | MSP | Tissue | 13 | 37 | 7 | 31 | Heterogeneous |
| Supic et al28 | 2011 | Serbia | Caucasian | MSP | Tissue | 20 | 47 | 6 | 47 | Autologous |
| Nagata et al44 | 2012 | Japan | Asian | MSP | Rinse | 32 | 34 | 5 | 24 | Heterogeneous |
| Xu et al47 | 2012 | People’s Republic of China | Asian | MSP | Tissue | 22 | 60 | 2 | 50 | Heterogeneous |
| Asokan et al45 | 2014 | India | African | MSP | Tissue | 6 | 10 | 0 | 5 | Heterogeneous |
| Mielcarek-Kuchta et al46 | 2014 | Poland | Caucasian | MSP | Tissue | 24 | 53 | 14 | 53 | Autologous |
| Choudhury and Ghosh15 | 2015 | India | African | MSP | Tissue | 23 | 71 | 4 | 45 | Autologous |
| Hasegawa et al50 | 2002 | USA | Caucasian | MSP | Tissue | 29 | 80 | na | na | na |
| Calmon et al48 | 2007 | Brazil | Caucasian | MSP | Tissue | 38 | 43 | na | na | na |
| Dikshit et al49 | 2007 | France | Caucasian | MSP | Tissue | 82 | 190 | na | na | na |
| Marsit et al51 | 2008 | USA | Caucasian | MSP | Tissue | 113 | 340 | na | na | na |
| Supic et al52 | 2009 | Serbia | Caucasian | nMSP | Tissue | 33 | 77 | na | na | na |
| Pannone et al21 | 2014 | Italy | Caucasian | MSP | Tissue | 14 | 48 | na | na | na |
Notes: Autologous: control from the HNSCC group; Heterogeneous: control from other individuals.
Abbreviations: M, methylation; MSRE, methylation-sensitive restriction endonuclease; MSP, methylation-specific polymerase chain reaction; nMSP, nested methylation-specific polymerase chain reaction; na, not available; HNSCC, head and neck squamous cell carcinoma.
Association between CDH1 promoter methylation and HNSCC risk
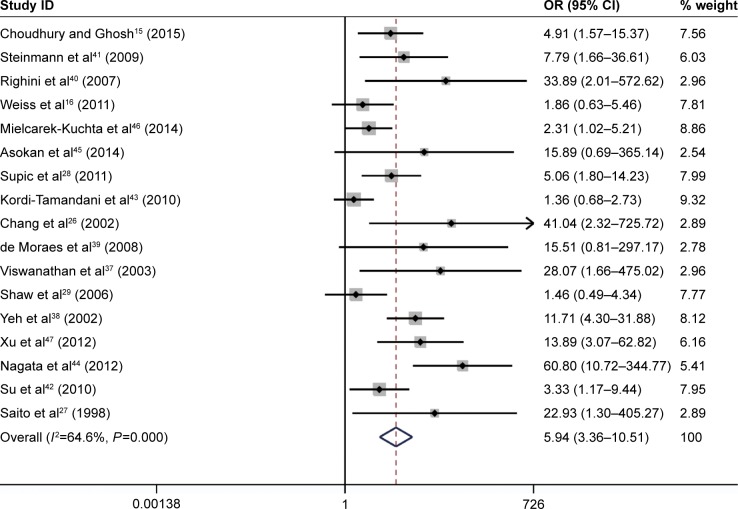
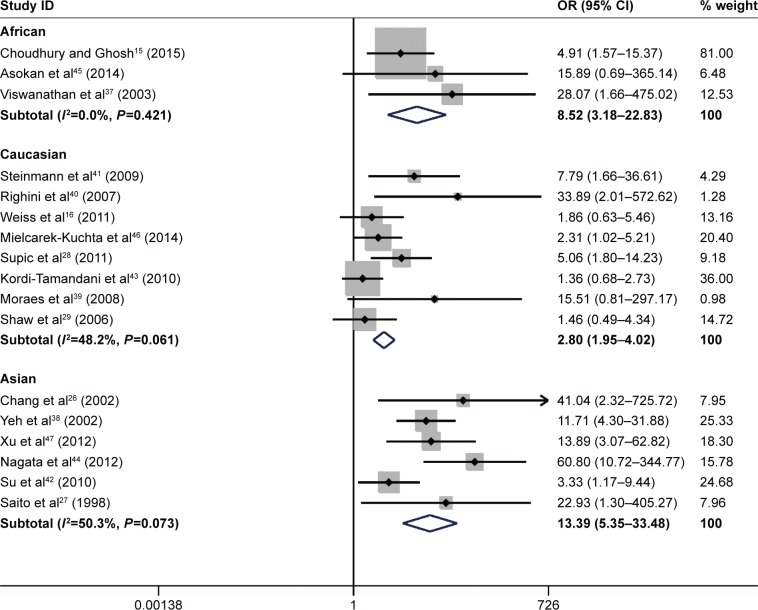
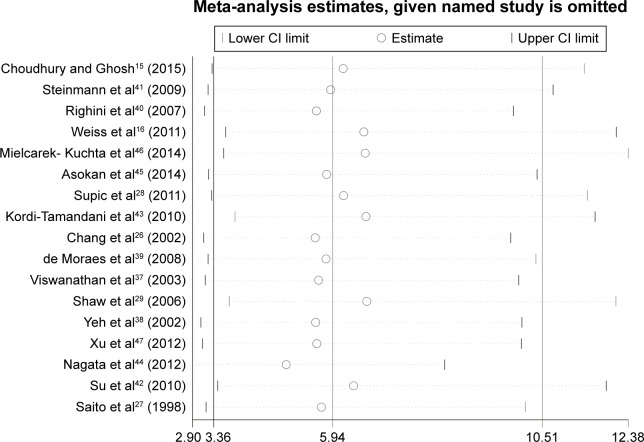
The results of the meta-analysis indicated that the frequency of CDH1 methylation in patients diagnosed with HNSCC was significantly elevated when compared with normal controls (OR =5.94, 95% CI: 3.36–10.51, P<0.001, Figure 2). There was significant heterogeneity across the included studies (I2=64.6%, P<0.001). The potential sources of heterogeneity were investigated by applying meta-regression analysis and subgroup analysis. However, the source of heterogeneity was not identified by meta-regression analysis (Table 2). The subgroup analysis was performed based on ethnicity, control types, sample size, methods for detecting methylation, and the study publication year. In the ethnicity-based stratified analyses, the pooled OR for CDH1 methylation in HNSCC compared with normal controls in the Asian group was 13.39 (95% CI: 5.35–33.48, P<0.001), and was greater than that in the African (OR =8.52, 95% CI: 3.18–22.83, P<0.001) and Caucasian groups (OR =2.80, 95% CI: 1.95–4.02, P<0.001). Furthermore, the degree of heterogeneity was reduced in all the three ethnic subgroups (Figure 3). The heterogeneity did not change remarkably in the other subgroup analysis. The detailed subgroup analysis results are shown in Table 3. Therefore, sensitivity analysis was performed by omitting each study in turn, under the random effects model, which demonstrated that no single study could essentially influence the overall pooled ORs, supporting the robust nature of the meta-analysis (Figure 4).
Figure 2.
Forest plot for evaluating the association between CDH1 promoter methylation and head and neck squamous cell carcinoma (HNSCC) by application of the random-effect model.
Note: Weights are from random effects analysis.
Abbreviations: CI, confidence interval; OR, odds ratio; HNSCC, head and neck squamous cell carcinoma.
Table 2.
Meta-regression analysis based on publication year, ethnicity, detection method, control type, case size
| Heterogeneity sources | Coefficient | 95% CI
|
P-value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Publication year | −0.058 | −0.307 | 0.191 | 0.613 |
| Ethnicity | ||||
| African | −0.102 | −2.89 | 2.686 | 0.936 |
| Caucasian | −1.046 | −2.797 | 0.706 | 0.21 |
| Detection method | ||||
| MSP | −0.578 | −4.056 | 2.89 | 0.716 |
| Pyrosequence | −1.645 | −5.465 | 2.176 | 0.356 |
| Control type | 0.209 | −1.24 | 1.658 | 0.752 |
| Case size | <0.001 | −0.043 | 0.043 | 0.996 |
Abbreviations: CI, confidence interval; MSP, methylation-specific polymerase chain reaction.
Figure 3.
Forest plot for the subgroup analyses by ethnicity.
Abbreviations: CI, confidence interval; OR, odds ratio; HNSCC, head and neck squamous cell carcinoma.
Table 3.
Subgroup analyses of CDH1 promoter methylation in HNSCC
| Subgroup | Case
|
Control
|
M–H pooled OR
|
D–L pooled
|
Heterogeneity
|
|||
|---|---|---|---|---|---|---|---|---|
| M | U | M | U | OR (95% CI) | OR (95% CI) | I2 (%) | P-value | |
| Total | 450 | 499 | 105 | 450 | 4.82 (3.64–6.39) | 5.94 (3.36–10.51) | 64.6 | <0.001 |
| Race | ||||||||
| African | 64 | 116 | 4 | 71 | 8.52 (3.18–22.83) | 6.893 (2.53–18.78) | 0 | 0.42 |
| Caucasian | 216 | 258 | 66 | 198 | 2.80 (1.95–4.02) | 2.803 (1.56–5.03) | 48.2 | 0.06 |
| Asian | 170 | 125 | 35 | 181 | 11.84 (6.74–20.79) | 13.39 (5.35–33.48) | 50.3 | 0.07 |
| Control types | ||||||||
| Autologous | 258 | 358 | 60 | 312 | 5.22 (3.61–7.56) | 4.975 (2.83–8.75) | 46 | 0.05 |
| Heterogeneous | 192 | 141 | 45 | 138 | 4.27 (2.76–6.61) | 8.69 (2.36–31.96) | 78.4 | <0.001 |
| Methods | ||||||||
| MSP | 267 | 271 | 79 | 277 | 4.55 (3.36–6.15) | 5.56 (3.05–10.14) | 65.8 | <0.001 |
| No MSP | 119 | 173 | 6 | 100 | 6.58 (2.98–14.56) | 29.763 (5.72–154.95) | 71.8 | 0.01 |
| Sample size | ||||||||
| <60 | 216 | 196 | 62 | 257 | 5.44 (3.73–7.94) | 5.64 (3.08–10.33) | 56.6 | 0.01 |
| ≥60 | 234 | 303 | 43 | 193 | 4.20 (2.75–6.41) | 10.95 (1.25–96.12) | 73.2 | 0 |
| Published year | ||||||||
| <2010 | 242 | 288 | 24 | 188 | 9.21 (5.24–16.18) | 9.947 (3.77–26.23) | 50.8 | 0.05 |
| ≥2010 | 208 | 211 | 81 | 262 | 3.56 (2.52–4.91) | 4.36 (2.23–8.55) | 67.7 | 0 |
Note: The pooled OR with 95% CI was calculated by appropriate effect model based on heterogeneity and highlighted in boldface.
Abbreviations: CI, confidence interval; HNSCC, head and neck squamous cell carcinoma; M, methylation; MSP, methylation-specific polymerase chain reaction; OR, odds ratio; U, unmethylation.
Figure 4.
Sensitivity analysis of pooled OR for CDH1 promoter methylation and HNSCC under the random effects model.
Abbreviations: CI, confidence interval; OR, odds ratio; HNSCC, head and neck squamous cell carcinoma.
Association between CDH1 promoter methylation and the clinicopathological features of HNSCC
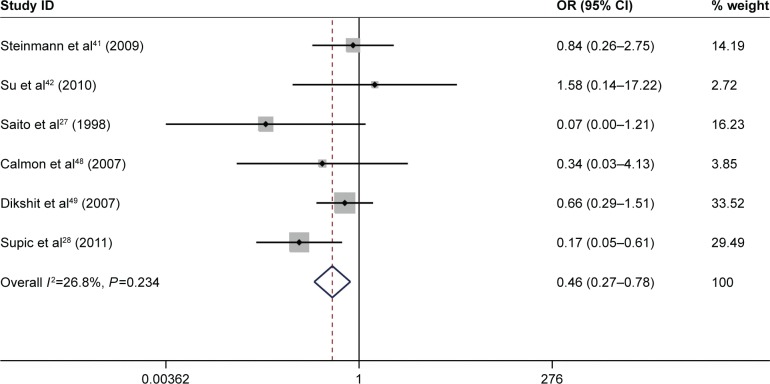
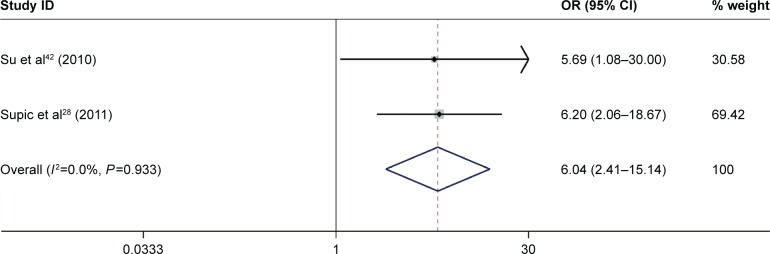
A total of 10 studies, which included 991 patients, were performed to analyze the associations between CDH1 promoter methylation and the HNSCC clinicopathological features, including age, gender, smoking behavior, alcohol consumption, tumor stage, clinical stage, histological tumor grade, and the presence of lymph node metastasis (Table 4). The result showed that the CDH1 promoter methylation was significantly associated with tumor stage (pooled OR =0.46, 95% CI: 0.27–0.78, P=0.004, Figure 5) and alcohol consumption (pooled OR =6.04, 95% CI: 2.41–15.14, P<0.001, Figure 6). However, there was no association between other clinicopathological characteristics and CDH1 promoter methylation in HNSCC.
Table 4.
The association between and CDH1 promoter methylation and the clinicopathological features in HNSCC
| Characteristics | Noa | Case/control types | Cases/controls | OR (95% CI) | P-value | I2% |
|---|---|---|---|---|---|---|
| Age | 4 | Older/younger | 354/329 | 0.84 (0.61–1.15) | 0.277 | 0 |
| Gender | 5 | Male/female | 560/156 | 0.82 (0.55–1.20) | 0.306 | 0 |
| Smoking behavior | 5 | Yes/no | 523/128 | 1.14 (0.76–1.70) | 0.539 | 29.8 |
| Alcohol consumption | 2 | Yes/no | 40/62 | 6.04 (2.41–15.14) | <0.001 | 0 |
| Differentiation grade | 4 | Well/moderate or poor | 61/115 | 0.42 (0.08–2.24) | 0.312 | 56.7 |
| Tumor stage | 6 | T1+2/T3+4 | 139/234 | 0.46 (0.27–0.78) | 0.004 | 26.8 |
| Clinical stage | 4 | I + II/III + IV | 65/170 | 0.63 (0.33–1.18) | 0.149 | 25 |
| Lymph node metastasis | 6 | Yes/no | 191/182 | 0.93 (0.56–1.56) | 0.794 | 41.4 |
Note:
The number of included studies.
Abbreviations: CI, confidence interval; OR, odds ratio; HNSCC, head and neck squamous cell carcinoma.
Figure 5.
Forest plot for the associations between CDH1 promoter methylation and tumor stage in HNSCC.
Abbreviations: CI, confidence interval; OR, odds ratio; HNSCC, head and neck squamous cell carcinoma.
Figure 6.
Forest plot for the associations between CDH1 promoter methylation and alcohol consumption in HNSCC.
Abbreviations: CI, confidence interval; OR, odds ratio; HNSCC, head and neck squamous cell carcinoma.
Diagnostic value of CDH1 promoter methylation for HNSCC
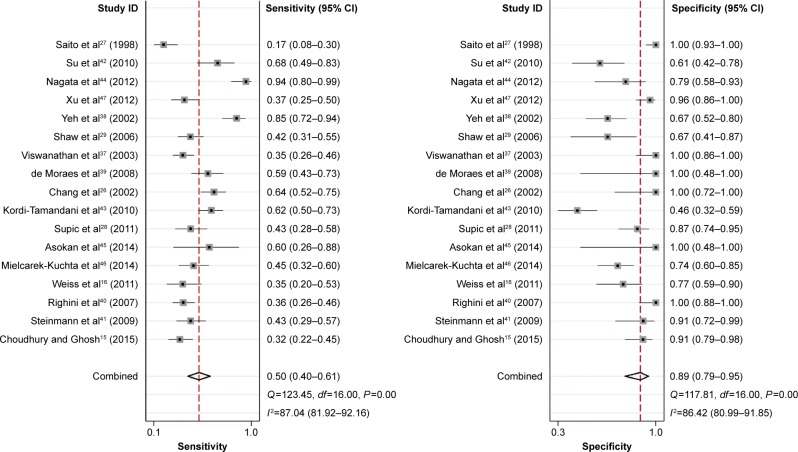
In the current analysis, 17 eligible case–control studies were included to assess the diagnostic value of CDH1 promoter methylation for HNSCC. Figure 7 shows the pooled sensitivity and specificity for all included studies, which were 0.50 (95% CI: 0.40–0.61) and 0.89 (95% CI: 0.79–0.95). The AUC of the summary ROC was 0.74 (95% CI: 0.70–0.78) (Figure 8), indicating that the detection of CDH1 methylation was associated with a diagnosis of HNSCC, representing a potential diagnostic biomarker.
Figure 7.
Forest sensitivity and specificity of CDH1 promoter methylation for head and neck squamous cell carcinoma (HNSCC).
Abbreviations: CI, confidence interval; HNSCC, head and neck squamous cell carcinoma.
Figure 8.
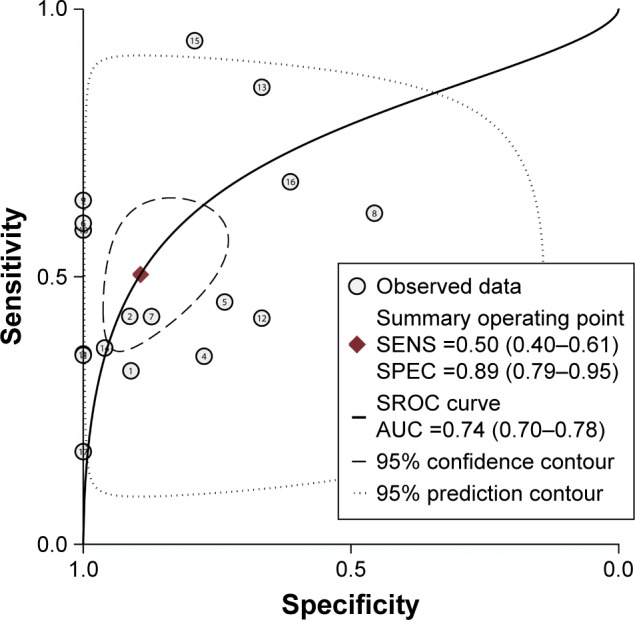
SROC plot with best-fitting asymmetric curve of methylated CDH1 for the diagnosis of HNSCC.
Abbreviations: AUC, area under curve; HNSCC, head and neck squamous cell carcinoma; SENS, sensitivity; SPEC, specificity; SROC, summary of receiver operating characteristic.
Publication bias
A Begg’s funnel plot was performed to assess the publication bias of literatures. Figure 9 shows that the shape of the funnel plot showed no evidence of publication bias (P=0.077). Furthermore, we conducted an Nfs to assess the efficacy of the meta-analysis (Nfs0.05=611, Nfs0.01=181), which indicated that our results were robust.
Figure 9.
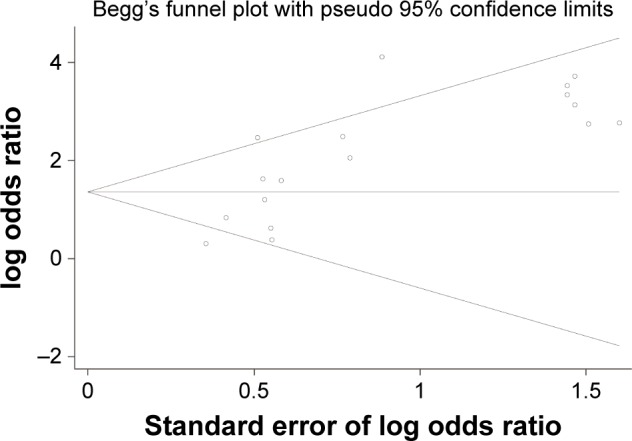
Begg’s funnel plot of publication bias.
Discussion
Previous studies have shown that hypermethylation of TSG promoters in many cancers can contribute to tumor progression.53,54 Specifically, CDH1, which encodes the cell adhesion protein E-cadherin, is an important TSG.55 Studies have shown that loss of CDH1 expression by promoter hypermethylation is involved in several types of cancer, including colorectal,56 lung,57 breast,23 and gastric cancers.58 This meta-analysis was done to resolve some of the inconsistent reports of the association between CDH1 promoter methylation and HNSCC.15,16
In this meta-analysis, a total of 23 studies included 1,727 cases of HNSCC (and 555 control cases). The results showed that the frequency of CDH1 promoter methylation in HNSCC was statistically greater than in controls (OR =5.94, 95% CI: 3.36–10.51, P<0.001). In reported cases of HNSCC, CDH1 promoter methylation was associated with tumor stage (OR =0.46, 95% CI: 0.27–0.78, P=0.004) and a history of alcohol consumption (OR =6.04, 95% CI: 2.41–15.14, P<0.001). The findings demonstrated almost sixfold greater level of CDH1 methylation in the HNSCC patient group compared with normal controls, indicating that hypermethylation of CDH1 was strongly associated with HNSCC, which would support its role as a diagnostic biomarker. The reduced value of I2 found in the stratified analysis by ethnicity indicated that ethnicity might account for some of the study heterogeneity. The OR of the Asian subgroup with HNSCC was greater than that of the Caucasian and African subgroups with HNSCC, indicating that the Asian population may be more susceptible to CDH1 promoter methylation, which is supported by a previous study.59
This study showed that there was an increased frequency of CDH1 promoter methylation with more advanced tumor stage HNSCC compared with early tumor stage disease, which may also support a role for CDH1 promoter methylation in the invasion progression of HNSCC. Alcohol consumption is a known predisposing factor for HNSCC60 and has been shown to induce DNA methylation in oncogenesis.61 In the current study, CDH1 promoter methylation was significantly increased in patients with high alcohol consumption, indicating that it may contribute to HNSCC via the induction of hypermethylation of CDH1. These findings support the need for further controlled studies with large patient sample sizes to evaluate these ethnic, social, and etiological factors involved in the etiology and pathogenesis of HNSCC.
The findings of this meta-analysis support a possible diagnostic role for CDH1 promoter methylation evaluation in HNSCC, with pooled sensitivity and specificity of 0.5 and 0.89, respectively. Previous studies have shown that the combination of several methylation biomarkers can improve the sensitivity and specificity of diagnosis testing for cancers, including HNSCC.62–64 Therefore, it would be logical to combine CDH1 methylation testing with other epigenetic biomarkers. This combined diagnostic approach requires further studies to determine the diagnostic power in HNSCC. When the AUC of the ROC is close to 1.0, this signifies a good risk predictor,65,66 and in this study, the AUC for detection of CDH1 promoter methylation in HNSCC was 0.74, indicating a qualified diagnostic accuracy for CDH1 promoter methylation in HNSCC.
The present meta-analysis had several limitations. First, it must be acknowledged that studies with positive findings on CDH1 promoter methylation in HNSCC are more likely to be those that are published, resulting in possible publication bias. Second, a significant heterogeneity was observed in the data analysis, which means that the findings should be interpreted with caution. Third, although studies in all languages were included, it is possible that relevant studies published in other languages may have been missed. Therefore, in future, we recommend that an updated meta-analysis, including more high quality studies with larger sample sizes, should be done to support or add to the findings of this present study.
Conclusion
In summary, our meta-analysis results have supported the role of promoter methylation of CDH1 in the diagnosis of HNSCC. These findings may have implications for a future role of this biomarker in the diagnosis of HNSCC.
Acknowledgments
The research was supported by grants from the Zhejiang Provincial Natural Science Foundation of China (LY14H160003), the Scientific Innovation Team Project of Ningbo (2012B82019), the Ningbo Social Developmental Key Research Project (2012C5015), the Ningbo Natural Science Foundation (2012A610208 and 2012A610217), and the Medical, and Health Research Project of Zhejiang Province (2012ZDA042 and 2014PYA017).
Footnotes
Author contributions
ZSS and CCZ conceived and designed the experiments. CCZ and JYL performed the experiments. CCZ and JW analyzed the data. CCZ, HXD, and QL contributed analysis tools. CCZ and JYL wrote the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Vokes EE, Weichselbaum RR, Lippman SM, Hong WK. Head and neck cancer. N Engl J Med. 1993;328(3):184–194. doi: 10.1056/NEJM199301213280306. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Cmelak AJ, Arneson K, Chau NG, Gilbert RW, Haddad RI. Locally advanced head and neck cancer. Am Soc Clin Oncol Educ Book. 2013:237–244. doi: 10.14694/EdBook_AM.2013.33.237. [DOI] [PubMed] [Google Scholar]
- 5.Matar N, Haddad A. New trends in the management of head and neck cancers. J Med Liban. 2011;59(4):220–226. [PubMed] [Google Scholar]
- 6.Pai SI, Westra WH. Molecular pathology of head and neck cancer: implications for diagnosis, prognosis, and treatment. Annu Rev Pathol. 2009;4:49–70. doi: 10.1146/annurev.pathol.4.110807.092158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345(26):1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 8.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 9.Mao L, Hong WK, Papadimitrakopoulou VA. Focus on head and neck cancer. Cancer Cell. 2004;5(4):311–316. doi: 10.1016/s1535-6108(04)00090-x. [DOI] [PubMed] [Google Scholar]
- 10.Milet PR, Mallet Y, El Bedoui S, Penel N, Servent V, Lefebvre JL. Head and neck cancer surgery in the elderly–does age influence the postoperative course? Oral Oncol. 2010;46(2):92–95. doi: 10.1016/j.oraloncology.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Chor JS, Vlantis AC, Chow TL, et al. The role of human papillomavirus in head and neck squamous cell carcinoma: a case control study on a southern Chinese population. J Med Virol. 2016;88(5):877–887. doi: 10.1002/jmv.24405. [DOI] [PubMed] [Google Scholar]
- 12.van Imhoff LC, Kranenburg GG, Macco S, et al. Prognostic value of continued smoking on survival and recurrence rates in patients with head and neck cancer: a systematic review. Head Neck. 2016;38(Suppl 1):E2214–E2220. doi: 10.1002/hed.24082. [DOI] [PubMed] [Google Scholar]
- 13.Lechner M, Fenton TR. The genomics, epigenomics, and transcriptomics of HPV-associated oropharyngeal cancer–understanding the basis of a rapidly evolving disease. Adv Genet. 2016;93:1–56. doi: 10.1016/bs.adgen.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Menendez ST, Villaronga MA, Rodrigo JP, et al. HERG1A potassium channel is the predominant isoform in head and neck squamous cell carcinomas: evidence for regulation by epigenetic mechanisms. Sci Rep. 2016;6:19666. doi: 10.1038/srep19666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhury JH, Ghosh SK. Promoter hypermethylation profiling identifies subtypes of head and neck cancer with distinct viral, environmental, genetic and survival characteristics. PLoS One. 2015;10(6):e0129808. doi: 10.1371/journal.pone.0129808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss D, Basel T, Sachse F, Braeuninger A, Rudack C. Promoter methylation of cyclin A1 is associated with human papillomavirus 16 induced head and neck squamous cell carcinoma independently of p53 mutation. Mol Carcinog. 2011;50(9):680–688. doi: 10.1002/mc.20798. [DOI] [PubMed] [Google Scholar]
- 17.Arantes LM, de Carvalho AC, Melendez ME, Carvalho AL, Goloni-Bertollo EM. Methylation as a biomarker for head and neck cancer. Oral Oncol. 2014;50(6):587–592. doi: 10.1016/j.oraloncology.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Hartley R, Reiss B, et al. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell Mol Life Sci. 2012;69(16):2779–2789. doi: 10.1007/s00018-012-0951-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moriyama N, Ishihara S, Hirose M, Watanabe S, Sato N, Kinoshita Y. E-cadherin is essential for gastric epithelial restitution in vitro: a study using the normal rat gastric mucosal cell line RGM1. J Lab Clin Med. 2001;138(4):236–242. doi: 10.1067/mlc.2001.118177. [DOI] [PubMed] [Google Scholar]
- 20.Mayer B, Johnson JP, Leitl F, et al. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993;53(7):1690–1695. [PubMed] [Google Scholar]
- 21.Pannone G, Santoro A, Feola A, et al. The role of E-cadherin down-regulation in oral cancer: CDH1 gene expression and epigenetic blockage. Curr Cancer Drug Targets. 2014;14(2):115–127. doi: 10.2174/1568009613666131126115012. [DOI] [PubMed] [Google Scholar]
- 22.Yu Z, Sun M, Jin F, et al. Combined expression of ezrin and E-cadherin is associated with lymph node metastasis and poor prognosis in breast cancer. Oncol Rep. 2015;34(1):165–174. doi: 10.3892/or.2015.3967. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Sun X, Qin S, et al. CDH1 promoter methylation correlates with decreased gene expression and poor prognosis in patients with breast cancer. Oncol Lett. 2016;11(4):2635–2643. doi: 10.3892/ol.2016.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XW, Shi BY, Yang QL, et al. Epigenetic regulation of CDH1 exon 8 alternative splicing in gastric cancer. BMC Cancer. 2015;15:954. doi: 10.1186/s12885-015-1983-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michailidi C, Theocharis S, Tsourouflis G, et al. Expression and promoter methylation status of hMLH1, MGMT, APC, and CDH1 genes in patients with colon adenocarcinoma. Exp Biol Med (Maywood) 2015;240(12):1599–1605. doi: 10.1177/1535370215583800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang HW, Chow V, Lam KY, Wei WI, Yuen A. Loss of E-cadherin expression resulting from promoter hypermethylation in oral tongue carcinoma and its prognostic significance. Cancer. 2002;94(2):386–392. doi: 10.1002/cncr.10211. [DOI] [PubMed] [Google Scholar]
- 27.Saito Y, Takazawa H, Uzawa K, Tanzawa H, Sato K. Reduced expression of E-cadherin in oral squamous cell carcinoma: relationship with DNA methylation of 5′ CpG island. Int J Oncol. 1998;12(2):293–298. doi: 10.3892/ijo.12.2.293. [DOI] [PubMed] [Google Scholar]
- 28.Supic G, Kozomara R, Jovic N, Zeljic K, Magic Z. Prognostic significance of tumor-related genes hypermethylation detected in cancer-free surgical margins of oral squamous cell carcinomas. Oral Oncol. 2011;47(8):702–708. doi: 10.1016/j.oraloncology.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Shaw RJ, Liloglou T, Rogers SN, et al. Promoter methylation of P16, RARbeta, E-cadherin, cyclin A1 and cytoglobin in oral cancer: quantitative evaluation using pyrosequencing. Br J Cancer. 2006;94(4):561–568. doi: 10.1038/sj.bjc.6602972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 31.Coory MD. Comment on: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2010;39(3):932. doi: 10.1093/ije/dyp157. author reply 933. [DOI] [PubMed] [Google Scholar]
- 32.Zintzaras E, Ioannidis JP. HEGESMA: genome search meta-analysis and heterogeneity testing. Bioinformatics. 2005;21(18):3672–3673. doi: 10.1093/bioinformatics/bti536. [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–145. doi: 10.1016/j.cct.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. [PubMed] [Google Scholar]
- 35.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295(6):676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 36.Deville WL, Buntinx F, Bouter LM, de Vet HC, van der Windt DA, Bezemer PD. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer. 2003;105(1):41–46. doi: 10.1002/ijc.11028. [DOI] [PubMed] [Google Scholar]
- 38.Yeh KT, Shih MC, Lin TH, et al. The correlation between CpG methylation on promoter and protein expression of E-cadherin in oral squamous cell carcinoma. Anticancer Res. 2002;22(6C):3971–3975. [PubMed] [Google Scholar]
- 39.de Moraes RV, Oliveira DT, Landman G, et al. E-cadherin abnormalities resulting from CPG methylation promoter in metastatic and nonmetastatic oral cancer. Head Neck. 2008;30(1):85–92. doi: 10.1002/hed.20666. [DOI] [PubMed] [Google Scholar]
- 40.Righini CA, de Fraipont F, Timsit JF, et al. Tumor-specific methylation in saliva: a promising biomarker for early detection of head and neck cancer recurrence. Clin Cancer Res. 2007;13(4):1179–1185. doi: 10.1158/1078-0432.CCR-06-2027. [DOI] [PubMed] [Google Scholar]
- 41.Steinmann K, Sandner A, Schagdarsurengin U, Dammann RH. Frequent promoter hypermethylation of tumor-related genes in head and neck squamous cell carcinoma. Oncol Rep. 2009;22(6):1519–1526. doi: 10.3892/or_00000596. [DOI] [PubMed] [Google Scholar]
- 42.Su PF, Huang WL, Wu HT, Wu CH, Liu TY, Kao SY. p16(INK4A) promoter hypermethylation is associated with invasiveness and prognosis of oral squamous cell carcinoma in an age-dependent manner. Oral Oncol. 2010;46(10):734–739. doi: 10.1016/j.oraloncology.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Kordi-Tamandani DM, Moazeni-Roodi AK, Rigi-Ladiz MA, Hashemi M, Birjandian E, Torkamanzehi A. Promoter hypermethylation and expression profile of MGMT and CDH1 genes in oral cavity cancer. Arch Oral Biol. 2010;55(10):809–814. doi: 10.1016/j.archoralbio.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Nagata S, Hamada T, Yamada N, et al. Aberrant DNA methylation of tumor-related genes in oral rinse: a noninvasive method for detection of oral squamous cell carcinoma. Cancer. 2012;118(17):4298–4308. doi: 10.1002/cncr.27417. [DOI] [PubMed] [Google Scholar]
- 45.Asokan GS, Jeelani S, Gnanasundaram N. Promoter hypermethylation profile of tumour suppressor genes in oral leukoplakia and oral squamous cell carcinoma. J Clin Diagn Res. 2014;8(10):ZC09–ZC12. doi: 10.7860/JCDR/2014/9251.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mielcarek-Kuchta D, Paluszczak J, Seget M, et al. Prognostic factors in oral and oropharyngeal cancer based on ultrastructural analysis and DNA methylation of the tumor and surgical margin. Tumour Biol. 2014;35(8):7441–7449. doi: 10.1007/s13277-014-1958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C, Zhao J, Loo WT, et al. Correlation of epigenetic change and identification of risk factors for oral submucous fibrosis. Int J Biol Markers. 2012;27(4):e314–e321. doi: 10.5301/JBM.2012.9937. [DOI] [PubMed] [Google Scholar]
- 48.Calmon MF, Colombo J, Carvalho F, et al. Methylation profile of genes CDKN2A (p14 and p16), DAPK1, CDH1, and ADAM23 in head and neck cancer. Cancer Genet Cytogenet. 2007;173(1):31–37. doi: 10.1016/j.cancergencyto.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 49.Dikshit RP, Gillio-Tos A, Brennan P, et al. Hypermethylation, risk factors, clinical characteristics, and survival in 235 patients with laryngeal and hypopharyngeal cancers. Cancer. 2007;110(8):1745–1751. doi: 10.1002/cncr.22975. [DOI] [PubMed] [Google Scholar]
- 50.Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21(27):4231–4236. doi: 10.1038/sj.onc.1205528. [DOI] [PubMed] [Google Scholar]
- 51.Marsit CJ, Posner MR, McClean MD, Kelsey KT. Hypermethylation of E-cadherin is an independent predictor of improved survival in head and neck squamous cell carcinoma. Cancer. 2008;113(7):1566–1571. doi: 10.1002/cncr.23770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Supic G, Kozomara R, Brankovic-Magic M, Jovic N, Magic Z. Gene hypermethylation in tumor tissue of advanced oral squamous cell carcinoma patients. Oral Oncol. 2009;45(12):1051–1057. doi: 10.1016/j.oraloncology.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Gong C, Fujino K, Monteiro LJ, et al. FOXA1 repression is associated with loss of BRCA1 and increased promoter methylation and chromatin silencing in breast cancer. Oncogene. 2015;34(39):5012–5024. doi: 10.1038/onc.2014.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gyorffy B, Bottai G, Fleischer T, et al. Aberrant DNA methylation impacts gene expression and prognosis in breast cancer subtypes. Int J Cancer. 2016;138(1):87–97. doi: 10.1002/ijc.29684. [DOI] [PubMed] [Google Scholar]
- 55.Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- 56.Almeida FG, de Aquino PF, de Souza AD, et al. Colorectal cancer DNA methylation patterns from patients in Manaus, Brazil. Biol Res. 2015;48:50. doi: 10.1186/s40659-015-0042-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu PW, Xu HY, Liu XN, et al. Aberrant promoter methylation of cell adhesion-related genes associated with clinicopathologic features in non-small cell lung cancer in China. Cancer Biomark. 2013;13(2):115–122. doi: 10.3233/CBM-130341. [DOI] [PubMed] [Google Scholar]
- 58.Lee KH, Hwang D, Kang KY, et al. Frequent promoter methylation of CDH1 in non-neoplastic mucosa of sporadic diffuse gastric cancer. Anticancer Res. 2013;33(9):3765–3774. [PubMed] [Google Scholar]
- 59.Chmielewski J, Jaremin B, Gandurski P. Intravascular clotting syndrome in malaria treated with heparin. Pol Tyg Lek. 1974;29(16):667–668. Polish. [PubMed] [Google Scholar]
- 60.Zhang Y, Wang R, Miao L, Zhu L, Jiang H, Yuan H. Different levels in alcohol and tobacco consumption in head and neck cancer patients from 1957 to 2013. PLoS One. 2015;10(4):e0124045. doi: 10.1371/journal.pone.0124045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moon JW, Lee SK, Lee YW, et al. Alcohol induces cell proliferation via hypermethylation of ADHFE1 in colorectal cancer cells. BMC Cancer. 2014;14:377. doi: 10.1186/1471-2407-14-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen K, Sawhney R, Khan M, et al. Methylation of multiple genes as diagnostic and therapeutic markers in primary head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133(11):1131–1138. doi: 10.1001/archotol.133.11.1131. [DOI] [PubMed] [Google Scholar]
- 63.Shan M, Yin H, Li J, et al. Detection of aberrant methylation of a six-gene panel in serum DNA for diagnosis of breast cancer. Oncotarget. 2016;7(14):18485–18494. doi: 10.18632/oncotarget.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang K, Tian Y, Xu H. Improved noninvasive bladder cancer diagnosis using urine sediments and novel DNA methylation biomarker panels. Clin Lab. 2016;62(3):327–336. doi: 10.7754/clin.lab.2015.150602. [DOI] [PubMed] [Google Scholar]
- 65.Jones CM, Athanasiou T. Summary receiver operating characteristic curve analysis techniques in the evaluation of diagnostic tests. Ann Thorac Surg. 2005;79(1):16–20. doi: 10.1016/j.athoracsur.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 66.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561–577. [PubMed] [Google Scholar]