Abstract
While aryl pyrrolidinoamido thioureas derived from α-amino acids are effective catalysts in a number of asymmetric transformations, they exist as mixtures of slowly interconverting amide rotamers. Herein, the compromising role of amide bond isomerism is analyzed experimentally and computationally. A modified catalyst structure that exists almost exclusively as a single amide rotamer is introduced. This modification is shown to result in improved reactivity and enantioselectivity by minimizing competing reaction pathways.
Graphical abstract
Dual hydrogen-bond (H-bond) donors, such as chiral urea and thiourea derivatives, have come to define an important class of catalysts for a range of highly enantioselective transformations.1 Catalysts bearing the 2-aryl pyrrolidino amide motif have been of particular utility for promoting transformations via anion-binding or anion-abstraction pathways.2,3 However, reactions enabled by these catalysts are frequently hampered by low efficiencies, requiring high catalyst loadings (5–20 mol %), long reaction times (> 24 hours), and/or dilute conditions (≤ 0.1 M in initial substrate concentration) to achieve optimal results.
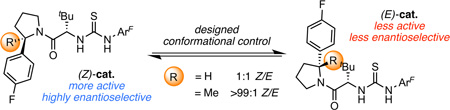
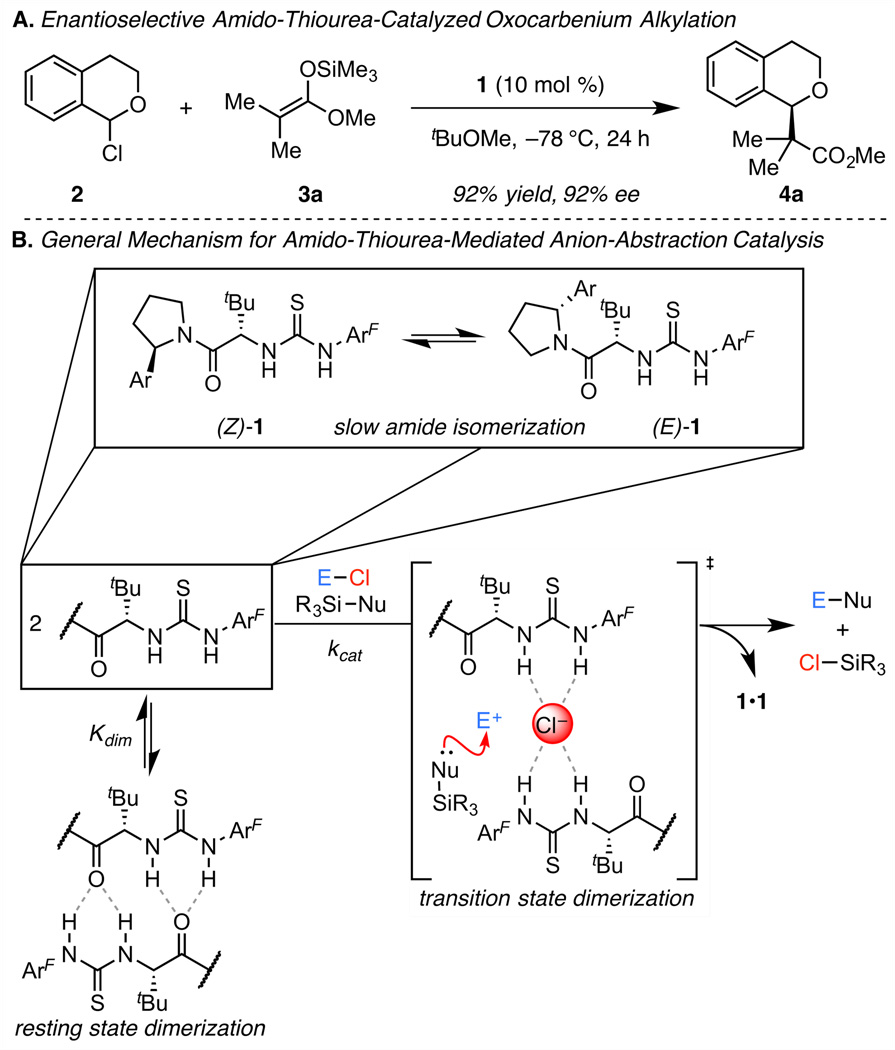
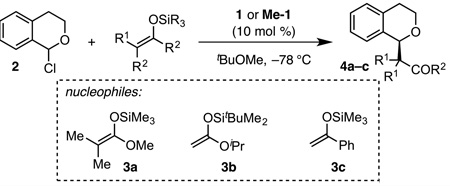
In an effort to elucidate the basis for these practical limitations to H-bond donor-mediated anion-abstraction catalysis, we recently reported a detailed mechanistic study of a representative transformation,4 the enantioselective amido-thioureacatalyzed alkylation of α-chloroisochroman (2, Scheme 1A).3a This study revealed several key features of the reaction system, including: 1) The catalyst resting state under typical reaction conditions (0.1 M in substrate, 10 mol % of catalyst) is a non-productive dimeric aggregate; 2) Two molecules of thiourea catalyst 1 are involved in the cooperative activation of the α-chloroether electrophile; 3) Catalyst 1 exists as a mixture of slowly interconverting (E)- and (Z)-amide rotamers in solution (Scheme 1B).
Scheme 1. Chiral Amido-Thiourea-Mediated Anion-Abstraction Catalysis.
ArF = 3,5-bis(trifluoromethyl)phenyl, Ar =4-fluorophenyl, E = generic electrophile, Nu = generic nucleophile
Taken together, these observations imply that each of the pairwise combinations of the catalyst amide rotamers could participate in the alkylation transition state (ZZ-TS, EE-TS, ZE-TS, and EZ-TS)5 and would likely make different—and potentially conflicting—contributions to the overall rate and enantioselectivity of the reaction. As such, we conjectured that constraining this catalyst system to operate through a single conformation could result in improved efficiency by funneling reactivity through a single enantioselective pathway. Herein we report the realization of this proposal through the mechanism-driven design and development of a conformationally biased catalyst that exhibits improved activity and enantioselectivity.
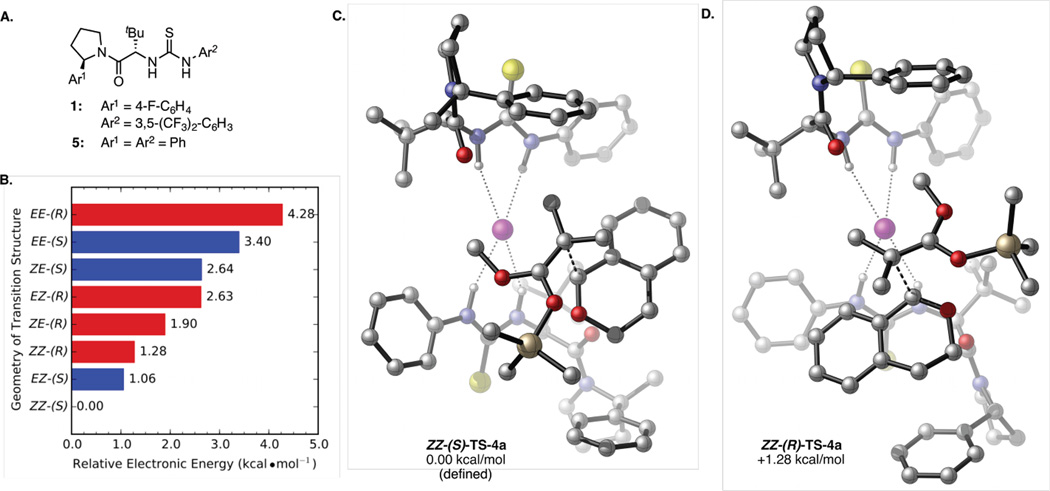
We sought first to identify the catalyst rotamer(s) responsible for controlling reactivity and enantioselectivity in the amido-thiourea-catalyzed alkylation of α-chloroisochroman (Scheme 1A). While only the (Z)-amide rotamer of catalyst 1 is observed in the solid state,6 1D and 2D 1H NMR analysis provides evidence that (Z,Z), (Z,E), and (E,E) dimeric catalyst complexes all exist in solution.4a,c Density functional theory (DFT) calculations with simplified catalyst 5 (Figure 1A)7 were employed to identify transition structures with each of the possible dimeric rotamer combinations.8,9 Energetically accessible transition structures possessing a single imaginary frequency along the reaction coordinate were located in all cases, lending credence to the hypothesis that multiple reaction pathways are competing under typical reaction conditions. Comparison of the lowest energy transition structures en route to the major and minor enantiomers of product 4a from each of the dimeric catalyst complexes (Figure 1B) revealed that the lowest energy structures (ZZ-(S)-TS-4a and ZZ-(R)-TS-4a) are accessed when both catalyst units are in the (Z)-conformation (Figure 1C, D).
Figure 1.
A. Simplified catalyst 5 was utilized as a proxy for 1 in computational investigation. B. A comparison of the relative energies of the lowest energy transition structures involving each of the four possible homodimeric and heterodimeric complexes of catalyst 5. See ref 5. Lowest energy computed transition structures involving the homodimeric (Z)-5/(Z)-5 catalyst complex en route to the C. major and D. minor enantiomers of isochroman product 4a. All calculations performed with B3LYP/6-31G+(d,p)//B3LYP/6-31G(d). Atom coloring scheme: C = silver, H = white, O = red, N = blue, S = yellow, Cl = magenta, Si = gold. Carbon-bound H atoms omitted for clarity.
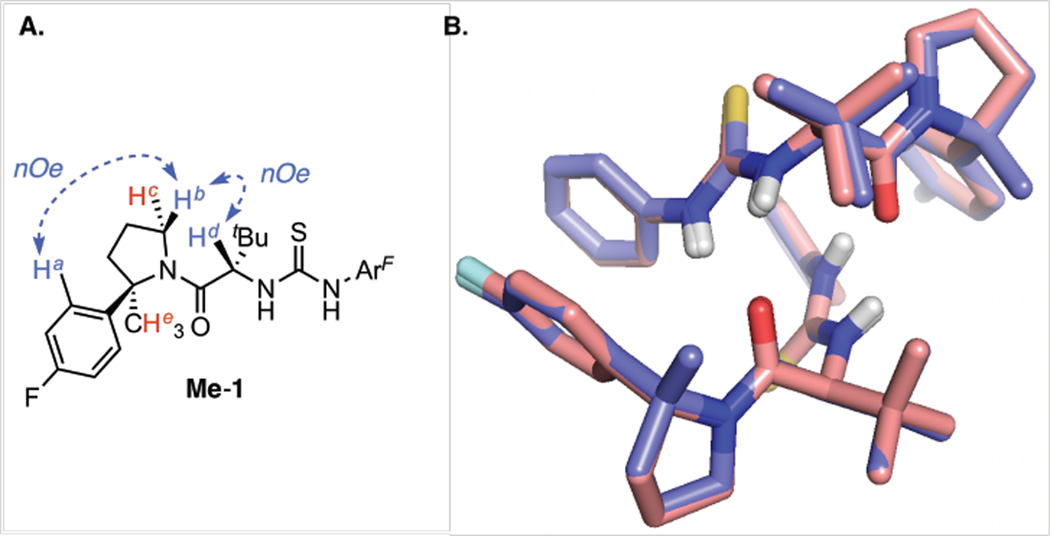
Given these insights, we next sought to design an analogous catalyst scaffold that exists exclusively in the (Z)-rotameric form. The relative energies of the (E)- and (Z)-rotamers of amido-thiourea catalysts bearing a variety of substituents on the pyrrolidine moiety were determined computationally. From this analysis, the 2-aryl-2-methylpyrrolidine-derived amido-thiourea Me-1 (Figure 2) was identified as a particularly promising candidate; this structure is predicted to favor the (Z)-amide rotamer by 2.8 kcal/mol. This energetic preference can be attributed to destabilizing interactions between the methyl substituent on the pyrroldine and the tert-butyl side-chain in the (E)-amide conformation.10 Furthermore, the optimized transition structure obtained with simplified catalyst Me-5 is calculated to possess a very similar conformation to ZZ-(S)-TS-4a (vide infra.)
Figure 2.
A. Select nOe correlations utilized to assign Me-1 as the (Z)-amide conformation. Correlations Ha↔Hb and Hb↔Hd are observed; correlations Hd↔He and Hc↔Hd are not. B. Overlay of the H-bonded (Z)-1•(Z)-1 (light pink) and Me-1•Me-1 (slate blue) homodimeric complexes determined by X-ray crystallographic analysis. CF3 groups and carbon-bound H atoms omitted for clarity.
In order to test these computational predictions, catalyst Me-1 was prepared,11 and its structural and functional properties were evaluated experimentally. Unlike catalyst 1, only a single amide rotamer of Me-1 can be observed by 1D 1H and 13C NMR in a variety of solvents. The structural assignment of the (Z)-amide rotamer was corroborated by 2D NOESY experiments, in which diagnostic nOe correlations are observed (Figure 2A). Furthermore, the solid-state conformations of 1 and Me-1 determined by single crystal X-ray diffraction analysis are nearly perfectly superimposable (Figure 2B).
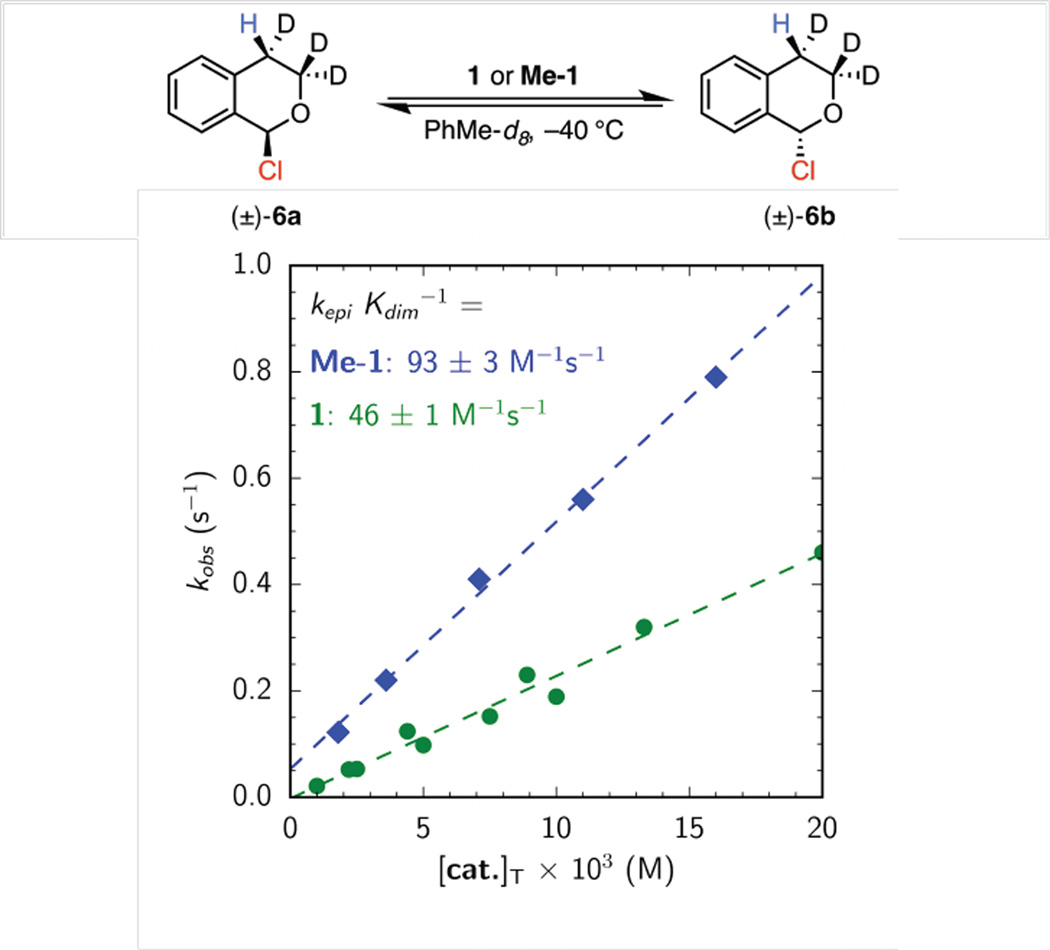
The new thiourea derivative, Me-1, was evaluated in the model oxocarbenium alkylation reaction. This conformationally rigid catalyst was found to exhibit improved activity and enantioselectivity relative to 1, affording 4a with quantitative conversion in 97% ee after only 4 hours (Table 1, entries 1 & 2).12 Catalyst Me-1 also displays a two-fold rate enhancement relative to catalyst 1 for epimerization of α-chloroisochroman-d3 (Figure 3).4b,c This rate acceleration can be rationalized by the fact that (Z)-1 and (E)-1 exist in a 1:1 mixture under these conditions. If it is assumed that (E)-1 is inactive in the epimerization process, then (Z)-1 and Me-1 can be concluded to display the same activity. This analysis lends credence to the assumption that Me-1 operates through the same anionabstraction mechanism elucidated to be operative with 1.
Table 1.
Enantioselective Oxocarbenium Alkylation Enabled by Designed Catalyst Me-1.a
 | |||||
|---|---|---|---|---|---|
| entry | nucleophile | time (h) | cat. | conv (%)b |
ee (%) |
| 1 | 3a | 4 | 1 | 66 | 92c |
| 2 | Me-1 | >95 | 97c | ||
| 3 | 3b | 1 | 1 | 81 | 85d |
| 4 | Me-1 | 91 | 91d | ||
| 5 | 3c | 24 | 1 | 82 | 88d |
| 6 | Me-1 | >95 | 96d | ||
Reactions were run on 0.2 or 0.3 mmol scale and quenched after the designated time with 0.5 M NaOMe in MeOH. See footnote 12.
Conversion was determined from the relative integration of the 1H NMR resonances of the product and quenched α-methoxyisochroman.
Determined by CSP-GC.
Determined by CSP-HPLC.
Figure 3.
Observed first-order rate constant for epimerization of α-chloroisochroman-d3 (6) via anion abstraction catalyzed by 1 (green circles) or Me-1 (blue diamonds). In all cases [6a] + [6b] = 0.10 M.
In principle, the improved activity observed with catalyst Me-1 could be due either to an increase in the effective concentration of the active (Z)-rotameric form of the catalyst or to deaggregation of nonproductive ground state dimers. In order to differentiate between these possibilities, the self-dimerization equilibrium constants for catalysts 1 and Me-1 were determined from the changes in the chemical shifts of diagnostic 1H NMR signals observed upon serial dilution.13 Catalyst Me-1 was determined to possess a similar dimerization constant (Kdim = 3.1 ± 0.1 × 102 M−1) to those determined for (E)- and (Z)-1 (Kdim = 4.6 ± 1.2 × 102 M−1 for (E)-1 and Kdim = 2.0 ± 0.3 × 102 M−1 for (Z)-1).14 Thus, increased activity cannot be ascribed to selective deaggregation of the catalyst, and must rather be attributed to the increased population of the more active (Z)-amide conformation.15
Similarly, the improved enantioselectivity could, in principle, be due either to minimization of competing pathways involving rotameric forms of the catalysts (as originally proposed), or to intrinsically higher stereoinduction imparted by introduction of the methyl substituent. These possibilities were distinguished by comparing the lowest energy transition structures for C–C bond formation located with catalysts (Z)-5 and Me-5 (B3LYP/6-31G+(d,p)//B3LYP/6-31G(d)). Catalyst methylation was found to have very little effect on the geometry of the transition structure leading to the major enantiomer of the product (Figure 1C; see Supporting Information for an overlay of the two computed structures), and the differential energy of activation was found to be similar between Me-5 (ΔΔE‡ = 1.13 kcal/mol) and (Z)-5. (ΔΔE‡ = 1.28 kcal/mol).
These results suggest that improvement in the observed enantioselectivity with Me-1 can be ascribed entirely to minimization of competing pathways involving the (E)-rotamer. This conclusion is corroborated by the observation that the enantioselectivity differences between catalysts 1 and Me-1 are greatest under conditions in which the Z/E rotamer ratio of 1 is low.16
We anticipate that the conformational-control strategy outlined above will prove broadly beneficial in enantioselective anion-abstraction pathways. For example, Me-1 is found to be significantly superior to 1 in the alkylation of 2 with a silyl enol ether nucleophile (3c, Table 1, entries 5 & 6).
In conclusion, experimental and theoretical analyses have shed light on the detrimental role that catalyst amide rotamers play in anion-abstraction catalysis by aryl pyrrolidinoamidothiourea derivatives. We have demonstrated the successful application of these mechanistic insights to the design of a new catalyst in which amide conformational control enables improved reaction efficiency and enantioselectivity. This design is likely to provide a general strategy for suppressing competing pathways in other transformations catalyzed by chiral amido-thioureas and related H-bond donor catalysts. The application of this design principle to development of highly efficient transformations is the focus of ongoing research.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM-43214) and by fellowships to D.L. (NSERC PDF), D.D.F. (Eli Lilly and Co.), and A.J.B. and C.R.K. (NSF DGE1144152).We thank Dr. Shao-Liang Zheng (Harvard X-Ray Laboratory) for X-ray crystallographic structure determination and Erica D’Amato (Harvard University) for experimental assistance.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Full experimental procedures, characterization data including NMR spectra for all new compounds, geometries and energies of all calculated stationary points. (PDF)
Crystallographic data for Me-1 (CCDC 1477622 & 1478394), catalyst analogs (CCDC 1478392, 1478393, 1478395), and a synthetic intermediate en route to Me-1 (CCDC 1477623). (CIF)
Author Contributions
The manuscript was written through contributions of all authors. / All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interests.
REFERENCES
- 1.(a) Schreiner PR. Chem. Soc. Rev. 2003;32:289–296. doi: 10.1039/b107298f. [DOI] [PubMed] [Google Scholar]; (b) Takemoto Y. Org. Biomol. Chem. 2005;3:4299–4306. doi: 10.1039/b511216h. [DOI] [PubMed] [Google Scholar]; (c) Doyle AG, Jacobsen EN. Chem. Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]; (d) Knowles RR, Jacobsen EN. Proc. Natl. Acad. Sci. U.S.A. 2010;107:20678–20685. doi: 10.1073/pnas.1006402107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.For reviews, see: Zhang Z, Schreiner PR. Chem. Soc. Rev. 2009;38:1187–1198. doi: 10.1039/b801793j. Brak K, Jacobsen EN. Angew. Chem. Int. Ed. 2012;52:534–561. doi: 10.1002/anie.201205449. Phipps RJ, Hamilton GL, Toste FD. Nature Chem. 2012;4:603–614. doi: 10.1038/nchem.1405. Beckendorf S, Asmus S, Mancheño OG. Chem. Cat. Chem. 2012;4:926–936. Seidel D. Synlett. 2014;25:783–794.
- 3.For select examples, see: Reisman SE, Doyle AG, Jacobsen EN. J. Am. Chem. Soc. 2008;130:7198–7199. doi: 10.1021/ja801514m. Knowles RR, Lin S, Jacobsen EN. J. Am. Chem Soc. 2010;132:5030–5032. doi: 10.1021/ja101256v. Birrell JA, Desrosiers J-N, Jacobsen EN. J. Am. Chem. Soc. 2011;133:13872–13875. doi: 10.1021/ja205602j. Lin S, Jacobsen EN. Nature Chem. 2012;4:817–824. doi: 10.1038/nchem.1450. Bergonzini G, Schindler CS, Wallentin C-J, Jacobsen EN, Stephenson CRJ. Chem. Sci. 2014;5:112–116. doi: 10.1039/C3SC52265B. Yeung CS, Ziegler RE, Porco JA, Jr, Jacobsen EN. J. Am. Chem. Soc. 2014;136:13614–13617. doi: 10.1021/ja508523g. Liu RY, Wasa M, Jacobsen EN. Tetrahedron Lett. 2015;56:3428–3430. doi: 10.1016/j.tetlet.2015.01.124.
- 4.(a) Ford DD, Lehnherr D, Kennedy CR, Jacobsen EN. J. Am. Chem. Soc. 2016 doi: 10.1021/jacs.6b04686. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ford DD, Lehnherr D, Kennedy CR, Jacobsen EN. ACS Catal. 2016 doi: 10.1021/acscatal.6b01384. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ford DD. Ph.D. Thesis. Harvard University; 2013. The Role of Catalyst-Catalyst Interactions in Asymmetric Catalysis with (salen)Co(III) Complexes and H-Bond Donors. [Google Scholar]
- 5.The (E)-1 and (Z)-1 monomers in the heterodimeric catalyst complexes are conformationally inequivalent. Two distinct transition state classes are defined based on the orientation of each monomer relative to the reacting substrate. ZE-TS = Transition structures in which the amide oxygen of the (E)-1 monomer interacts with the oxocarbenium ion EZ-TS = Transition structures in which the amide oxygen of the (Z)-1 monomer interacts with the oxocarbenium ion. See Supporting Information for additional information.
- 6.X-ray diffraction analysis of single crystals of 1 in the presence and absence of bound tetramethylammonium chloride enable assignment of dimeric structures of (Z)-1.
- 7.Catalyst 5 affords isochroman 4a in 81% ee under standard reaction conditions and provides a significantly reduced computational cost relative to catalyst 1.
- 8. Frisch MJ. Gaussian 09, Revision A.02. Wallingford, CT: Gaussian Inc.; 2009. See Supporting Information for full citation.
- 9.(a) Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; (b) Lee C, Yang W, Parr RG. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]; (c) Miehlich B, Savin A, Stoll H, Preuss H. Chem. Phys. Lett. 1989;157:200–206. [Google Scholar]; (d) Frisch MJ, Pople JA, Binkley JS. J. Chem. Phys. 1984;80:3265–3269. [Google Scholar]
- 10.For a discussion of syn-pentane interactions in hydrocarbon backbones, see: Hoffman RW, Stahl M, Schopfer U, Frenking G. Chem. Eur. J. 1998;4:559–566.
- 11.See Supporting Information for synthetic details.
- 12.These reactions were quenched with sodium methoxide after the indicated time to emphasize the rate enhancement achieved with Me-1 versus 1.
- 13.For discussion of this technique, see: Tan HKS. J. Chem. Soc. Faraday Trans. 1994;90:3521–3525. Nogales DF, Ma J-S, Lightner DA. Tetrahedron. 1993;49:2361–2372.
- 14.These values, which were determined in CD2Cl2, are in good agreement with the values determined previously in toluene-d8. See refs 4a, c.
- 15.See Supporting Information for discussion and data.
- 16.For example, in toluene at −78 °C, catalyst 1 exists as a 1:1 mixture of (Z)-1 and (E)-1 and affords 4a in 76% ee. Me-1 affords 4a in 91% ee.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.