Abstract
Individual differences, the importance of which was identified by Darwin more than 150 years ago, are evident in multiple domains. This review discusses the role of temperament in health-related outcomes in rhesus monkeys. Temperament is proposed as affecting health outcomes via behavioral means, and also via physical means either through its direct association with variation in physiological systems (a “main effects” model), or through its impact on functioning when organisms are in stressful circumstances (an “interaction effects” model). The specific temperament factor described is Sociability, which reflects a tendency to affiliate, and which is associated with positive affect, and with differences in sensitivity of brain dopamine systems. Data are reviewed showing that individual differences in Sociability in rhesus monkeys are related to variation in sympathetic innervation of lymphoid tissue (a main effect), as well as to patterns of coping in socially stressful circumstances (an interaction effect). Results such as these have implications for studies in behavioral ecology, medicine, and even for management practices in captive colonies of nonhuman primates.
Keywords: temperament, personality, sociability, AIDS, sympathetic nervous system, coping
INTRODUCTION
Differences between individuals in a species are evident in multiple domains, including those associated with physical characteristics (height, weight) and psychological characteristics (cognitive ability, temperament), as well as those associated with health (e.g., not all individuals get sick during the flu season). The idea of individual differences was, of course, central to Darwin’s ideas on natural selection and evolution. As he wrote in The Origin of Species:
The many slight differences… being observed in the individuals of the same species inhabiting the same confined locality, may be called individual differences. No one supposes that all the individuals of the same species are cast in the same actual mould. These individual differences are of the highest importance for us, for they are often inherited, as must be familiar to every one; and they thus afford materials for natural selection to act on and accumulate… [Darwin, 1872/1975].
Of the three domains that I listed above, individual differences in health are likely to be of great importance, inasmuch as health is intimately tied into survival, and survival can have important implications for reproduction and fitness. But the three domains are not independent of each other. In terms of infectious disease, it’s important to remember that while it is cells that become infected, it is individuals that become ill—characteristics of the individual that are not directly involved in cellular-level processes can affect the infection and its sequelae. It is well known, for example, that differences in physical functioning are associated with health and disease; there is now growing evidence that individual differences in psychological functioning influence the process by which an infection becomes an illness, and what the course of that illness will be.
Which psychological differences are important? Both human and nonhuman studies have demonstrated that emotional functioning is of critical importance. Although it is true that all members of a species are very likely able to express the same emotions, such as fear and anger, the important variation is likely to lie in higher order phenomena reflected in issues such as these:
Should an emotion be expressed in this situation or not?
If the choice is made to express an emotion, with which one should the individual respond—anger or fear?
How soon should the emotion be expressed—should a response be given immediately or should it be delayed?
In what manner should it be displayed—should the response be of high intensity immediately or should the response be escalated over a period of time as needed?
For how long should the emotion be expressed—should it be terminated after a certain time or after another individual has displayed a certain behavior?
If the expression is terminated after the other individual displays a certain behavior, which behavior must it be, how intensely must it be displayed, and for how long must the animal display it?
“Temperament” is the term that is usually applied to the phenomena just described. Allport [1937; p 54], for example, defined temperament as “the characteristic phenomena of an individual’s emotional nature, including his susceptibility to emotional stimulation, his customary strength and speed of response, the quality of his prevailing mood, and all peculiarities of fluctuation and intensity in mood…” Historically, the concept of temperament has been fairly distinct from a related concept, personality, although this distinction is becoming less clear [Clark & Watson, 2008]. In the animal literature, these two terms are often used interchangeably, and both are related to a third term that has recently emerged in behavioral ecology, namely “behavioral syndromes” [Sih et al., 2004]. All of these terms, however, reflect the same basic phenomenon: consistencies in patterns of response (and its presumed psychological and emotional underpinnings) between individuals across different contexts.
TEMPERAMENT AND HEALTH
The idea that temperament could be related to health is an old one [Capitanio, 2008]. In Greek and Roman times, for example, physicians postulated that there were four humors that filled the body and when these humors were in balance, the individual experienced good health and well-being. If one humor predominated, however, physical health could be compromised. This humoral theory of health was extended to describe differences in character, and the idea arose that physical health and temperament shared underlying causes. A predominance of black bile, for example, led to a melancholy temperament, manifested as fear, depression, and discontent with life, as well as physical disease of abdominal organs with symptoms manifesting as flatulence and digestive disturbances [Jackson, 1978]. Later formulations of a health and personality/temperament link, as in the development of the field of Psychosomatic Medicine, were more scientific. Although a typology of personality “profiles” associated with specific disease outcomes emerged in the scientific literature of the early 20th century [see Capitanio, 2008], Franz Alexander [1950] thought that the more productive domain to explore was emotional states, not personality “types” per se: “The true psychosomatic correlations are between emotional constellations and vegetative responses” (p 75). The focus here was on how an individual’s pattern of emotional responsiveness influenced his or her adjustments to the conditions of life—does the person typically respond in a hostile fashion, in a passive, dependent fashion, or in a withdrawn fashion? Interestingly, the idea that consistent patterns of emotional and behavioral responses could be related to health was recently echoed by the former Director of the National Institutes of Health, in testimony before Congress:
…NIH is strategically investing in research to further our understanding of the fundamental causes of diseases at their earliest molecular stages so that we can reliably predict how and when a disease will develop and in whom. Because we now know that individuals respond differently to environmental changes according to their genetic endowment and their own behavioral responses, we can envision the ability to precisely target treatment on a personalized basis. Ultimately, this individualized approach, completely different than how we treat patients today, will allow us to preempt disease before it occurs [Zerhouni, 2006; emphasis added].
How might individual differences in temperament influence health? One set of mechanisms exists at the behavioral level. In humans, for example, one of the best-known relationships between personality and health is that of conscientiousness and longevity. Conscientiousness is a major personality factor that includes such traits as organization, reliability, dutifulness, order, and self-discipline. A recent meta-analysis of 19 studies [Kern & Friedman, 2008] revealed that the average effect size was considerable: at any given age, individuals that were higher on this trait were less likely to die than were individuals that were lower on this trait. How conscientiousness affects longevity is not known for sure, but there is good evidence that some of the effect of this personality factor is mediated through health behaviors; not surprisingly, conscientious people tend to take better care of themselves [Bogg & Roberts, 2004] and this can translate into longer lifespan. This example, then, illustrates a mechanism that is principally behavioral.
Other ways in which temperament could affect health are more directly physical and could involve, broadly speaking, two models [Capitanio, 2008]. The first is a “main effects” model and asks whether individuals that are high or low on some fundamental trait are “built” differently in a way that can affect a disease process. The second is an “interaction” model, in which traits (such as temperament/personality) influence the way an individual copes during encounters with stressful stimuli. The three models (behavioral, main effects, and interaction models) are not necessarily mutually exclusive; rather, this categorization is proposed simply as a heuristic device to organize discussion and investigation. Below, I describe data from our laboratory supporting both the main effects and the interaction models. First, however, I turn to a brief discussion of some data suggesting that social relationships are associated with health and fitness, followed by a discussion of a personality factor, Sociability, which we have been studying for more than a decade that is likely involved in mediating the health effects of social relationships.
SOCIAL CONNECTEDNESS AND HEALTH
It has been known for decades that social connectedness is related to better health and reduced mortality [e.g., Berkman & Breslow, 1983; House et al., 1988]. In a rare experimental study in humans, for example, 276 adults were given nasal drops that contained rhinoviruses and were monitored for the development of a common cold. In addition to recording a variety of health and immune measures, the investigators also recorded the number of social ties reported by the individuals; specifically, does the subject speak (either in person or on the phone) at least once every 2 weeks to anyone within a set of 12 different classes of people (e.g., spouses, parents, neighbors, friends, workmates). If a subject does speak that frequently or more to someone in a class, he or she was given a score of 1; thus, the range of scores was from 0 to 12. The likelihood of contracting a cold was directly related to this measure of social integration: individuals with only 1–3 types of social connections had approximately double the rate of colds compared with those with 6 or more connections [Cohen et al., 1997].
Recent studies have shown that social connectedness can have fitness consequences as well. Silk et al. [2003] reported that adult female yellow baboons at the Amboseli National Park, Kenya, who spent more time grooming and maintaining proximity with other adults, had offspring that were more likely to survive up to 1 year of age than did adult females that spent less time engaged in such affiliation. Importantly, this relationship was maintained after controlling for dominance rank and environmental conditions. In a follow-up analysis with chacma baboons in Botswana, Silk et al. [2009] restricted their analysis to adult females that did not have offspring at the time, to eliminate the possibility that social connectedness is an artifact of others’ attraction to infants that have survived. In addition, only relationships with adult females were considered, inasmuch as relationships with males could have been responsible for the earlier result; a “friendship” with a male could enhance offspring survival in the event of an immigrating male’s attempts at infanticide, for example. Their results confirmed the earlier results—females with stronger social bonds had offspring that survived longer than did females with weaker social bonds, and this relationship was independent of rank and number of available kin.
SOCIABILITY AND EMOTION
Social connectedness is clearly associated with beneficial health outcomes, lower mortality, and in baboons, fitness-related measures. So what contributes to variation in social connectedness? Among the most important factors is social motivation: an interest in others and a desire to affiliate with them. In fact, studies of human and nonhuman primate personality (as well as many studies of nonprimate animals) typically find a factor reflecting a “tendency to affiliate” [Gosling & John, 1999]. In the Five Factor Model of human personality, this factor is Extraversion [McCrae & Costa, 2008], and in various studies of nonhuman primates, this factor is often labeled Sociability [e.g., Capitanio, 1999; Stevenson-Hinde & Zunz, 1978]. At its simplest, individuals high on this trait tend to seek out companionship, whereas those low on this trait do not.
But what constitutes the emotional foundation for Sociability/Extraversion? In humans, it has been long known that Extraversion is correlated with positive affect—extraverts are generally “happier.” (In fact, it is worth noting that, in the first quantitative study of personality in chimpanzees, Crawford [1938] reported a high correlation between “friendliness” and “cheerfulness”; similar relationships have been found in more recent primate work as well [e.g., Weiss et al., 2009].) Behavioral data suggest that while extraverts engage in some types of social activity more than do introverts, this amount of social activity does not mediate the relationship between extraversion and positive affect: extraverts still report more positive affect than do introverts, even after controlling for differences in the amount of social activity. Nor does Extraversion moderate the relationship between social activity and positive affect—both introverts and extraverts seem to get the same “boost” in positive affect from social interaction [Lucas et al., 2008]. These data suggest that there is a more fundamental dimension underlying Extraversion, and it has been suggested that this dimension is probably related to reward sensitivity, and is reflected in variation in sensitivity of brain dopamine systems associated with reward [Depue & Collins, 1999]. In fact, our own research has suggested not only that high Sociability in adult male rhesus monkeys is associated with more sensitive central dopamine function [Ruys et al., 2002], but that Sociability is also associated with a more global interest in novelty [Kinnally et al., 2008], both of which results are similar to those found in humans [e.g., Netter, 2006; Reuter & Hennig, 2005]. Together, results such as these have suggested that the defining characteristic of Extraversion may not be sociability, but rather positive affect [discussed in Lucas et al., 2008]. Nevertheless, in social group-living animals, social objects as a class present numerous fitness-related challenges; so, there is some benefit in focusing on this particular instantiation of the expression of positive emotionality.
SOCIABILITY AND HEALTH: A MECHANISM FOR THE MAIN EFFECTS MODEL
Earlier, I indicated that one means by which a temperament dimension, such as Sociability, could be related to a health outcome may be if variation in such a trait is associated with differences in physiological functioning—if High Sociable (HS) animals are somehow “built” differently than Low Sociable (LS) animals. Sociability does seem to be associated with variation in central neurobiology; in both rhesus monkeys (Sociability) and humans (Extraversion), high values on the trait are related to more sensitive dopaminergic systems [Netter, 2006; Ruys et al., 2002]. Recently, we demonstrated that Sociability in adult male rhesus monkeys is associated with variation in peripheral neurobiology as well [Sloan et al., 2008], and this may be a relatively direct mechanism through which affiliative dispositions facilitate health. (We note that the work to be described below has been conducted with adult male rhesus monkeys; important questions reflecting the roles of species differences, sex differences, and development remain to be answered.)
Lymph nodes are scattered throughout the body and serve as important sites of interaction between immune cells and pathogens. What is generally not appreciated is that lymph nodes (in fact, all primary and secondary lymphoid tissues) are innervated by fibers of the sympathetic nervous system. This is the branch of the autonomic nervous system that is responsible for the global “fight or flight” response, involving sympathetic stimulation and inhibition of various target tissues and release of epinephrine into the circulation from the adrenal medulla. The sympathetic fibers in lymph nodes, which contain norepinephrine, enter the nodes along with the blood supply and then radiate into parenchymal tissue [Bellinger et al., 2001]. When stimulated, the fibers release micromolar doses of norepinephrine nonsynaptically. Most cells of the immune system contain α- or β-adrenergic receptors that respond to the local release of norepinephrine (or availability of epinephrine from the blood compartment) with a consequence of altered immune function [Nance & Sanders, 2007]. (As an interesting aside, there is no anatomical evidence for parasympathetic innervation of any immune organ; it seems that it was more important, in an evolutionary sense, for the immune system to know quickly when things are about to “heat up” than it was to know when things are operating in a calm and steady [i.e., parasympathetic] fashion.)
In general, release of norepinephrine in lymphoid tissue tends to inhibit T-cell function and natural killer (NK) cell function [Madden, 2001]. Because NK cells are important in killing virus-infected cells, one might expect that secretion of norepinephrine would accelerate viral replication through its inhibition of NK function. In fact, in vitro work suggests this is true—Cole et al. [1998] found that administration of physiologic doses of epinephrine or norepinephrine to cultures of cells infected with the HIV results in an acceleration in HIV replication through stimulation of β-adrenergic receptors, activation of the cyclic AMP/protein kinase-A signaling pathway, and subsequent upregulation of viral co-receptor expression on the cell surface, HIV gene expression, and inhibition of antiviral cytokines such as IL-10 and interferon-gamma. That this is attributed to the stimulation of β-adrenergic receptors is evident from pharmacologic studies—when these receptors are blocked, the stimulating effect of norepinephrine is abrogated [Cole et al., 1998].
That this effect can have real consequences was evident from in vivo work conducted during a study of social stress and simian immunodeficiency virus (SIV) infection in our laboratory [Capitanio et al., 1998]. In collaboration with Steve Cole and Erica Sloan at UCLA, we obtained lymph nodes from both SIV-infected and control animals at 37 weeks postinoculation. Nodes were sectioned and stained for the presence of catecholaminergic neural fibers and the presence of SIV replication was determined by in situ hybridization. Next, a grid was overlaid on the node sections and the presence of SIV replication was noted for 250 μm2 quadrats of parenchymal tissue in which noradrenergic fibers were either present or absent. We found that the odds of SIV replication were nearly four times greater in quadrats that contained a noradrenergic fiber than in those that did not [Sloan et al., 2006], confirming in vivo Cole’s earlier in vitro findings. Furthermore, we noted a remarkable plasticity in innervation patterns when we examined nodes from non-SIV-infected animals [Sloan et al., 2007]: lymph nodes from animals in stressful social conditions had nearly double the density of catecholaminergic fibers compared with nodes from animals in nonstressful social conditions, and this was particularly evident in paracortical regions, which are populated primarily by T-cells and macrophages. The degree of sympathetic innervation was significantly associated with mRNA production of nerve growth factor (NGF), the principal neurotrophin that supports innervation of tissue by the sympathetic nervous system, as well as with decreased mRNA expression of interferon-β and one of its effectors, IFI27. Together, these studies strongly suggest that stress can affect the dynamics of nervous system-immune interactions in lymphoid tissue in ways that can have important consequences for viral disease progression.
But what of the role of Sociability? We reexamined the lymph node data from seven non-SIV-infected control animals, to determine whether innervation patterns might be related to Sociability. Animals had been classified as “HS” or “LS” based upon ratings performed in the animals’ natal cages, years earlier [Capitanio, 1999], and analysis of behavioral data confirmed that HS animals did show significantly higher frequencies of affiliative behaviors [Sloan et al., 2008]. Examination of the nodes from these animals revealed that nodes from LS animals had a 2.8-fold greater density of innervation compared with nodes from HS animals, and the increased innervation was also related to greater expression of mRNA for NGF [Sloan et al., 2008]. These results helped us explain an earlier, puzzling finding [Maninger et al., 2003]: after the animals’ personality was assessed in their natal cages, they were relocated from their large, outdoor field cages to individual housing indoors, in order to eventually be enrolled in the SIV study. Shortly after the relocation, and well before any of our experimental work began, we gave the animals a booster vaccination with tetanus toxoid and assessed tetanus-specific antibody (IgG), to test the hypothesis that removal from the rich social environment to individual caging might have different “meaning” to animals that were Low vs. High in Sociability. In fact, a significant effect emerged, although it was in the direction opposite to what we initially expected; although the IgG response increased for all animals after the boost, it increased more for the HS animals. This was particularly evident at the 9-month follow-up blood sample, where the tetanus-specific IgG response was significantly lower for the LS animals [Maninger et al., 2003]. When we reexamined these data in light of the innervation density data, we discovered that lower IgG levels were found in animals with the highest innervation density and, as described above, these were LS animals. Statistically, we found that innervation density mediated a significant part of the relationship between Sociability and IgG response, although there remained a significant residual, indicating that other factors also mediate the effects of Sociability on variation in antibody response [Sloan et al., 2008].
Together, these data suggest that animals that are high or low on the trait Sociability may indeed be “built” differently. The evidence (described earlier) is good that there are central nervous system differences, especially with respect to brain dopamine systems. Our data, however, suggest for the first time that differences also extend to the peripheral nervous system in a way that can have consequences for a disease process. We know that stress can lead to increased SNS innervation of lymph nodes [Sloan et al., 2007]; it’s possible that the higher innervation density for LS animals reflects a similar process—perhaps because of having a less sensitive dopaminergic reward system, LS animals may not find social interaction to be as pleasant/rewarding/stimulating as do HS animals, and in fact, may find it somewhat stressful. This could result in greater density of sympathetic nerves in various tissues but especially in lymphoid tissue, placing the animal at risk for increased morbidity, as has been found [e.g., Ironson et al., 2008]. It is, of course, unknown how such a pattern develops (though it could develop early in life) and whether the trajectory for low Sociability is set by genetic factors, prenatal factors, postnatal factors, or some combination of these.
SOCIABILITY AND HEALTH: INTERACTIONS WITH SOCIAL CONTEXT
A second way in which temperament could influence health is that it may affect how an individual copes with its circumstances. This “interaction” model suggests that the risk associated with a trait would be evident only in stressful circumstances. In terms of social temperament, then, low Sociability might not be risky for individuals in some contexts, but could be risky in other contexts. Evidence in our laboratory in support of the interaction model comes from a study of the effects of Sociability and social stress on early measures of SIV disease progression in adult male rhesus monkeys.
After a several month period of adaptation to individual indoor housing [Capitanio et al., 2006a], animals were socialized for 100 min/day in one of two conditions. In the Stable condition, animals met daily in groups of three, with the identity of the animals in a particular group remaining constant for the entire study. In the Unstable condition, animals also met in the same cages for the same period of time, but the number and identity of animals changed daily. There were two pools of nine animals assigned to the Unstable social condition in the study, and on any given day, a two-member, three-member, and a four-member unstable group was formed from each nine-member pool. Because group membership changed daily from within each pool, animals did not know from day-to-day with whom they were to be socialized. This resulted in a situation in which, on a daily basis, animals had to reestablish social relationships with each other depending on which other animals were present in the cage. A prior study that utilized this same manipulation confirmed that the Unstable social condition is stressful, as indicated by higher frequencies of agonistic behavior, and altered regulation of the hypothalamic–pituitary–adrenal axis [Capitanio et al., 1998].
All social groups, Stable or Unstable, were homogeneous in terms of personality composition: LS animals were socialized only with LS animals and HS animals were socialized only with HS animals. As in our earlier study, all animals experienced three 100 min socialization sessions before SIV inoculation. This was done because, although animals in the Stable and Unstable conditions were equally unfamiliar with each other for the first socialization session, animals in the Stable condition quickly learned that their experience was predictable. In contrast, animals in the Unstable condition learned over the course of the first few sessions of the unpredictability of their social situation. By the fourth session, behavior in the Stable and Unstable social groups was already quite different. In fact, data analysis revealed that, as expected, monkeys in Unstable social conditions showed increased frequencies of fear and conflict (grimaces, threats, chases, and contact aggression) and decreased amounts of affiliation (grooming and groom solicitation) compared with monkeys in Stable social conditions. After the three initial socialization sessions, animals were inoculated either with SIVmac251 (n = 12 LS animals and n = 12 HS animals) or with saline (n = 6 LS animals; n = 6 HS animals). Saline-inoculated controls principally served to insure that sufficient animals were present to maintain the social conditions once they developed disease and were euthanized; for example, each Stable LS group comprised two SIV-inoculated animals and one saline-inoculated animal.
Social groups were typically formed 4 days per week for 100 min/day, and on alternate weeks each animal was chemically restrained (ketamine hydrochloride 10 mg/kg) and given a complete physical examination. Blood samples were drawn at this time to assess viral load [using real-time PCR: Leutenegger et al., 2001] and SIV-specific IgG [using ELISA: Lu et al., 1998]. Additional blood samples were drawn from the animals at 4-week intervals without use of anesthesia—made possible by the fact that these animals had been trained before the start of the study to present their arms for venipuncture. The armpull samples (all of which were drawn at 15:00–15:30 hr while monkeys were in their individual living cages) were assayed for plasma cortisol concentrations using radioimmunoassay. Also, a complete blood count with manual differential and three-color flow cytometry was performed to track disease. Animals did not receive socialization on days when they experienced venipuncture either via armpull or chemical restraint. Finally, euthanasia decisions were made by veterinary staff using a set of standardized clinico-pathological criteria developed specifically for retrovirus-infected monkeys. In our studies, the most common criterion for euthanasia was weight loss greater than 15% in 2 weeks or 30% in 2 months. The principal outcome measure for our analysis was viral set-point, as indexed by viral RNA (vRNA) measured at 8–10 weeks p.i. This set-point reflects a relatively stable value resulting from the individual’s immune response achieving a sort of balance with the virus. Set-point is a good proxy measure of survival, inasmuch as the correlation between set-point and survival (i.e., days to euthanasia) in both this and an earlier study [Capitanio et al., 1998] was from r = −0.7 to −0.8. Among HIV infected patients, the use of antiretroviral medication is aimed specifically at keeping set-point low, inasmuch as human studies have also demonstrated the link between virus concentrations in blood and survival [Mellors et al., 1996].
We found set-point vRNA to be significantly related to three immune measures, which together accounted for 66.4% of the variance in vRNA [Capitanio et al., 2008], but, here I will focus on only one of these measures, interferon-stimulated gene expression. As background, most know that a virus, such as HIV or SIV, is an intracellular parasite; it must infect a cell and then commandeer the cell’s machinery to replicate itself. When it does this, the immune system eventually can mobilize cytotoxic T-cells that will detect the infected cells and kill them. But this branch of the immune system, a component of “specific” immunity, can take weeks to develop. Until then, the body has mechanisms for combat that are associated with the other major branch of the immune system, the “innate” immune response, which gets mobilized immediately upon infection. One mechanism of innate immunity involves the production of proteins called interferons by both the virally infected cells themselves and by populations of immune cells. Interferon-α (IFNA) is one such substance. When cells are infected, IFNA production (i.e., transcription of the IFNA gene, leading to production of the IFNA protein) increases. This is a transient increase, however, because a principal function of the IFNA protein is to stimulate transcription of a variety of downstream genes, the proteins of which will become the effector molecules that will “do the work” of the innate immune response. These downstream genes are collectively referred to as “interferon-stimulated genes” (ISG), and there are dozens of them. Unfortunately, the role of interferons in immunodeficiency virus infection is not clear, but evidence suggests that activation of the IFNA system is inadequate to control SIV/HIV replication [Abel et al., 2002]. And in fact, what we found was that greater ISG expression in peripheral blood cells was indeed associated with higher viral load.
The interesting thing about the ISG results in our study was that we saw a pattern of biobehavioral responses that were associated with ISG expression, and it was driven by variation in the personality factor, Sociability—but only for animals in Unstable social conditions, illustrating the interaction effect described above. Specifically, for animals in Unstable conditions, LS animals showed more submissive behaviors in the form of present-sex postures, showed lower concentrations of basal plasma cortisol (which, for this model, we have reported as reflecting altered regulation of the pituitary–adrenal axis [Capitanio et al., 1998], and so is not a good thing), and higher ISG expression. All measures (Sociability, cortisol, present-sex, and ISG expression) were significantly intercorrelated, but only for animals in the Unstable condition; no correlations among these measures were evident for animals in the Stable condition (Fig. 1).
Fig. 1.
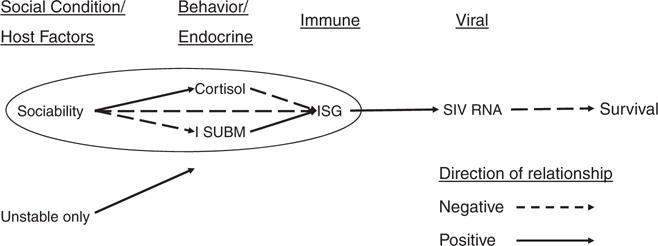
Influence of Sociability on behavior, immune, and endocrine measures is moderated by social condition. Adapted from Capitanio et al. [2009]. ISUBM, initiation of submissive behavior (i.e., present-sex posture); ISG, interferon-stimulated genes.
The results of this study suggest that temperament may exert its influence on health and disease through its interaction with the individual’s social context: Sociability was associated with a set of biobehavioral relationships only for individuals in the stressful social context. What was the role of Sociability specifically? In the Unstable groups, periodic eruptions of aggression were more frequent and the tension was more sustained than was the case in Stable social groups. Insuring that one is not harmed during periodic eruptions would be very important and there are different ways one can accomplish this. One way of coping with this tension is to keep a “low profile,” by avoiding interaction, gaze averting quickly, and when necessary sending clear nonthreatening signals, such as moving out of the way or by exhibiting fear grimaces from a distance. Animals higher in Sociability seem to use this strategy when encountering unfamiliar animals [Capitanio, 1999, 2002]. LS animals, however, handled the tension in Unstable groups by displaying more “present-sex” postures, and our mediational analysis revealed that this submissive behavior mediated the relationship between Sociability and higher ISG activity. “Present-sex” is a behavior that is usually not given at a distance, but rather occurs after a higher ranked animal has already approached. Other data from our laboratory suggest that LS animals have poorer social skills; for example, when presented with videotaped displays of an unfamiliar monkey displaying aggressive behaviors, LS animals sit and stare—they show significantly less activity, tend to have higher durations of looking at the videotaped animal, and take nearly twice as long to gaze avert as do HS animals [Capitanio, 2002]. Sustained displays of submission by LS animals early in the study, then, could reflect a more passive style of coping in response to the aggression and tension seen in the early Unstable group formations. This more passive style of coping may be a manifestation of LS animals’ lower social motivation—they may be less willing to work on managing the situation actively, such as HS animals do, to achieve a positive outcome, because they are simply not that interested in, skilled at, or presumably, stimulated by social interaction.
DISCUSSION
It’s clear that a personality dimension reflecting a tendency to affiliate with conspecifics exists broadly in the animal world, at least among social species. There is growing evidence that this dimension reflects a broader underlying trait associated with seeking out stimulating situations and finding them rewarding. For such an individual, social interaction provides almost unparalleled opportunities for reward: successful navigation of the complexities associated with changing moods of partners, meeting new people, the dynamics of a situation as people come and go, can all be quite energizing. Evidence suggests that such people may differ in fundamental ways in terms of central and peripheral neurobiology from those for whom social interaction is a less desirable way to spend time. And, when forced into a tricky (i.e., stressful) social situation, this factor may strongly influence how one responds. Together, anatomical and behavioral consequences of variation on this personality dimension may create an internal milieu that affects fundamental immune processes, and these in turn impact physical health, particularly if there is a mismatch between the person’s tendencies and the types of situations the individual finds herself in. LS individuals may thrive in nonsocial situations and be stressed in highly social situations, whereas HS individuals may show the opposite pattern.
This interpretation suggests the value of an ecological perspective that can have implications in multiple fields of inquiry. What may be of greater importance than the main effect model is the interaction model—the idea that risky patterns of emotional response are risky primarily in specific contexts: recall that being low in Sociability was not risky for animals in Stable social groups. It may be more fruitful to think about the person–situation fit than about the person or the situation, per se: how well do the individual’s characteristics mesh with the circumstances she finds herself in? From a behavioral ecological point of view, one might be interested in how particular traits can be adaptive in one context but maladaptive in another, and study how such a trade-off could have implications for fitness [Sih et al., 2004]; for example, because of their lower rate of interaction, LS animals may be at reduced risk from respiratory pathogens, which are often transmitted through close social interaction. From a human medicine point of view, one might want to tailor a treatment (or a prevention effort) to be maximally effective for an individual with a specific set of characteristics who finds herself repeatedly in a specific set of unpleasant circumstances (e.g., poor meshing); this is exemplified by a growing interest in identifying individuals in the military with particular characteristics who might be at risk for developing posttraumatic stress disorder when exposed to combat situations [e.g., Zohar et al., 2009]. And from a colony management perspective, one might recognize that “one size doesn’t fit all” and that animal welfare can best be promoted by considering the “fit” between the animal’s capabilities and the kinds of situations we put the animals in [Capitanio et al., 2006b]. All these examples draw attention to the fundamental fact that organisms exist in environments, and optimal health (physical and mental) and fitness will likely be facilitated when the fit between the organism and its circumstances is maximized.
Acknowledgments
The research described in this review was supported by NIH grant MH049033 (to J.P.C.) and RR000169 to the California National Primate Research Center. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California at Davis, and the work complies with all relevant laws of the United States. All research reported in this manuscript adhered to the American Society of Primatologists principles for the ethical treatment of nonhuman primates. I thank my collaborators, staff, and students for their efforts in this research program, and the staff of CNPRC for their excellent care of the animals. I am grateful to Erin Kinnally, who provided valuable comments on an earlier draft of this manuscript, and I thank Doree Fragaszy for the invitation to participate in the symposium at the 2009 meeting of the American Society of Primatologists, upon which this article was based.
References
- Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. Journal of Virology. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander F. Psychosomatic medicine: its principles and applications. New York: Norton; 1950. [Google Scholar]
- Allport GW. Personality: a psychological interpretation. New York: Henry Holt; 1937. [Google Scholar]
- Bellinger DL, Lorton D, Lubahn C, Felten DL. Innervation of lymphoid organs—association of nerves with cells of the immune system and their implications in disease. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3rd. NY: Academic; 2001. pp. 55–111. [Google Scholar]
- Berkman LF, Breslow L. Health and ways of living: the Alameda county study. NY: Oxford Press; 1983. [Google Scholar]
- Bogg T, Roberts BW. Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin. 2004;130:887–919. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality dimensions in adult male rhesus macaques: prediction of behaviors across time and situation. American Journal of Primatology. 1999;47:299–320. doi: 10.1002/(SICI)1098-2345(1999)47:4<299::AID-AJP3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Sociability and responses to video playbacks in adult male rhesus monkeys (Macaca mulatta) Primates. 2002;43:169–178. doi: 10.1007/BF02629645. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Personality and disease. Brain, Behavior, and Immunity. 2008;22:647–650. doi: 10.1016/j.bbi.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proceedings of the National Academy of Sciences. 1998;95:4714–4719. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, Fairbanks LA. Considerations in the selection and conditioning of Old World monkeys for laboratory research: animals from domestic sources. ILAR Journal. 2006a;47:294–306. doi: 10.1093/ilar.47.4.294. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery rearing of nonhuman primates in the 21st Century. New York: Springer; 2006b. pp. 191–213. [Google Scholar]
- Capitanio JP, Abel K, Mendoza SP, Blozis SA, McChesney MB, Cole SW, Mason WA. Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain, Behavior, and Immunity. 2008;22:676–689. doi: 10.1016/j.bbi.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Lerche NW, Sloan EK, Cole SW. Stress, coping, and AIDS: insights from a monkey model. In: Singh PP, Donahoe RM, editors. Proceedings of the International Conference on Biotechnological Approaches to Neuroimmunomodulation and Infectious Diseases. SAS Nagar: NIPER; 2009. pp. 1–18. [Google Scholar]
- Clark LA, Watson D. Temperament: an organizing paradigm for train psychology. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality: theory and research. 3rd. New York: Guilford; 2008. pp. 265–286. [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM., Jr Social ties and susceptibility to the common cold. Journal of the American Medical Association. 1997;277:1940–1944. [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. Journal of Immunology. 1998;161:610–616. [PubMed] [Google Scholar]
- Crawford MP. A behavior rating scale for young chimpanzees. Journal of Comparative Psychology. 1938;26:79–91. [Google Scholar]
- Darwin C. The origin of species. 6th. Franklin Center, PA: Franklin Library; 1872/1975. [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Gosling SD, John OP. Personality dimensions in nonhuman animals: a cross-species review. Current Directions in Psychological Science. 1999;8:69–75. [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Ironson GH, O’Cleirigh C, Weiss A, Schneiderman N, Costa PT., Jr Personality and HIV disease progression: role of NEO-PI-R openness, extraversion, and profiles of engagement. Psychosomatic Medicine. 2008;70:245–253. doi: 10.1097/PSY.0b013e31816422fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SW. Melancholia and the waning of humoral theory. Journal of the History of Medicine and Allied Sciences. 1978;33:367–376. doi: 10.1093/jhmas/xxxiii.3.367. [DOI] [PubMed] [Google Scholar]
- Kern ML, Friedman HS. Do conscientious individuals live longer? A Quantitative Review. Health Psychology. 2008;27:505–512. doi: 10.1037/0278-6133.27.5.505. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Whiteman HJ, Mason WA, Mendoza SP, Capitanio JP. Dimensions of response to novelty are associated with social engagement and aggression in adult male rhesus macaques. Journal of Comparative Psychology. 2008;122:195–203. doi: 10.1037/0735-7036.122.2.195. [DOI] [PubMed] [Google Scholar]
- Leutenegger CM, Higgins J, Matthews TB, Tarantal AF, Luciw PA, Pedersen NC, North TW. Real-time TaqMan PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Research and Human Retroviruses. 2001;17:243–251. doi: 10.1089/088922201750063160. [DOI] [PubMed] [Google Scholar]
- Lu X, Kiyono H, Lu D, Kawabata S, Torten J, Srinivasan S, Dailey PJ, McGhee JR, Lehner T, Miller CJ. Targeted lymph-node immunization with whole inactivated simian immunodeficiency virus (SIV) or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS. 1998;12:1–10. doi: 10.1097/00002030-199801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RE, Le K, Dyrenforth PS. Explaining the extraversion/positive affect relation: sociability cannot account for extraverts’ greater happiness. Journal of Personality. 2008;76:3. doi: 10.1111/j.1467-6494.2008.00490.x. [DOI] [PubMed] [Google Scholar]
- Madden KS. Catecholamines, sympathetic nerves, and immunity. In: Ader R, Felten DL, Cohen N, editors. Psychoneuroimmunology. 3rd. New York: Academic Press; 2001. pp. 197–216. [Google Scholar]
- Maninger N, Capitanio JP, Mendoza SP, Mason WA. Personality influences tetanus-specific antibody response in adult male rhesus macaques after removal from natal group and housing relocation. American Journal of Primatology. 2003;61:73–83. doi: 10.1002/ajp.10111. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. The five factor theory of personality. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality. 3rd. New York: Guilford Press; 2008. pp. 159–181. [Google Scholar]
- Mellors JW, Rinaldo CR, Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science. 1996;272:1167–1170. doi: 10.1126/science.272.5265.1167. [DOI] [PubMed] [Google Scholar]
- Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987–2007) Brain, Behavior, and Immunity. 2007;21:736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netter P. Dopamine challenge tests as an indicator of psychological traits. Human Psychoparmacology Clinical and Experimental. 2006;21:91–99. doi: 10.1002/hup.754. [DOI] [PubMed] [Google Scholar]
- Reuter M, Hennig J. Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. Neuroreport. 2005;16:1135–1138. doi: 10.1097/00001756-200507130-00020. [DOI] [PubMed] [Google Scholar]
- Ruys JD, Capitanio JP, Mendoza SP. Individual differences in personality and neuroendocrine responses to pharmacological treatment in adult male rhesus macaques (Macaca mulatta). Paper presented at the 25th annual meeting of American Society of Primatologists, Oklahoma City, OK, June 2002. American Journal of Primatology. 2002;57(S1):78. [Google Scholar]
- Sih A, Bell AM, Johnson JC, Ziemba RE. Behavioral syndromes: an integrative overview. The Quarterly Review of Biology. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proceedings, Biological Sciences. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced SIV replication adjacent to catecholaminergic varicosities in primate lymph nodes. Journal of Virology. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. Journal of Neuroscience. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Nguyen CT, Cox BF, Tarara RP, Capitanio JP, Cole SW. SIV infection decreases sympathetica innervation of primate lymph nodes: the role of neurotrophins. Brain, Behavior, and Immunity. 2008;22:185–194. doi: 10.1016/j.bbi.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Zunz M. Subjective assessment of individual rhesus monkeys. Primates. 1978;19:473–482. [Google Scholar]
- Weiss A, Inoue-Murayama M, Hong K-W, Inoue E, Udono S, Ochiai T, Matsuzawa T, Hirata S, King JE. Assessing chimpanzee personality and subjective well-being in Japan. American Journal of Primatology. 2009;71:283–292. doi: 10.1002/ajp.20649. [DOI] [PubMed] [Google Scholar]
- Zerhouni E. Testimony before US House of Representatives Subcommittee on Labor April 2006. 2006 http://www.nih.gov/about/director/budgetrequest/fy2007directorsbudgetrequest.htm.
- Zohar J, Fostick L, Cohen A, Bleich A, Dolfin D, Weissman Z, Doron M, Kaplan Z, Klein E, Shalev AY. Israeli consortium on PTSD. Risk factors for the development of posttraumatic stress disorder following combat trauma: a semiprospective study. Journal of Clinical Psychiatry. 2009;70:1629–1635. doi: 10.4088/JCP.08m04378blu. [DOI] [PubMed] [Google Scholar]


