Abstract
Background
Combined treatment with an angiotensin-converting enzyme inhibitor and a mineralocorticoid receptor (MR) antagonist improved cardiac and skeletal muscle function and pathology in a mouse model of Duchenne muscular dystrophy. MR is present in limb and respiratory skeletal muscles and functions as a steroid hormone receptor.
Objective
The goals of the current study were to compare the efficacy of the specific MR antagonist eplerenone with the non-specific MR antagonist spironolactone, both in combination with the angiotensin-converting enzyme inhibitor lisinopril.
Methods
Three groups of n=18 dystrophin-deficient, utrophin-haploinsufficient male mice were given chow containing: lisinopril plus spironolactone, lisinopril plus eplerenone, or no drug, from four to 20 weeks-of-age. Eighteen C57BL/10 male mice were used as wild-type controls. In vivo measurements included cardiac magnetic resonance imaging, conscious electrocardiography, and grip strength. From each mouse in the study, diaphragm, extensor digitorum longus, and cardiac papillary muscle force was measured ex vivo, followed by histological quantification of muscle damage in heart, diaphragm, quadriceps, and abdominal muscles. MR protein levels were also verified in treated muscles.
Results
Treatment with specific and non-specific MR antagonists did not result in any adverse effects to dystrophic skeletal muscles or heart. Both treatments resulted in similar functional and pathological improvements across a wide array of parameters. MR protein levels were not reduced by treatment.
Conclusions
These data suggest that spironolactone and eplerenone show similar effects in dystrophic mice and support the clinical development of MR antagonists for treating skeletal muscles in Duchenne muscular dystrophy.
Keywords: spironolactone, eplerenone, lisinopril, Duchenne muscular dystrophy, mineralocorticoid receptors
INTRODUCTION
Duchenne muscular dystrophy (DMD) is the most common genetic muscle disease in children and results in progressive degeneration of skeletal and cardiac muscles due to absence of the dystrophin protein [1]. Dystrophin normally links the subsarcolemmal cytoskeleton to a transmembrane glycoprotein complex and contributes to the stabilization of muscle membranes. The translation of dystrophin replacement therapy from animal models to patients has been hampered by many challenges over the past 3 decades since the dystrophin gene was cloned. Dystrophin surrogates and pharmacological and protein-based approaches targeting downstream pathogenic mechanisms are being investigated as alternative therapeutic approaches [1]. The current standard-of-care treatment in DMD is prednisone, a glucocorticoid that delays loss of ambulation by an average of two years, but has numerous serious side effects [2]. Several labs have also demonstrated that prednisone actually worsens muscle damage in both skeletal and cardiac muscles in DMD mouse models [3–5]. There is a critical need for safe and efficacious treatment strategies that can prolong and improve DMD patients’ quality of life.
Since DMD patients exhibit a cardiomyopathy that slowly progresses to heart failure, our team previously investigated whether prophylactic introduction of standard-of-care heart failure drugs could prevent or delay the progression of cardiomyopathy in a DMD mouse model. Surprisingly, a combination treatment with the angiotensin-converting enzyme inhibitor (ACEi) lisinopril and the mineralocorticoid receptor (MR) antagonist spironolactone, led to improved function and pathology not only in the heart, but also in skeletal muscles [6]. We then showed that mineralocorticoid receptors, not previously investigated in skeletal muscles, were present in limb and respiratory muscles from wild-type and dystrophic mice [7]. The endogenous mineralocorticoid receptor agonist aldosterone was able to induce a large number of gene expression changes in normal human differentiated myotubes, supporting MR functions as a steroid hormone receptor in skeletal muscles. These data suggest that ACEi and MR antagonists can have a direct therapeutic effect on skeletal muscles. We have also shown that lisinopril plus spironolactone can improve the prednisolone-induced damage to dystrophic mouse muscles [4]. However, lisinopril treatment alone benefits histopathology, but not function of dystrophic skeletal muscles in mice [8].
Our team has now demonstrated in a one–year, double-blind placebo-controlled clinical trial that addition of the specific MR antagonist eplerenone to cardiac standard-of-care ACEi in DMD patients at an early stage of cardiac disease was able to slow the progression of cardiomyopathy [9]. Since cardiomyopathy develops after skeletal myopathy most of the patients in this trial were non-ambulatory, so the effect of MR antagonist treatment on dystrophic skeletal muscles was not assessed.
Spironolactone was the first MR antagonist developed and has been used clinically in cardiology for decades. It has high affinity for MR, but is non-specific and can also bind other steroid hormone receptors including the androgen and progesterone receptors, and with lower affinity, glucocorticoid receptors [10]. Eplerenone is a second-generation selective MR antagonist, which specifically binds MR but with lower affinity than spironolactone [10]. Both drugs are used at the same dosages interchangeably for cardiac disease, but typically not until late stage heart failure. Since MR was not previously identified in skeletal muscles, use of MR antagonists for skeletal myopathies has not been clinically investigated [7]. It is not known whether the functional benefit on dystrophic skeletal muscles is due to MR antagonism by spironolactone or from off-target effects on the other steroid hormone receptors that it can bind. Additionally, it is not known whether spironolactone binding to these other receptors in skeletal muscle may limit its efficacy compared to more specific MR antagonism.
The goal of the current study was to compare the efficacy of a specific versus non-specific MR antagonist combined with an ACEi. This preclinical study is important for optimizing the clinical development of MR antagonists for treatment of dystrophic skeletal muscles.
MATERIALS AND METHODS
Mice and preclinical treatment
All protocols were approved by the Institutional Animal Care and Use Committee of The Ohio State University, are in compliance with the laws of The United States of America, and conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Dystrophin-deficient, utrophin haplo-insufficient (utrn +/− ; mdx) “het” male mice [11] were bred and genotyped in house as previously described [12], housed 2 per cage, and were used for treated groups or untreated controls (n = 18 per group). C57BL/10 (C57) (Harwell) mice bred in-house were used as wild-type controls (n = 18) for all analyses with the exception of MRI. Het groups were given Teklad Rodent Chow #7912 containing either 133 mg/kg lisinopril (SBH Medical Ltd. CAS# 83915-83-7) and 666.66 mg/kg spironolactone (Sigma S3378) (LS) or 133 mg/kg lisinopril and 2000 mg/kg Eplerenone (Pfizer Compound Transfer Program) (EL) (prepared by Research Diets, Inc.) or the chow alone (untreated). Medicated pellets were replaced every week; mice were weighed and the amount of pellets consumed was recorded to ensure mice were receiving the estimated dosage of Lisinopril (20 mg/kg x day), Spironolactone (100 mg/kg x day) and Eplerenone (200 mg/kg x day). The dosages were based on those previously described in preclinical experiments that demonstrated efficacy for these drugs in other models [13–17] and recommended by Ellen McMahon, PhD, developer of eplerenone at Pfizer (personal communication). Eplerenone and spironolactone are used at different dosages in mice due to different clearance rates and potency on MR. We used drug-laden chow since eplerenone is not soluble in drinking water, the vehicle used for LS delivery in our previous studies. Het mice were distributed evenly between the groups over time, to control for any environmental factors during the time-course of the experiment. Mice were treated from 4 to 20 weeks-of-age or left untreated and then analyzed by personnel not involved in genotyping or treating the animals and blinded to the treatment and genotypes of the animals, which were only identifiable by a tag number at analysis. Each measurement described below was performed by the same individual, to limit variability. Mice were euthanized by cervical dislocation without anesthesia as per IACUC approval and the AMVA guidelines on Euthanasia to avoid chemically contaminated tissues. A single mouse in the EL group died of causes not related to the study. At the end of the treatment period, 30 grams of the custom pellets were analyzed using GC/MS (Cornerstone Laboratories, LLC), which confirmed pellets contain the predicted amount of both drugs.
In vivo cardiac and forelimb grip strength measurements
Within 4 days of the animals reaching 20 weeks of age, magnetic resonance imaging (MRI) was performed on het untreated and treated mice using a 9.4 Tesla 30 mm bore system (Bruker Biospin) with electrocardiographic (ECG) leads while under body temperature control (37°C), as described previously [6]. Myocardial strain and strain rate were computed using vector-based tracking software (Vector Velocity Imaging, Siemens).
On the day of sacrifice, the body weight of each mouse was recorded and resting, non-anesthetized, non-invasive electrocardiographic recordings were taken using the ECGenie system (Mouse Specifics Inc.) as previously described [8]. Analysis of QT- interval, heart rate, and heart rate variability was done using time intervals when paws were in contact with the electrodes and heart rate (HR) remained consistent. Forelimb grip strength was then assessed as described in Treat-NMD SOP DMD_M.2.2.001 and previously [8]. The highest value (N) of three measurements separated by one minute rest periods was reported.
In vitro cardiac, diaphragm and extensor digitorum longus (EDL) contraction force measurements
In vitro force measurements of linear cardiac papillary muscles, diaphragm muscle strips, and extensor digitorum longus limb muscles were conducted in parallel.
In vitro contraction measurements in small cardiac papillary muscles were performed as previously described [18]. Briefly, small intact papillary muscles were dissected from the right ventricle, stretched to optimal length, and paced at 4 Hz at 37°C. Length-dependent activation was assessed by measuring developed force at 4 different lengths, roughly encompassing the cardiac physiological range from end-systolic (85% of optimal length) to end-diastolic length (optimal length). Frequency-dependent activation was measured by assessment of force development at 4, 6, 8, 10, 12, and 14 Hz, encompassing the entire in vivo heart rate range of the mouse. Finally, the response to the beta-adrenergic agonist isoproterenol was assessed, in semi-log steps from 1 nM to 1 μM. Specific forces were calculated per unit of cross-sectional area (CSA) and expressed in mN/mm2.
For diaphragm force measurements, two linear strips of muscle approximately 2–3 mm in width were carefully dissected from the center of each diaphragm. Single electrical stimulation pulses (180 Hz for 4 ms) were delivered via two parallel platinum-iridium electrodes surrounding the muscle to determine the “optimal length” for measuring maximum twitch force. The muscles were subsequently allowed to rest for 10 minutes, and then subjected to a series of tetanic contractions. Six tetanic contractions, one at each 20, 50, 80, 120, 150, and 180 Hz (250 ms duration each) were performed with 2 minutes of rest between stimulations. Five minutes after the final tetanic measurement, a fatigue protocol (stimuli at a frequency of 100 Hz and 250 ms duration each second for a total of 66 seconds) was performed. After a 20 minute rest the protocol was repeated. Force measurements are expressed per unit CSA (normalized isometric force or tension: mN/mm2) as previously described [19].
Sutures were knotted to each tendon prior to the removal of each EDL muscle. Muscles were stretched to optimal length using twitch contractions (evoked by a single 4 ms pulse) as previously described [4, 19]. After 10 minutes, a tetanic contraction was performed (150 Hz for 250 ms). After another 5 min rest period, 10 eccentric contractions (ecc, 150 Hz for 450 ms, subjected to a 3% stretch for the final 200 ms of contraction) were done with two minutes of rest between stimulations. To differentiate between damage and fatigue, a final eccentric contraction was performed after allowing the muscle to rest for 15 minutes after the 10th eccentric contraction. Specific forces were calculated per unit of CSA and ecc values are presented as the percentage of the initial tetanus for each group in Table 1. Force recordings and analysis were done using custom-made LabView (National Instruments) programs. If more than one diaphragm strip was assessed from the same mouse, values were averaged and treated as n = 1 for statistical analyses [4]. For EDL, only the first muscle analyzed was used for analyses.
TABLE I.
Comparison of preclinical outcome measures.
| Control C57 Mean ±SEM (n) |
Untreated Het Mean ±SEM (n) |
EL treated Mean ±SEM (n) |
LS treated Mean ±SEM (n) |
P-value | Best het group or closest to C57 | |||
|---|---|---|---|---|---|---|---|---|
| ANOVA | Dunnett Untreated vs EL | Dunnett Untreated vs LS | ||||||
| Body Weight (g) | 28.3 ± 0.5 (18) | 32.2 ± 0.8 (18) | 30.1 ± 0.5 (17) | 31.0 ± 1.1 (18) | 0.00446 | 0.1438 | 0.5684 | EL treated |
| Grip Strength (N) | 1.5 ± 0.05 (18) | 1.1 ± 0.07 (18) | 1.1 ± 0.07 (17) | 1.2 ± 0.07 (18) | < .0001 | 0.798 | 0.8083 | LS treated |
| GS/BW (N/mg) * | 53.5 ± 1.6 (18) | 35.3 ± 2.5 (18) | 34.9 ± 1.9 (17) | 38.3 ± 1.9 (18) | < .0001 | 0.997 | 0.5852 | LS treated |
| Heart Weight (g) | 0.14 ± 0.004 (18) | 0.17 ± 0.006 (18) | 0.13 ± 0.003 (17) | 0.15 ± 0.005 (17) | < .0001 | < .0001 | 0.0079 | EL treated |
| HW/BW (mg/g) * | 5.0 ± 0.1 (18) | 5.2 ± 0.1 (18) | 4.5 ± 0.1 (17) | 4.7 ± 0.1 (17) | < .0001 | < .0001 | 0.0058 | EL treated |
| Heart Rate (bpm) | 592 ± 16 (18) | 605 ± 20 (18) | 613 ± 18 (17) | 591 ± 23 (18) | 0.83173 | 0.9797 | 0.9256 | LS treated |
| QT (ms) * | 51.1 ± 2.2 (18) | 51.2 ± 1.8 (18) | 47.8 ± 2.7 (17) | 49.9 ± 4.0 (18) | 0.82311 | 0.7341 | 0.9723 | EL treated |
| QTc (ms) | 49.2 ± 1.8 (18) | 52.0 ± 2.9 (18) | 49.7 ± 0.8 (17) | 52.5 ± 3.4 (18) | 0.71999 | 0.849 | 0.9985 | EL treated |
| MID_PeakSysSR_Mean (endocardial circumferential S−1) | ND | 0.42 ± 0.04 (12) | 0.50 ± 0.05 (12) | 0.52 ± 0.05 (11) | 0.25608 $ | 0.3292 | 0.2187 | LS treated |
| BASE-PeakSysSR_Mean (endocardial circumferential S−1) * | ND | 0.37 ± 0.03 (12) | 0.40 ± 0.03 (11) | 0.49 ± 0.05 (11) | 0.08108 $ | 0.8309 | 0.0584 | LS treated |
| Cardiac Fdev (mN/mm−2) * | 11.6 ± 1.9 (17) | 9.5 ± 2.2 (16) | 4.9 ± 0.7 (12) | 15.4 ± 4.4 (14) | 0.07549 | 0.4972 | 0.2736 | LS treated |
| Cardiac Fmax (mN/mm−2) | 16.6 ± 2.7 (17) | 16.2 ± 3.8 (16) | 10.0 ± 3.3 (12) | 18.2 ± 4.8 (14) | 0.49797 | 0.5318 | 0.965 | LS treated |
| Diaphragm spec. force * (mN/mm−2) | 142.2 ± 10.0 (16) | 114.8 ± 7.9 (18) | 116.4 ± 8.2 (16) | 103.1 ± 8.3 (18) | 0.01863 | 0.9985 | 0.6352 | EL treated |
| Diaphragm Absolute Recovery (mN/mm−2) * | 13.5 ± 2.2 (15) | 7.9 ± 0.9 (17) | 10.1 ± 1.0 (15) | 8.5 ± 1.2 (16) | 0.02681 | 0.5241 | 0.9773 | EL treated |
| Diaphragm Relative Recovery (%) | 31.7 ± 4.4 (15) | 17.8 ± 1.5 (17) | 21.8 ± 1.2 (15) | 21.0 ± 2.0 (16) | 0.00213 | 0.5442 | 0.6897 | EL treated |
| EDL tet (mN/mm−2) * | 443 ± 21 (18) | 352 ± 19 (13) | 420 ± 28 (16) | 384 ± 34 (15) | 0.09242 | 0.203 | 0.7521 | EL treated |
| ecc1 (% tet) | 63 ± 2 (17) | 55 ± 6 (12) | 62 ± 4 (14) | 59 ± 5 (14) | 0.52856 | 0.4953 | 0.8805 | EL treated |
| ecc2 (% tet) * | 30 ± 3 (17) | 21 ± 5 (12) | 28 ± 4 (14) | 30 ± 5 (13) | 0.41442 | 0.5474 | 0.3446 | LS treated |
| ecc5 (% tet) | 10 ± 1 (17) | 5 ± 1 (12) | 8 ± 2 (13) | 12 ± 3 (13) | 0.13579 | 0.648 | 0.068 | LS treated |
| ecc10 (% tet) | 4 ± 1 (17) | 2 ± 1 (12) | 3 ± 1 (13) | 6 ± 2 (12) | 0.15307 | 0.6245 | 0.0729 | LS treated |
| Post-rest ecc 11 (% tet) | 8 ± 1 (15) | 3 ± 1 (10) | 7 ± 2 (13) | 8 ± 2 (11) | 0.1744 | 0.3851 | 0.1653 | LS treated |
| ((ecc11 - ecc10) / ecc10) x 100% (% ecc10) * | 128 ± 17 (15) | 107 ± 14 (10) | 130 ± 16 (13) | 153 ± 37 (11) | 0.59542 | 0.823 | 0.3847 | LS treated |
| Quad IgG (% CSA) * | 0.6 ± 0.1 (18) | 9.7 ± 1.0 (18) | 8.9 ± 0.9 (17) | 9.9 ± 1.5 (18) | < .0001 | 0.8912 | 0.9991 | EL treated |
| Heart IgG (% CSA) * | 0.1 ± 0.03 (18) | 2.7 ± 0.4 (18) | 1.7 ± 0.5 (16) | 1.3 ± 0.3 (18) | < .0001 | 0.0701 | 0.0059 | LS treated |
| Dia IgG (% CSA) * | 0.5 ± 0.1 (18) | 14.5 ± 0.6 (18) | 11.8 ± 0.8 (17) | 10.8 ± 0.8 (18) | < .0001 | 0.0117 | 0.0003 | LS treated |
| Abd IgG (% CSA) * | 0.5 ± 0.1 (18) | 8.8 ± 0.7 (18) | 8.3 ± 1.6 (17) | 6.3 ± 0.6 (18) | < .0001 | 0.9717 | 0.1225 | LS treated |
indicates independent parameters used for the stochastic analyses
indicates ANOVA P-values calculated without a C57BL/10 wild-type control group
Measurements for 26 parameters from untreated het, LS treated and EL treated groups, compared with control C57 wild-type mice. ANOVA and Dunnett post-hoc P-values are shown for each parameter. The group with the closest value to wild-type or the best value if wild-type was not included (for MRI) is bolded and listed in the last column. P-values of < 0.05 are underlined.
Histopathology and quantification
For each mouse, remaining portions of the heart and diaphragm in addition to quadriceps and rectus abdominis muscles were embedded in optimal-cutting temperature (OCT) medium and frozen on liquid-nitrogen cooled isopentane for histological analyses. Eight μm cryosections were stained with Hematoxylin and Eosin (H&E) to verify section quality or with an antibody against mouse immunoglobulin (Ig)G (Alexa 488 goat anti-mouse IgG, 1:200; Life Technologies) to quantify ongoing muscle damage as previously described [8]. Damage is reported as a percentage of CSA. Longitudinal quadriceps and diaphragm sections were excluded from analysis.
Western blot analysis
Snap frozen mouse tissues were pulverized and resuspended in cellular extract buffer as described previously [7]. Briefly, tissue homogenates were sonicated on ice, centrifuged, and total protein was quantified. 50 μg per lane of total protein was used to detect MR with a combination of MR-specific monoclonal antibodies, MRN 2B7 & rMR 1–18 1D5 [20] or GAPDH (Proteintech).
Data and statistical analysis
All data was included for statistical calculations and analyzed using one-way ANOVA. If the overall ANOVA indicated statistical significance, a non-parametric Dunnett post-hoc test was used to test for significant differences between each treated group compared with the untreated het group. In addition, student’s t-tests were used to directly compare 2 groups. Summary values are presented as mean ± SE. Two-tailed P values < 0.05 were accepted as significant. A multi-parameter stochastic analysis was performed to assess the overall effectiveness of treatment. Only 14 independent parameters were considered in this analysis (identified by * in Table 1). If treatment was not effective, the probability that the untreated group (when compared to the two treatment groups) displays the best outcome would be one-third for each parameter and the probability that it is not the best outcome would be two-thirds. Probability statistics determined the likelihood of treatments not being effective by testing the number of parameters for which untreated het mice did not show the best outcome in relation to the number of tested parameters.
RESULTS
The specific goal of this study was to determine whether the non-specific mineralocorticoid receptor antagonist spironolactone, added to the cardiac standard-of-care lisinopril (LS), was equally beneficial as the more expensive specific mineralocorticoid receptor antagonist eplerenone plus lisinopril (EL). Dystrophin-deficient, utrophin-haploinsufficient “het” male mice were treated from four to 20 weeks-of-age with chow containing LS, EL, or no drug (n=18). At 20 weeks-of-age, cardiac MRI, conscious electrocardiograms, and grip strength measurements were performed. From each mouse, ex vivo force measurements were made from diaphragm, extensor digitorum longus, and cardiac papillary muscles, and tissue samples from diaphragm, quadriceps, abdominal muscles, and heart were collected for histological and biochemical analyses.
Dunnett post-hoc tests showed no difference between the groups treated with spironolactone or eplerenone, except for developed cardiac force (Fdev), where the force of the EL group was lower than the untreated (Table 1). For each of the 26 parameters measured, either the LS or EL treatment group was closest to wild-type control values and the group of untreated het mice never had the best values (Table 1). To determine the overall effectiveness of treatment, we performed a multi-parameter stochastic analysis. In the 14 independent parameters tested (indicated with * in Table 1) the untreated group was never the best. The statistical probability of this occurrence is calculated as (2/3)14, returning a P-value of 0.0034. This same statistical analysis also did not detect a significant difference <0.05 between the LS and EL treatment groups.
Myocardial circumferential strain rate at both the base and mid region of the left ventricle showed an increased magnitude with both treatments, with the most improved strain rate in the LS treatment group (Table 1). To directly compare the LS treatment group with the untreated group, a t-test was performed and showed a P-value of 0.046 for base myocardial strain rate. In vitro measurement of cardiac force also exhibited the largest improvement with LS treatment. Although baseline developed force and maximal force in untreated het mice were 82% and 97% of wild-type forces, the LS treatment group showed values for forces above wild-type values at 15.4±4.4 and 18.2±4.8 mN/mm2, respectively (Table 1). Staining for serum IgG was used to detect ongoing damage and accumulation of interstitial and replacement fibrosis of dystrophic tissues and was used as a more straightforward measurement that morphometric histological analysis. IgG accumulates intracellularly and marks ongoing cellular damage and accumulates extracellularly in regions of fibrotic replacement of muscle tissue. Cardiac damage detected by IgG localization was also significantly reduced (P = 0.0059) in the LS treatment group to 54% of that in the untreated het group after normalizing for wild-type IgG staining (Fig. 1).
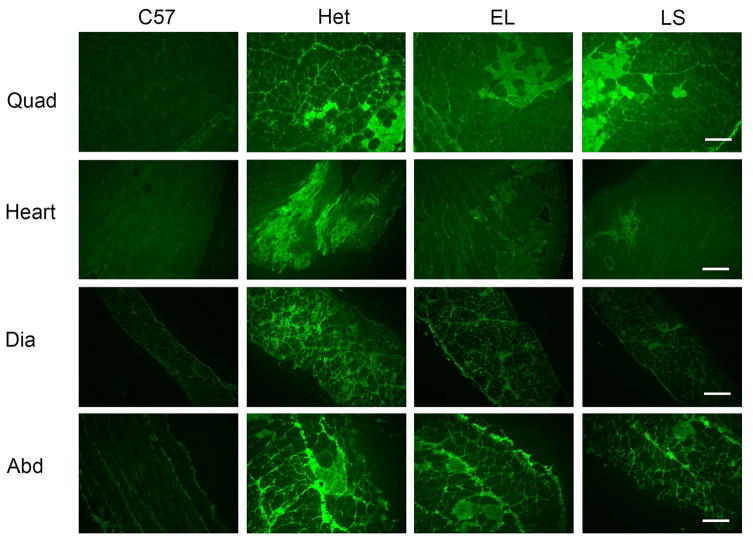
Fig. 1. Myofiber damage in quadriceps, heart, diaphragm and abdominal muscles.
Representative images of immunofluorescence stain for serum IgG on quadriceps, heart, diaphragm and abdominal sections. Sections from treated mice groups (EL and LS) show less ongoing damage than untreated het mice (LS heart compared to het, P = 0.0059; LS and EL diaphragm sections compared to het, P = 0.0003 and P = 0.0117 respectively, see also Table 1). C57BL/10 (C57) wild-type control mice had no visible muscle damage. Bar = 200 μm.
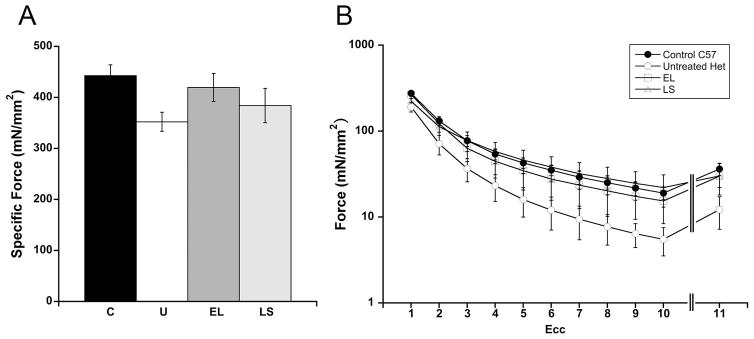
Dystrophic limb muscles are known to show increased susceptibility to injury compared to wild-type mice. We measured the baseline force of an EDL limb muscle (EDL tet) from each mouse, followed by force measurements at the beginning of each of 10 lengthening or eccentric contractions (ecc 1–10) (Table 1 and Fig. 2). After a rest period to recover from fatigue, a final force measurement was taken to differentiate between muscle damage and fatigue (ecc 11) (Table 1 and Fig. 2). EDL tet for untreated het mice was 79% of wild-type C57 values, compared to 40% in our previous published study (Fig. 2A) [6]. EDL tet forces for the treated groups were 95% and 87% of C57 values for EL and LS, respectively. The differential susceptibility to damage in het dystrophic muscles was more evident after additional contractions with ecc1, 2, 5, and 10, which generated 69%, 53%, 37%, and 32% of wild-type forces for the same measurements (Fig. 2B). This rapid decline was decreased in the EL treatment group, which generated forces for ecc1, 2, 5, and 10 that were 96%, 92%, 81% and 79% of C57 control values. For the LS treatment group, the values for ecc1, 2, 5, and 10 were 81%, 86%, 107%, and 116% of C57 control values. T-tests comparing LS to untreated detected significant differences for ecc5 (P = 0.05) and ecc11 (P = 0.042).
Fig. 2. EDL force measurements.
(A) EDL specific force shows a larger average force generation in the LS and EL treated groups compared with the untreated het group. C: C57BL/10 wild-type control mice, U: untreated het mice, and LS: lisinopril / spironolactone treated mice (n = 18 per group); and EL: lisinopril / eplerenone treated mice (n = 17). (B) EDL forces measured during 10 eccentric contractions (ecc1-10) and during a final contraction (ecc 11) after a rest period recovery to allow recovery from fatigue, show lower force generation in the untreated het group compared to C57 control mice. Force for LS and EL treated groups had values closer to wild-type values. Force are represented mean ± SEM.
In diaphragm, het mice produced a force of 81% of C57 control values at baseline, but a recovery of only 59% of the wild-type force after fatiguing contractions (Table 1). The EL treatment group showed 82% and 75% of wild-type values at baseline and after recovery, supporting that EL treatment may benefit recovery after fatigue rather than improve baseline force. A t-test comparing relative recovery of EL compared to untreated detected a significant difference of P= 0.048.
Myofiber damage in diaphragm was significantly improved in both LS and EL treatment groups and was 26% and 19% lower (P = 0.0003 and 0.0117), respectively, compared to untreated het mice (Fig. 1). Abdominal muscles that help to support respiration, had a 30% lower amount of damage in the LS treated group compared with the untreated het group (t-test P = 0.01). Surprisingly, and in contrast to our previous studies with either LS or lisinopril alone [6, 8], quadriceps showed only a 9% reduction of damage and only in the EL group, but not the LS group. However ongoing damage in this untreated het cohort was less than 10% overall, and fibrosis has not accumulated in quadriceps of het mice at this age (Fig. 1).
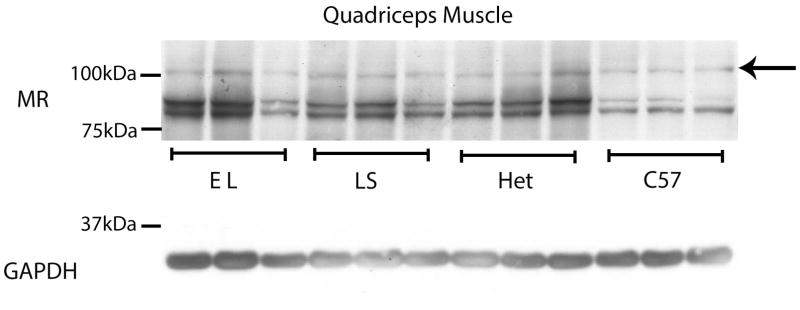
Since other therapeutic targets have been found to be reduced during drug treatment in muscular dystrophy trials [21], we investigated the levels of mineralocorticoid receptors from treated mice. Western blots demonstrated equivalent levels of mineralocorticoid receptors in untreated, LS, and EL treated mice (Fig. 3). This data supports that MR is not reduced by either LS or EL during the 16-week treatment time-course.
Fig. 3. MR protein levels are maintained in quadriceps muscles from het mice after 16 weeks of LS or EL treatment.
Representative western blots of quadriceps muscles from 3 biological replicates are shown comparing MR protein levels from equivalent amounts (50 μg) of protein homogenates from: C57BL/10 wild-type mice (C57), dystrophin-deficient; utrophin haplo-insufficient mice (Het), lisinopril plus spironolactone treated Het mice (LS) and eplerenone plus lisinopril treated HET mice (EL). Western blots used a combination of MR-specific monoclonal antibodies MR1-18 1D5 & MRN 2B7 (full length MR predicted molecular weight ~107kDa, arrow) or a GAPDH antibody (loading control; predicted molecular weight ~36kDa). MR blots detected an additional lower molecular weight (~90 kDa) doublet, which are known degradation products resulting from snap freezing samples prior to processing [22].
DISCUSSION
Treatment with both LS and EL resulted in overall improvements in function and pathology of dystrophic skeletal and cardiac muscles. A difference in overall efficacy between the inclusion of a non-specific versus specific MR antagonist was not detected. These data support that MR, rather than the other steroid hormone receptors bound by spironolactone, is the therapeutic target conferring efficacy of these drugs on skeletal muscles. Mineralocorticoid receptor levels were also maintained in skeletal muscles after 16 weeks of treatment. These data, together with the successful translation of preclinical MR antagonist efficacy to DMD cardiomyopathy, supports that targeting MR may also confer skeletal muscle benefits to DMD patients.
Analysis indicated that both treatment groups improved overall performance in all 14 independent outcome parameters: in none of the 14 assessed parameters was the untreated group quantitatively comparable with treatment. Although we powered the group sizes based on our previous published study using this mouse model [6], and wild-type C57 values were improved compared to untreated het mice in each of the 26 parameters, significant differences were not detected by ANOVA for several of the parameters (Table 1). During the original published study using these mice there was ongoing construction in the building housing the vivarium and the noise and vibration may have exacerbated the phenotype of the dystrophic mice accounting for larger differences between dystrophic and wild-type measurements. Stress levels of corticosterone may have activated MR at times in the circadian rhythm when circulating glucocorticoids are normally low.
In addition to environmental effects on dystrophic mice, we have also modified our EDL dissection technique. In the previous study, EDL muscles were removed and sutures were tied to each tendon to mount the muscle on the force transducer, as per TREAT-NMD SOP DMD_M.1.2.002 guidelines. However, this method causes muscle damage during dissection and leads to sutures with a large amount of compliance that compensate for much of the increased length when performing eccentric contraction measurements. Therefore, we now tie the suture to the tendons in situ prior to removing the muscle from the leg. This technique has resulted in increased baseline forces in both C57 wild-type controls (443±21 versus 334±39 mN/mm2) and het dystrophic muscles (352 ±19 versus 145 ± 24 mN/mm2) compared to our own previously published studies [6]. Diaphragm force values at baseline in het mice in our previous study were also only 40% of wild-type values, and LS treatment resulted in 80% of wild-type values. Eighty percent of normal diaphragm force may represent the upper limit of the therapeutic effect from these drugs and would be difficult to detect when forces of untreated het dystrophic mice in the current cohort are already near that value.
It is clear from the comprehensive analyses of muscle and heart function and pathology, that neither MR antagonist shows any detrimental effect on these tissues and that both have benefits in combination with lisinopril. Since lisinopril targets the renin-angiotensin-aldosterone pathway upstream from MR, but high dosages of lisinopril did not have the same benefit on all of the parameters as observed with the addition of MR antagonists preclinically and clinically [8, 9], these studies support the continued clinical development of MR antagonists for treating muscular dystrophy. A limitation of this preclinical study is the inability to assess gynecomastia, a major side-effect of spironolactone in boys older than 8 or 9 years-of-age due to its anti-androgenic effects. Hyperkalemia, another side-effect of MR antagonists in humans, is typically much less prevalent in mice and was therefore not assessed in this study. If MR antagonists are demonstrated to be efficacious for dystrophic skeletal muscles in clinical trials, the choice of MR antagonist use in individual DMD patients will ultimately need to be based on considerations of age, side-effects, cost, and access. In summary, MR antagonists represent a novel therapeutic approach for all muscle types affected by DMD and have the potential to be used in combination treatments that ultimately improve morbidity and mortality in muscular dystrophy patients.
Acknowledgments
We would like to thank Anna Bratasz and Jessica Pyles of the Small Animal Imaging Core for conducting the MRI scans and Ellen McMahon for helpful discussions. This study was funded by NIH R01 HL116533, R01 NS082868, and DOD MD120063. Eplerenone was provided by the Pfizer Compound Transfer Program. Part of the diaphragm studies were supported by P30 core grant NINDS P30 NS045758.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest relevant to this study.
References
- 1.Guiraud S, Aartsma-Rus A, Vieira NM, Davies KE, van Ommen GJ, Kunkel LM. The Pathogenesis and Therapy of Muscular Dystrophies. Annu Rev Genomics Hum Genet. 2015;16:281–308. doi: 10.1146/annurev-genom-090314-025003. [DOI] [PubMed] [Google Scholar]
- 2.Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 3.Heier CR, Damsker JM, Yu Q, Dillingham BC, Huynh T, Van der Meulen JH, Sali A, Miller BK, Phadke A, Scheffer L, Quinn J, Tatem K, Jordan S, Dadgar S, Rodriguez OC, Albanese C, Calhoun M, Gordish-Dressman H, Jaiswal JK, Connor EM, McCall JM, Hoffman EP, Reeves EK, Nagaraju K. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO molecular medicine. 2013;5:1569–85. doi: 10.1002/emmm.201302621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janssen PM, Murray JD, Schill KE, Rastogi N, Schultz EJ, Tran T, Raman SV, Rafael-Fortney JA. Prednisolone attenuates improvement of cardiac and skeletal contractile function and histopathology by lisinopril and spironolactone in the mdx mouse model of Duchenne muscular dystrophy. PLoS One. 2014;9:e88360. doi: 10.1371/journal.pone.0088360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sali A, Guerron AD, Gordish-Dressman H, Spurney CF, Iantorno M, Hoffman EP, Nagaraju K. Glucocorticoid-treated mice are an inappropriate positive control for long-term preclinical studies in the mdx mouse. PLoS One. 2012;7:e34204. doi: 10.1371/journal.pone.0034204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rafael-Fortney JA, Chimanji NS, Schill KE, Martin CD, Murray JD, Ganguly R, Stangland JE, Tran T, Xu Y, Canan BD, Mays TA, Delfin DA, Janssen PM, Raman SV. Early treatment with lisinopril and spironolactone preserves cardiac and skeletal muscle in duchenne muscular dystrophy mice. Circulation. 2011;124:582–8. doi: 10.1161/CIRCULATIONAHA.111.031716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chadwick JA, Hauck JS, Lowe J, Shaw JJ, Guttridge DC, Gomez-Sanchez CE, Gomez-Sanchez EP, Rafael-Fortney JA. Mineralocorticoid receptors are present in skeletal muscle and represent a potential therapeutic target. FASEB J. 2015;29:4544–54. doi: 10.1096/fj.15-276782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowe J, Wodarcyk AJ, Floyd KT, Rastogi N, Schultz EJ, Swager SA, Chadwick JA, Tran T, Raman SV, Janssen PM, Rafael-Fortney JA. The angiotensin converting enzyme inhibitor lisinopril improves muscle histopathology but not contractile function in a mouse model of Duchenne muscular dystrophy. J Neuromusc Diseases. 2015;2:257–68. doi: 10.3233/JND-150099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raman SV, Hor KN, Mazur W, Halnon NJ, Kissel JT, He X, Tran T, Smart S, McCarthy B, Taylor MD, Jefferies JL, Rafael-Fortney JA, Lowe J, Roble SL, Cripe LH. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14:153–61. doi: 10.1016/S1474-4422(14)70318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Young MJ. Mineralocorticoid receptor antagonists-pharmacodynamics and pharmacokinetic differences. Curr Opin Pharmacol. 2016;27:78–85. doi: 10.1016/j.coph.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Rafael-Fortney JA, Huang P, Zhao XS, Cheng G, Zhou X, Kaminski HJ, Liu L, Ransohoff RM. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci. 2008;264:106–11. doi: 10.1016/j.jns.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–27. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 13.Pojoga LH, Adamova Z, Kumar A, Stennett AK, Romero JR, Adler GK, Williams GH, Khalil RA. Sensitivity of NOS-dependent vascular relaxation pathway to mineralocorticoid receptor blockade in caveolin-1-deficient mice. Am J Physiol Heart Circ Physiol. 2010;298:H1776–88. doi: 10.1152/ajpheart.00661.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Susic D, Varagic J, Ahn J, Matavelli L, Frohlich ED. Long-term mineralocorticoid receptor blockade reduces fibrosis and improves cardiac performance and coronary hemodynamics in elderly SHR. Am J Physiol Heart Circ Physiol. 2007;292:H175–9. doi: 10.1152/ajpheart.00660.2006. [DOI] [PubMed] [Google Scholar]
- 15.Qin W, Rudolph AE, Bond BR, Rocha R, Blomme EA, Goellner JJ, Funder JW, McMahon EG. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 16.Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation. 2008;117:2253–61. doi: 10.1161/CIRCULATIONAHA.107.748640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Q, Saito Y, Naya N, Imagawa K, Somekawa S, Kawata H, Takeda Y, Uemura S, Kishimoto I, Nakao K. The specific mineralocorticoid receptor blocker eplerenone attenuates left ventricular remodeling in mice lacking the gene encoding guanylyl cyclase-A. Hypertens Res. 2008;31:1251–6. doi: 10.1291/hypres.31.1251. [DOI] [PubMed] [Google Scholar]
- 18.Janssen PM, Hiranandani N, Mays TA, Rafael-Fortney JA. Utrophin deficiency worsens cardiac contractile dysfunction present in dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2005;289:H2373–8. doi: 10.1152/ajpheart.00448.2005. [DOI] [PubMed] [Google Scholar]
- 19.Murray JD, Canan BD, Martin CD, Stangland JE, Rastogi N, Rafael-Fortney JA, Janssen PM. The force-temperature relationship in healthy and dystrophic mouse diaphragm; implications for translational study design. Front Physiol. 2012;3:422. doi: 10.3389/fphys.2012.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–8. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- 21.Witting N, Kruuse C, Nyhuus B, Prahm KP, Citirak G, Lundgaard SJ, von Huth S, Vejlstrup N, Lindberg U, Krag TO, Vissing J. Effect of sildenafil on skeletal and cardiac muscle in Becker muscular dystrophy. Annals of neurology. 2014;76:550–7. doi: 10.1002/ana.24216. [DOI] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez CE, Warden M, Gomez-Sanchez MT, Hou X, Gomez-Sanchez EP. Diverse immunostaining patterns of mineralocorticoid receptor monoclonal antibodies. Steroids. 2011;76:1541–5. doi: 10.1016/j.steroids.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]