Abstract
Purpose of Review
To highlight recent studies which have examined the cell signalling mechanisms responsible for the amino acid (primarily leucine and the essential amino acids) stimulation of human skeletal muscle protein synthesis.
Recent Findings
Ingestion of a leucine-enriched essential amino acid nutrient solution rapidly and potently activates the mammalian target of rapamycin (mTOR) signalling pathway and protein synthesis in human skeletal muscle. Further, mTOR signalling and muscle protein synthesis are enhanced when leucine-enriched nutrients are ingested following resistance exercise. The addition of leucine to regular meals may improve the ability of feeding to stimulate protein synthesis in old human muscle.
Summary
Leucine and essential amino acids appear to stimulate human muscle protein synthesis primarily by activating the mTOR signalling pathway. How human muscle cells sense an increase in leucine and/or essential amino acids to activate mTOR signalling is currently unknown. However, recent work suggests that the kinases hVps34 and MAP43K may be involved. Leucine-enriched essential amino acid ingestion, in combination with resistance exercise in some cases, may be a useful intervention to promote mTOR signalling and protein synthesis in an effort to counteract a variety of muscle wasting conditions (e.g. sarcopenia, cachexia, AIDS, inactivity/bed-rest, sepsis, kidney failure, and trauma).
Keywords: amino acids, human skeletal muscle, leucine, mTOR, protein synthesis
Introduction
Muscle wasting is common in conditions such as cancer, AIDS, trauma, and sepsis, and is particularly prevalent in the elderly and during periods of physical inactivity including bed-rest, weightlessness and bone fractures [1]. Skeletal muscle comprises about 40% of body weight and contains 50-75% of all proteins in the human body [2]. The turnover of total body proteins, which is comprised of the simultaneous and energetic processes of protein synthesis and protein breakdown, may account for ~20% of resting energy expenditure [2]. In fact, quantitative estimates suggest that about 1-2% of all skeletal muscle is synthesized and broken down daily [3]. Even though skeletal muscle protein turnover is relatively slow in comparison to other tissues, the predominance of skeletal muscle means that it accounts for a large part of whole body protein turnover. A key role of skeletal muscle is to convert chemical energy into mechanical energy for movement. However, muscle is also an important regulator of metabolism by serving as a storage site of energy and nitrogen, providing gluconeogenic substrates and fuels for other tissues, and playing an important role in delivering substrate during malnutrition, starvation, injury, and disease [4].
In all conditions that lead to muscle wasting it is imperative to implement interventions to maintain and, possibly, increase skeletal muscle mass in order to reduce complications, improve function, and increase survival. Two useful interventions which can be used almost universally to counteract muscle loss with no major contraindications are nutrition and resistance exercise. However, for such interventions to be effective it is important to precisely understand the mechanisms by which they lead to muscle growth. There have been several recent studies which have begun to examine the cellular
mechanisms responsible for the activation of human skeletal muscle protein synthesis. The focus of the current review is to examine the cellular signalling mechanisms responsible for how nutrients (primarily leucine and leucine-enriched essential amino acid solutions) stimulate muscle protein synthesis in humans. In addition, we will also discuss recent resistance exercise studies and the role of providing nutrients following exercise to further promote muscle anabolism. The current review will primarily discuss the latest work in human skeletal muscle in vivo, however, it must be acknowledged that important work has been done examining the role of amino acids, signalling, and exercise in the regulation of muscle protein synthesis in non-human models [5-7].
Essential Amino acids and the Regulation of Human Muscle Protein Synthesis
It is well known that feeding stimulates protein synthesis [8;9]. However, the stimulation of muscle protein synthesis is primarily due to essential amino acids since protein synthesis is readily stimulated when only essential amino acids are ingested [10;11]. Furthermore, the rate of muscle protein synthesis is stimulated to a similar extent when the non-essential amino acids are removed from a mixed amino acid mixture [11]. In addition, infusion of leucine [12;13] and other essential amino acids such as phenylalanine and threonine, but not non-essential amino acids, into human subjects stimulates muscle protein synthesis [14]. Therefore, the current consensus is that essential amino acids (leucine in particular) are responsible for the amino acid stimulation of human muscle protein synthesis.
Signalling Pathways and the Control of Translation Initiation and Elongation
Several intracellular signalling pathways that sense amino acids, transmit the signal to the cell interior, and activate the initiation of translation of proteins from mRNA have been identified in animal studies [15;16]. The anabolic actions of leucine and insulin appear to activate independent intracellular signalling pathways which converge at the mammalian target of rapamycin (mTOR) and eventually affect translation initiation and elongation. Specifically insulin activates phosphatidylinositol 3-kinase (PI3K) and protein kinase B (PKB/Akt). Akt phosphorylates and inhibits tuberous sclerosis complex (TSC2) which relieves inhibition on Rheb (Ras homologue enriched in brain) and allows activation of mTOR. mTOR can then phosphorylate downstream effectors such as eukaryotic initiation factor 2, 4E binding protein 1 (4E-BP1), and ribosomal S6 kinase 1 (S6K1) to promote translation initiation and elongation [17].
The mechanisms of the anabolic actions of amino acids and/or leucine are elusive but are independent of Akt activation [18]. For instance, Anthony et al used a specific mTOR inhibitor (rapamycin) in rats and demonstrated that leucine was not able to stimulate muscle protein synthesis, binding of eIF4E with eIF4G, or phosphorylation of S6K1 [13] thus making a case that leucine stimulates muscle protein synthesis via the mTOR pathway. Recently it has been demonstrated that a class 3 PI3K, hVps34, could mediate the amino acid activation of mTOR and its downstream substrates [19;20]. In addition, a recent study in Drosophila has identified a unique MAP kinase (MAP4K3) which appears to sense amino acids and signal directly to mTOR independent of the insulin signalling pathway [21*]. These results indicate that mTOR acts as a nutrient sensor for leucine and essential amino acids. However, the cellular mechanisms for how hVps34 or MAP4K3 sense the amino acids and signal to mTOR are unknown. It is also not known if these proteins perform a similar function in human skeletal muscle.
Leucine and Essential Amino Acid Signalling in Skeletal Muscle
Several studies in rats and cells have shown the anabolic potency of amino acids and particularly leucine on mTOR signalling and skeletal muscle protein synthesis [13;15;22-24]. For example, it has been demonstrated in L6 myoblasts that leucine increased the phosphorylation of 4E-BP1, S6K1 and its downstream target S6, eukaryotic elongation factor 1A and protein synthesis. These findings were abolished with the administration of rapamycin [22]. In another study, Bolster et al. identified in rat skeletal muscle that the binding of eIF4E to eIF4G was enhanced and the phosphorylation of eIF4G increased after a supraphysiological dose of leucine [23].
However, in the last few years, these amino acid studies have been extended into human skeletal muscle [11;25-27] . For instance, infusion of leucine or an essential amino acid mixture in humans increases the phosphosphorylation of S6K1 and 4E-BP1 while increasing protein synthesis [28;29]. We have recently shown that an oral ingestion of amino acids is just as effective at increasing human muscle protein synthesis as when amino acids are provided intravenously [30]. Hence, when 10 grams of essential amino acids are ingested muscle protein synthesis and components of the mTOR signalling pathway are activated when measured 3 hours following ingestion [31].
Ingestion of an amino acid mixture with extra leucine has also gained attention due to the potency of leucine. Crozier et al. demonstrated that following incremental amounts of leucine administration in rats, a dose of 0.14 g leucine/kg body weight produced a near maximal increase in protein synthesis [24]. Increasing the amount of leucine did not further stimulate muscle protein synthesis although components of mTOR signalling tended to be dose-dependent [24]. In our laboratory we have conducted several human studies in which we provide an essential amino acid mixture enriched in leucine. The dose of leucine is somewhat equivalent to that of Crozier et al. and is approximately 0.12 g leucine/kg of lean body mass. In a recent investigation from our laboratory, 14 young subjects were studied before and 1 hour after an oral ingestion of a leucine-enriched essential amino acid and carbohydrate (EAA+CHO; ~20 grams of EAA + 30 grams of CHO) solution. We found that the phosphorylation status of Akt, mTOR, 4E-BP1 and S6K1 were increased while the phosphorylation status of eukaryotic elongation factor 2 (eEF2) was decreased [32**]. Of particular note was the rapid and very large increases detected in mTOR and S6K1 phosphorylation following the EAA+CHO ingestion. Muscle protein synthesis increased by ~100% concurrently with the increase in mTOR signaling [32]. Another interesting finding was the decreased phosphorylation of AMP-activated protein kinase (AMPK) and presumably its activity. AMPK is a well-known energy sensor and when activated it has been identified as an upstream negative regulator of mTOR [33]. We concluded that an oral ingestion of leucine enriched EAA+CHO is sensed in human skeletal muscle by both AMPK and mTOR which promotes signalling involved in the regulation of translation initiation as well as elongation.
Protein and Essential Amino Acids following Resistance Exercise
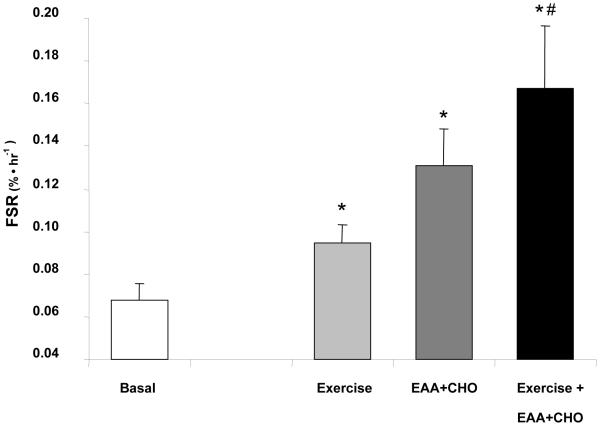
When essential amino acids are ingested following exercise [34] the rate of muscle protein synthesis increases more than when amino acids are ingested at rest or when resistance exercise is performed in the fasting state [11]. Several studies in humans have indicated the powerful effects of protein or essential amino acid ingestion on protein synthesis during post-exercise recovery [10;34-38]. However, only recently have studies investigated the mechanisms behind the increase in muscle protein synthesis [39-41]. Karlsson and colleagues demonstrated that the post-exercise S6K1 and S6 phosphorylation were greater following essential amino acid ingestion as compared to the effects of resistance exercise alone [39]. This finding is supported by Cuthbertson et al. in which they demonstrated a robust increase in S6K1 phosphorylation and muscle protein synthesis when large amounts of EAA+CHO were provided during recovery following a less intense type of exercise protocol [40*]. We have recently extended these findings by providing a leucine-enriched EAA+CHO mixture to young subjects 1 hour following a bout of heavy resistance exercise. At 2 hours post-exercise, muscle was sampled and analyzed for changes in muscle protein synthesis and several components of the mTOR signalling pathway. We found that both mTOR signalling and muscle protein synthesis were enhanced when the leucine-enriched EAA+CHO was ingested following resistance exercise [42*]. Specifically, the rate of muscle protein synthesis increased by 145% when the leucine-enriched EAA+CHO nutrient solution was ingested following resistance exercise as compared to only a 40% increase when nutrients were not ingested following exercise [43*]. In Figure 1, we show that the combination of resistance exercise followed by an oral ingestion of leucine-enriched EAA+CHO stimulated muscle protein synthesis to a greater extent (145% vs. 100%) than ingesting the same nutrients without the exercise stimulus [32]. A recent report by Koopman et al. has also shown that providing protein hydryolysate following a bout of resistance exercise enhances S6K1 phosphorylation in human muscle as compared to exercise alone which provides more support for the notion that essential amino acid provision post-exercise further increases human skeletal muscle protein synthesis by augmenting mTOR signalling [41*].
Figure 1.
Independent and combined effects of resistance exercise and leucine-enriched essential amino acid and carbohydrate (EAA+CHO) ingestion on muscle protein synthesis. Data are taken from three separate publications [32;42;43]. Baseline is the rate of muscle protein synthesis following an overnight fast in 13 young male subjects. Exercise data is the rate of muscle protein synthesis 2 hours following a bout of heavy leg resistance exercise (10 sets × 10 repetitions at 70% of 1-repetition maximum) in 7 young males. EAA+CHO is the rate of muscle protein synthesis 1 hour following the ingestion of a leucine-enriched EAA+CHO solution in 6 young males. The Exercise + EAA+CHO data is the rate of muscle protein synthesis 2 hours post-exercise when EAA+CHO was ingested at 1 hour following resistance exercise in 6 male subjects. Data are expressed as mean ± SE. *P<0.05 vs. Basal; #P<0.05 vs. 2 hr Post (Exercise).
Potential Use of Leucine-enriched Essential Amino Acids to Restore Muscle in Ageing
The thought of using a leucine-enriched essential amino acid mixture to attenuate muscle loss or augment muscle growth in old human muscle is intriguing. Several studies have shown that ageing is associated with muscle protein synthesis being resistant to the anabolic action of insulin [25;44] and also in response to small doses of oral essential amino acids [31]. Interestingly, a new study has shown that when the meals of elderly subjects are supplemented with leucine the ability of feeding to stimulate muscle protein synthesis appears to be restored [45*]. This has also been shown to be true for old rats [46;47].
Therefore, it appears that ageing is associated with a reduced sensitivity of muscle protein synthesis to insulin and/or essential amino acids [31;48]. However, if the proportion of leucine is increased in an oral essential amino acid solution the blunted muscle protein synthesis can be restored [27*]. In addition, increasing the total amount of essential amino acids to a minimum of 15-20 grams in an oral nutrient solution results in a similar increase in muscle protein synthesis between young and old human subjects [26;49;50].
However, one recent study has reported that co-ingestion of leucine with protein did not enhance post-exercise muscle protein synthesis in old men [51]. Future studies are needed to determine whether chronic ingestion of leucine-enriched purified amino acid nutrient solutions will augment muscle protein synthesis via enhanced mTOR signalling in old human skeletal muscle at rest or in combination with resistance exercise training.
Conclusion
In summary, an oral ingestion of leucine-enriched essential amino acids and carbohydrates rapidly and potently stimulates protein synthesis in human skeletal muscle. The effect appears to be mediated by enhanced activation of the mTOR signalling pathway. The combination of leucine-enriched nutrients and resistance exercise enhances both mTOR signalling and muscle protein synthesis. The cellular mechanism(s) responsible for the amino acid induced activation of mTOR is currently unknown but may involve two kinases known as hVps34 and/or MAP4K3. Long-term studies are needed to determine the effectiveness of leucine-enriched essential amino acid supplementation to augment muscle protein synthesis and restore or improve muscle mass in sarcopenia and other conditions associated with muscle wasting.
Acknowledgements
Supported by the U.S. National Institute of Arthritis and Musculoskeletal and Skin Disease grant # R01 AR049877 and National Institute of Aging grant # P30 AG024832.
References
- 1.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 2.Matthews DE. Proteins and amino acids. In: Shils M, Olson J, Shike M, Ross A, editors. Modern Nutrition and Health and Disease. 9th Williams and Wilkins; Baltimore, MD: 1999. pp. 11–48. [Google Scholar]
- 3.Welle S, Thornton C, Statt M, et al. Postprandial myofibrillar and whole body protein synthesis in young and old human subjects. Am J Physiol. 1994;267:E599–604. doi: 10.1152/ajpendo.1994.267.4.E599. [DOI] [PubMed] [Google Scholar]
- 4.Matthews DE, Battezzati A. Regulation of protein metabolism during stress. Curr Opin Gen Surg. 1993:72–77. [PubMed] [Google Scholar]
- 5.Avruch J, Hara K, Lin Y, et al. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. doi: 10.1038/sj.onc.1209882. [DOI] [PubMed] [Google Scholar]
- 6.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 7.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–234. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 8.Rennie MJ, Edwards RH, Halliday D, et al. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 9.McNurlan MA, Essen P, Milne E, et al. Temporal responses of protein synthesis in human skeletal muscle to feeding. Br J Nutr. 1993;69:117–126. doi: 10.1079/bjn19930014. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen BB, Tipton KD, Miller SL, et al. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 11.Volpi E, Kobayashi H, Sheffield-Moore M, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith K, Barua JM, Watt PW, et al. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol. 1992;262:E372–376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- 13.Anthony JC, Yoshizawa F, Anthony TG, et al. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 14.Smith K, Reynolds N, Downie S, et al. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 15.Anthony JC, Anthony TG, Kimball SR, et al. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 16.Jefferson LS, Kimball SR. Amino acid regulation of gene expression. J Nutr. 2001;131:2460S–2466S. doi: 10.1093/jn/131.9.2460S. discussion 2486S-2467S. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Campbell LE, Miller CM, et al. Amino acid availability regulates p70 S6 kinase and multiple translation factors. Biochem J. 1998;334:261–267. doi: 10.1042/bj3340261. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem. 2005;280:33076–33082. doi: 10.1074/jbc.M507201200. [DOI] [PubMed] [Google Scholar]
- 20.Nobukuni T, Joaquin M, Roccio M, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci U S A. 2005;102:14238–14243. doi: 10.1073/pnas.0506925102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Findlay GM, Yan L, Procter J, et al. A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J. 2007;403:13–20. doi: 10.1042/BJ20061881. This paper is the first to identify MAP4K3 as being an amino acid sensor and regulator of mTOR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimball SR, Shantz LM, Horetsky RL, et al. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–11652. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- 23.Bolster DR, Vary TC, Kimball SR, et al. Leucine regulates translation initiation in rat skeletal muscle via enhanced eIF4G phosphorylation. J Nutr. 2004;134:1704–1710. doi: 10.1093/jn/134.7.1704. [DOI] [PubMed] [Google Scholar]
- 24.Crozier SJ, Kimball SR, Emmert SW, et al. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135:376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- 25.Guillet C, Prod'homme M, Balage M, et al. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 26.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 27*.Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–387. doi: 10.1152/ajpendo.00488.2005. Increasing the proportion of leucine in a small oral ingestion of essential amino acids can overcome the resistance to amino acid stimulation of muscle protein synthesis in old human skeletal muscle. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Jahn LA, Long W, et al. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–2143. doi: 10.1210/jcem.86.5.7481. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Jahn LA, Wei L, et al. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab. 2002;87:5553–5558. doi: 10.1210/jc.2002-020424. [DOI] [PubMed] [Google Scholar]
- 30.Rasmussen BB, Wolfe RR, Volpi E. Oral and intravenously administered amino acids produce similar effects on muscle protein synthesis in the elderly. J Nutr Health Aging. 2002;6:358–362. [PMC free article] [PubMed] [Google Scholar]
- 31.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 32**.Fujita S, Dreyer HC, Drummond MJ, et al. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. This was the first study to show in humans that a leucine-enriched nutrient solution increased mTOR signaling and protein synthesis concurrently in human skeletal muscle. In addition, this study also reports that nutrients stimulate muscle protein synthesis by promoting both translation initiation and elongation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolster DR, Crozier SJ, Kimball SR, et al. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 34.Borsheim E, Tipton KD, Wolf SE, et al. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 35.Borsheim E, Aarsland A, Wolfe RR. Effect of an amino acid, protein, and carbohydrate mixture on net muscle protein balance after resistance exercise. Int J Sport Nutr Exerc Metab. 2004;14:255–271. doi: 10.1123/ijsnem.14.3.255. [DOI] [PubMed] [Google Scholar]
- 36.Tipton KD, Elliott TA, Cree MG, et al. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc. 2004;36:2073–2081. doi: 10.1249/01.mss.0000147582.99810.c5. [DOI] [PubMed] [Google Scholar]
- 37.Koopman R, Beelen M, Stellingwerff T, et al. Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. Am J Physiol Endocrinol Metab. 2007;293:E833–842. doi: 10.1152/ajpendo.00135.2007. [DOI] [PubMed] [Google Scholar]
- 38.Tipton KD, Elliott TA, Cree MG, et al. Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. Am J Physiol Endocrinol Metab. 2007;292:E71–76. doi: 10.1152/ajpendo.00166.2006. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson HK, Nilsson PA, Nilsson J, et al. Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab. 2004;287:E1–7. doi: 10.1152/ajpendo.00430.2003. [DOI] [PubMed] [Google Scholar]
- 40*.Cuthbertson DJ, Babraj J, Smith K, et al. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–738. doi: 10.1152/ajpendo.00415.2005. This paper demonstrated an increase in Akt and S6K1 phosphorylation and muscle protein synthesis post-exercise when subjects were studied in the fed state. [DOI] [PubMed] [Google Scholar]
- 41*.Koopman R, Pennings B, Zorenc AH, et al. Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr. 2007;137:1880–1886. doi: 10.1093/jn/137.8.1880. First paper to show that downstream components of mTOR signalling can be augmented in human skeletal muscle when protein is ingested following resistance exercise. [DOI] [PubMed] [Google Scholar]
- 42*.Dreyer HC, Drummond MJ, Pennings B, et al. Leucine-Enriched Essential Amino Acid and Carbohydrate Ingestion Following Resistance Exercise Enhances mTOR Signaling and Protein Synthesis in Human Muscle. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00582.2007. Leucine-enriched nutrients ingested orally following resistance exercise enhances human muscle protein synthesis concurrently with augmented mTOR, 4E-BP1, and S6K1 phosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43*.Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. First paper to show that human muscle protein synthesis is decreased during a bout of resistance exercise. Also demonstrates that the increase in human muscle protein synthesis during post-exercise recovery is associated with an activation of the mTOR signalling pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpi E, Mittendorfer B, Rasmussen BB, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45*.Rieu I, Balage M, Sornet C, et al. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. The addition of leucine to regular meals was able to restore the ability of feeding to stimulate muscle protein synthesis in old men. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dardevet D, Sornet C, Bayle G, et al. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- 47.Rieu I, Balage M, Sornet C, et al. Increased availability of leucine with leucine-rich whey proteins improves postprandial muscle protein synthesis in aging rats. Nutrition. 2007;23:323–331. doi: 10.1016/j.nut.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 48.Rasmussen BB, Fujita S, Wolfe RR, et al. Insulin resistance of muscle protein metabolism in aging. Faseb J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katsanos CS, Kobayashi H, Sheffield-Moore M, et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 50.Koopman R, Verdijk L, Manders RJ, et al. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84:623–632. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 51.Koopman R, Verdijk LB, Beelen M, et al. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2007:1–10. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]