Abstract
Formation of scar tissue may be reduced or prevented if wounds were locally treated with a combination of molecules tuned to the different healing phases, guiding tissue regeneration along a scar free path. To this end, drug delivery devices made of cellulose acetate phthalate and Pluronic F-127 were loaded with either quercetin or pirfenidone and plasticized with either triethyl citrate (TEC) or tributyl citrate (TBC). Quercetin inhibits oxidative stress, and pirfenidone has been shown to reduce production of pro-inflammatory and fibrogenic molecules. The combined effects of drug and plasticizer on erosion, release, and mechanical properties of the drug delivery films were investigated. TEC-plasticized films containing quercetin released drug at a slower rate than did TBC films. Pirfenidone-loaded films released drug at a faster rate than erosion occurred for both types of plasticizers. Higher plasticizer contents of both TEC and TBC increased the elongation and decreased the elastic modulus. In contrast, increased pirfenidone loading in both TEC and TBC films resulted in a significantly higher modulus, an anti-plasticizer effect. Adding pirfenidone significantly decreased elongation for all film types, but quercetin-loaded samples had significantly greater elongation with increasing drug content. Films containing quercetin elongated more than did pirfenidone-loaded films. Quercetin is over 1.5 times larger than pirfenidone, has water solubility over 12 times lower, and has 6 times more bonding sites than pirfenidone. These differences affected how the two drugs interacted with cellulose acetate phthalate and Pluronic F-127 and thereby determined polymer properties. Drug release, erosion, and mechanical properties of association polymer films can be tailored by the characteristics of the drugs and plasticizers included in the system.
Keywords: drug release, plasticizer, drug delivery film, erosion, cellulose acetate phthalate
Introduction
Large soft tissue defects are common in motor vehicle accidents as well as in military injuries. Open wounds accounted for 10.3% of the 5.3 million non-fatal injuries from traffic collisions in the U.S. in 2000, and about 50% of all military injuries involve musculoskeletal wounds to the extremities1, 2. Aberrant wound healing results in extended inflammatory phases leading to the formation of fibrotic tissue3–5. The greater the defect size, the longer the healing process will be6, and infection and ischemia further prolong inflammation7, 8. Wound location and the patient’s health and age also affect duration of the inflammatory phase9. Secondary union wounds, where exposed soft tissue requires re-epithelialization, can result in scar contractures with negative esthetic and functional consequences10–12. As such, there is a need for treatments that can simultaneously enhance wound healing while reducing fibrotic scar tissue formation.
Scar tissue formation could potentially be reduced or prevented if wounds were treated with individual or a combination of molecules targeted to the specific pathways of the healing process. The two drugs used in this study were quercetin and pirfenidone. Quercetin is a naturally occurring molecule that increases cell proliferation, decreases superoxide activity, and reduces wound contraction13–16. Reactive oxygen species are predominately released by infiltrating macrophages and act as pro-inflammatory mediators17. Thus, quercetin can inhibit oxidative stress to decrease inflammation. Pirfenidone is a pharmaceutical shown to reduce fibrosis and scarring by down-regulating adhesion molecules and certain fibrogenic cytokines and growth factors, including interleukin 1β and transforming growth factor-β118–22.
The drug delivery films examined in this study were an erodible, association polymer system of cellulose acetate phthalate (CAP) and Pluronic F-127 (PF127)23. As the polymer erodes, drugs can be released from the system in a zero-order manner24–26. CAP-PF127 films are rigid, glassy polymers, but the addition of plasticizer increases flexibility, potentially allowing the devices to contour to the shape of varying wounds27. The effects of plasticizers, in combination with different drugs, loaded in CAP-PF127 films have not been previously studied. Films containing quercetin or pirfenidone with either triethyl citrate (TEC) or tributyl citrate (TBC) were investigated to determine the combined effects of drug and plasticizer on erosion, release, and mechanical properties.
Materials and Methods
Film Fabrication
Cellulose acetate phthalate (Sigma-Aldrich, St. Louis, MO) and Pluronic F-127 (Sigma-Aldrich) were combined in a 70:30 weight ratio, respectively, for a total mass of 2 g per film. Either tributyl citrate (Sigma-Aldrich) or triethyl citrate (Sigma-Aldrich) was combined with the CAP and PF127 at 0, 10, or 20 wt%. Quercetin (10 or 100 mg; Sigma-Aldrich) or pirfenidone (6.1 or 61 mg; Tokyo Chemical Industries, Portland, OR) was added to the mixture (Figure 1). The low and high loadings of the two drugs are molar equivalents at 0.033 and 0.33 mol. A 25% (w/v) polymer solution was made by adding acetone to the CAP, Pluronic F-127, plasticizer, and drug. Mixtures were vortexed to ensure uniform dissolution of the components, cast into Teflon dishes, and the acetone allowed to evaporate at 10°C overnight. Films were desiccated overnight before analysis. Each sample is subsequently referenced by the average drug loading within individual samples (approximately 64 µg and 640 µg for quercetin and 39 µg and 390 µg for pirfenidone). The sample calculation below for 100 mg of quercetin shows how the average loadings were determined.
Figure 1.
Chemical structures of (a) quercetin, (b) pirfenidone, (c) cellulose acetate phthalate, (d) Pluronic F-127.
Erosion and Drug Release Studies
Five millimeter diameter discs were punched from the approximately 0.5 mm thick films, weighed to determine their initial mass, and placed individually in 24-well plates. Initial sample masses averaged around 15 mg. After adding phosphate-buffered saline (PBS), pH 7.4, to each well, the plates were gently agitated on an orbital shaker at 37°C. Three discs were collected and dried every hour, and their supernatants were saved for analysis of drug release. PBS in the remaining wells was replaced with fresh solution every hour. Dried samples were weighed to determine the final mass for calculating percentage mass loss.
High performance liquid chromatography (HPLC) analysis was performed using a Shimadzu Prominence system equipped with a Luna C-18 column (4.6 × 250 mm, 5 µm). For detection of quercetin, the mobile phase consisted of water containing 0.1% trifluoroacetic acid and methanol (30:70), with absorbance measurement at 254 nm. For detection of pirfenidone, the mobile phase consisted of water containing 0.2% acetic acid and acetonitrile (50:50), with detection at 310 nm. Injection volumes were 50 µl for all samples with a flow rate of 1 ml/min.
Mechanical Properties
Microtensile test samples were punched from the films using a dog bone die (ASTM D1708) and the width and thickness of each sample measured using digital calipers. Tensile tests were performed in displacement control mode at a rate of 0.5 mm/sec using a BOSE ELF 3300 system. From the force and displacement data, along with the dimensions of each sample, the elastic modulus (E), percent elongation normalized by the cross-sectional area, and ultimate tensile strength (UTS) were calculated.
Statistics
Samples of the same drug molar content, drug type, same plasticizer concentration, and plasticizer type were compared against each other. Results were analyzed using two-way ANOVA, and a p-value < 0.05 was considered statistically significant. Comparison of regression curves to a 45° line representing surface erosion was performed on slopes and intercepts of the release vs. erosion plots, with a p-value < 0.05 considered statistically significant.
Results
Erosion and Release Studies
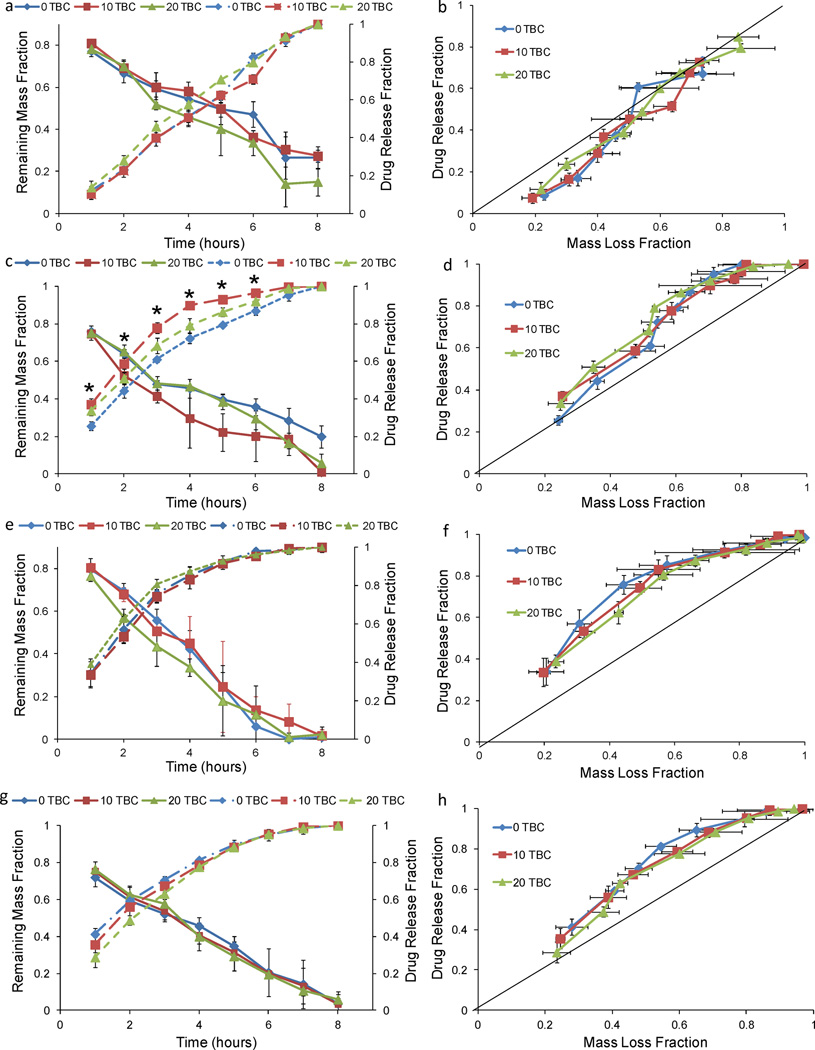
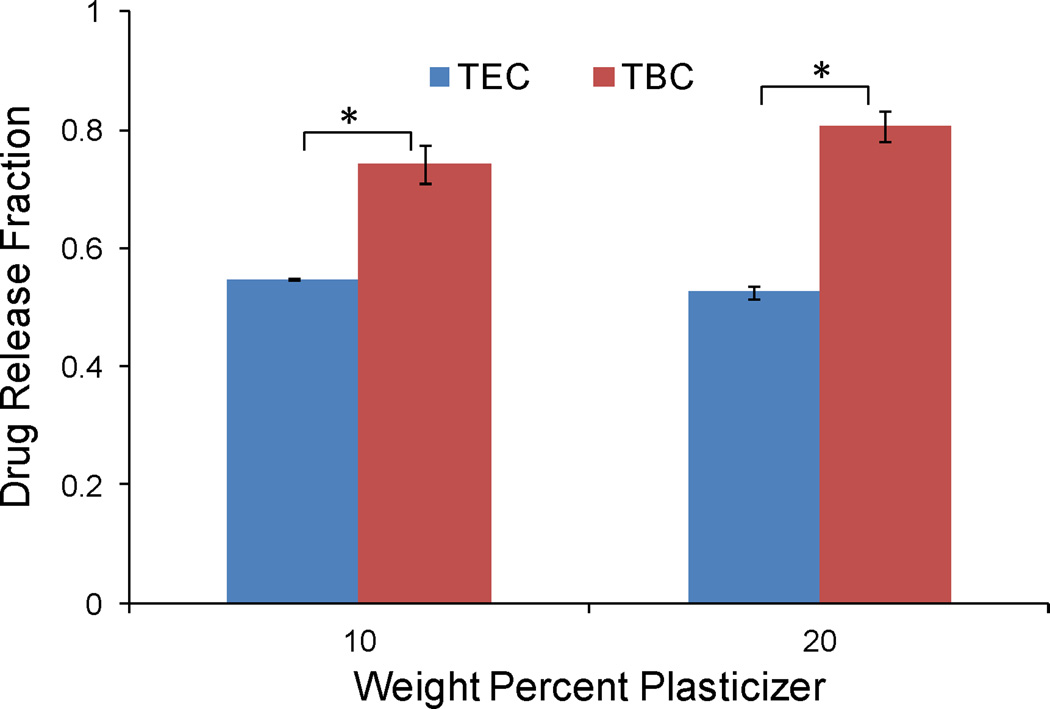
The mass loss and drug release profiles for 0 and 10 wt% TEC and 0, 10, and 20 wt% TBC films loaded with 64 µg quercetin were linear (Figures 2a and 3a), with drug release rates from 9.3 to 13.0%/hr and erosion rates from 7.1 to 9.3%/hr. When mass loss and release were plotted against each other, quercetin-loaded TEC and TBC films followed along or below the 45° line that represents drug release based on only surface erosion (Figures 2b and 3b). The mass loss and drug release profiles for 20 wt% TEC films loaded with 64 µg quercetin both appeared more curved than the others but followed the 45° surface erosion line when plotted against each other. When the same molar amount, 0.033 mol per film (39 µg per sample), of pirfenidone was added to the films, the cumulative drug release profile did not have the same linear behavior (Figures 2c and 3c). Over 9% more pirfenidone was released in the first six hours compared to the quercetin-loaded films (p < 0.001). None of the TBC-plasticized, 64 µg quercetin-loaded films reached 80% cumulative release at six hours, but all of the TBC-plasticized 39 µg pirfenidone-loaded samples had released over 93% of their total loading (p < 0.001) (Figures 3a and 3c). Similarly, with the TEC-plasticized films, the cumulative release at six hours was under 83% for all of the 64 µg quercetin-loaded samples and was over 92% for all 39 µg pirfenidone-loaded samples (p < 0.001) (Figures 2a and 2c). Pirfenidone release slowed significantly after six hours (Figures 2c and 3c). When release from the TEC-plasticized films loaded with 39 µg pirfenidone was plotted against erosion, the first three data points were not significantly different from the 45° line for 10 and 20% TEC. For the last five data points, however, drug release was faster than the rate of mass loss (Figures 2d). The erosion versus release slope in this later region was significantly different from the 45° line (p < 0.01) for both 10 and 20% TEC. The plot of release versus erosion for the TBC-plasticized, 39 µg pirfenidone-loaded films was initially above the 45° line, and even after the first half of the mass loss, release still increased faster than the films eroded (Figure 3d). When the quercetin loading was increased to 640 µg, TBC films released drug over 1.5 times faster than mass was lost, and the profile did not fit the erosion-based model (Figures 3e and 3f). TEC films loaded with quercetin released the drug at a significantly slower rate than did the TBC films: 52–56% of the quercetin had been released by three hours for TEC-plasticized films, while 74–80% had been released by TBC-plasticized films (Figure 4) (p < 0.001). TEC-plasticized films loaded with 390 µg pirfenidone released 98% or more of the drug at six hours, while the 640 µg quercetin-loaded films had a maximum cumulative release of 91% at that time (p < 0.001). Quercetin release from TEC-plasticized films better followed the 45° erosion-based drug release line (Figures 2e and 2f). TEC and TBC films loaded with 39 and 390 µg pirfenidone released drug at a faster rate than erosion occurred (Figures 2f, 2h, 3f, and 3h).
Figure 2.
Release and erosion of TEC films loaded with: (a) 64 µg quercetin, (c) 39 µg pirfenidone, (e) 640 µg quercetin, and (g) 390 µg pirfenidone. Release versus erosion of TEC films loaded with: (b) 64 µg quercetin, (d) 39 µg pirfenidone, (f) 640 µg quercetin, and (h) 390 µg pirfenidone. Data are mean ± standard deviation (n=3). The following symbol denotes statistical significance: * when p < 0.05.
Figure 3.
Release and erosion of TBC films loaded with: (a) 64 µg quercetin, (c) 39 µg pirfenidone, (e) 640 µg quercetin, and (g) 390 µg pirfenidone. Release versus erosion of TEC films loaded with: (b) 64 µg quercetin, (d) 39 µg pirfenidone, (f) 640 µg quercetin, and (h) 390 µg pirfenidone. Data are mean ± standard deviation (n=3). The following symbol denotes statistical significance: * when p < 0.05.
Figure 4.
Representative comparison of release TEC and TBC films. Data are for samples loaded with 640 µg quercetin and release measured at 3 hr. The following symbol denotes statistical significance: * when p < 0.001.
Mechanical Properties
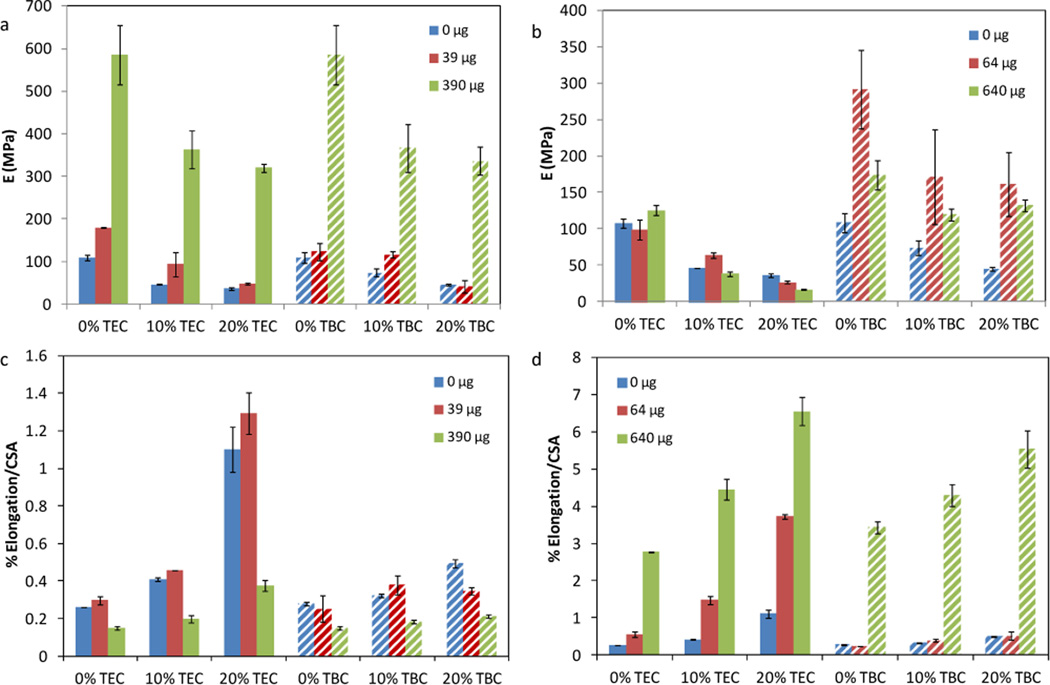
Higher plasticizer contents of both TEC and TBC increased the elongation and decreased the elastic modulus of CAP-PF127 films (Figure 5). Increased pirfenidone loading, from 0 to 390 µg, in both TEC and TBC films caused a significant increase in E (p < 0.001) (Figure 5a). TEC films with 20 wt% plasticizer showed a significant decrease in E when quercetin loading increased from 0 to 640 µg (p < 0.01) (Figure 5b). The modulus of films plasticized with TBC and loaded with quercetin increased with drug content from 0 to 64 µg and then decreased from 64 to 640 µg (p < 0.001 for 0 wt%; p < 0.01 for 10 and 20 wt%) (Figure 5b). Increasing pirfenidone loading from 0 to 39 µg significantly decreased elongation for all film types (p < 0.001) (Figure 5c). Quercetin-loaded samples had significantly greater elongation with increasing drug content (p < 0.001) (Figure 5d), and they elongated more than did pirfenidone-loaded films. For the same molar drug loading in 20 wt% TEC films, quercetin samples elongated 17 times more than did those containing pirfenidone (p < 0.001). For 20 wt% TBC, quercetin films elongated 26 times more than pirfenidone-loaded samples (p < 0.001).
Figure 5.
Elastic modulus of (a) pirfenidone-loaded and (b) quercetin-loaded films. Percent elongation normalized by the cross-sectional area of (c) pirfenidone loaded and (d) quercetin loaded films. Data are mean ± standard deviation (n=3).
Discussion
Two mechanisms govern drug release from CAP-PF127 films: erosion and diffusion28, 29. Erosion control occurs when the loaded drug is released at the same rate at which the polymer vehicle erodes, which is a zero-order process for CAP-PF127. In diffusion controlled release, water penetrates the material to dissolve the drug or disassociate it from the polymer, thus enabling drug to diffuse from the matrix. Erosion and diffusion control represent two ends of the spectrum; release from most degradable or erodible polymer systems falls somewhere between the two. The mechanism of drug release from unplasticized 70:30 CAP-PF127 films has been shown to be surface erosion23, 26, 30. Other drug delivery systems, such as poly(ε-caprolactone), poly(dl-lactide-co-glycolide), ethylene vinyl acetate, and starch, are diffusion-controlled and would leave behind polymer once release is complete31–35. In contrast, CAP-PF127 films release drug as they erode, so material will not remain in the healing wound to cause an inflammatory reaction after the therapeutic dose is released36, 37. Polyanhydrides can also be surface-eroding systems but require stabilizing co-monomers to increase hydrophobicity to slow hydrolysis and consequent drug release38, 39.
The effects of plasticizers on release of drugs from the CAP-PF127 system had not been previously studied. In other polymers, plasticizers can increase or decrease the rate of release by either increasing the surface area after plasticizer leaches out or by creating a better barrier to the dissolution media40, 41. The plasticizers, TEC and TBC, incorporated into the films are commonly used in pharmaceuticals and in biomedical devices42–44. Triethyl citrate is a hydrophilic plasticizer with an aqueous solubility of 12 mg/ml, but TBC exhibits limited solubility at 0.15 mg/ml45–47. Tributyl citrate is a larger molecule than TEC, with molar masses of 360.45 and 276.28 g/mol g/mol, respectively.
The two drugs explored, pirfenidone and quercetin, have different solubility in water, 4.4 and 0.36 mg/ml at 25 °C, respectively48, 49. The molar mass of quercetin is 302.24 g/mol, which is over 1.5 times that of pirfenidone at 185.22 g/mol. Quercetin also has 12 bonding sites (5 donor and 7 acceptor) that can interact and/or interfere with the association polymer system. For example, the phenol and enol groups of the drug can form hydrogen bonds with the ether oxygens in Pluronic F-12749, 50. Additionally, the ketones in quercetin may interact with the carboxylic acid of CAP. These associations could affect polymer erosion as well as the release of quercetin from the system. In contrast, pirfenidone has only two acceptor sites on the amide that could bond with the CAP-PF127 system48. Fewer acceptor sites, a smaller size, and increased hydrophilicity resulted in pirfenidone being released more easily from the micron-sized pores created as the system eroded27.
Samples containing pirfenidone exhibited a dual mechanism of release. Surface erosion controlled the first half of the 39 µg pirfenidone release from TEC-plasticized films. The first half of release from 39 µg loaded pirfenidone TBC-plasticized films also predominantly occurred via surface erosion, but after the fourth hour, release occurred at a faster rate than polymer mass loss, signifying a transition to diffusion control. The shift in the main mechanism of release did not occur for the other drug and plasticizer combinations. The 64 µg quercetin-loaded films all fit the 45° line closely, indicating that the mechanism of release was surface erosion. If some diffusion occurred, the effect was insignificant. Previous work found that film porosity, even in unplasticized films, increased significantly after a two hour incubation in PBS27. Development of pores increased the surface area by which drug was released from the surface-eroding system. As more drug was added to the system, the leading mechanism of release shifted from erosion to diffusion and increased the release rate. This shift has been seen in other drug release systems, including atenolol loaded ethylene-vinyl acetate films34. With increased drug loading, the concentration gradient is steeper and less CAP-PF127 is present to control the release. The voids left behind by the released drug allow molecules deeper in the device to also diffuse out. This was seen for higher loadings of both quercetin and pirfenidone.
As observed in previous studies, increasing the plasticizer content of both TEC and TBC led to decreased elastic modulus and increased elongation, resulting in a CAP-PF127 film that will conform better to the complex shapes of wounds27, 51, 52. Triethyl citrate plasticized the films to a greater degree than did TBC because it is a smaller molecule, and for the same mass, there were over 1.25 times as many TEC as TBC molecules present in the films. Although pirfenidone is a smaller molecule than either of the two plasticizers, it still may have physically interfered with the ability of TEC or TBC to plasticize the films by separating the polymer chains and interacting with the carboxylic acid of CAP. In the unplasticized films, this pirfenidone interaction reduced what little flexibility the CAP-PF127 films alone had, which resulted in an increased modulus and decreased elongation as pirfenidone content increased. It is not uncommon to see an “antiplasticizer” effect when small amounts of drug or plasticizer are added to polymer31, 51, 53. The chemical structures of pirfenidone and quercetin fit Jackson and Caldwell’s “antiplasticizer criteria” because they contain polar atoms, have at least two nonbridged rings, and have one dimension less than about 5.5 Å53. The side groups of quercetin likely interacted with both polymers in the CAP-PF127 system to result in synergistic effects on elongation and an increased modulus54–56. The changes in the mechanical properties caused by the plasticizers and drugs did not correlate with differences in the release profiles. Mechanical testing occurred when the films were dry at approximately 20 °C, and release occurred when the samples were wet at 37 °C, however. Because films composed of CAP and PF127 in a 70:30 weight ratio have a glass transition temperature around 80 °C, it is reasonable to expect that the mechanical properties do not significantly change at 37 °C23. Previous work found that devices incubated in saline for two hours leached plasticizer resulting in increased strength27. This phenomenon has been seen in other systems before, including sodium alginate-magnesium aluminum silicate films plasticized with polyethylene glycol 400 or glycerin57. The chemical and mechanical changes that occur once the films are wet reduced any observable difference in the release.
Conclusion
Release from drug delivery films can be controlled by the amount loaded and the drug properties, including size, hydrophobicity, and interactive side groups. The mechanical properties are also controlled by loading and the drug properties. Plasticizer can be introduced to further modulate films to achieve the desired mechanical properties. The combined effects of drug and plasticizer on the properties of drug delivery films can range from antagonistic to synergistic. Different drugs and plasticizers can be added to association polymer films, such as CAP-PF127, to tailor the erosion, release, and mechanical properties needed for various applications.
Acknowledgments
This research was funded by the NIH (DE019645 and AR060964). CJ was supported by NSF IGERT (DGE-0653710).
Footnotes
Declaration of Conflicting Interests
DAP has an equity interest in Regenera Materials, LLC, a start-up company for commercializing novel biomaterials for tissue regeneration.
References
- 1.World report on road traffic injury prevention. [[1/18/2015]];2002 Available from: http://www.who.int/violence_injury_prevention/publications/road_traffic/world_report/en/
- 2.Owens BD, Kragh JF, Wenke JC, Macaitis J, Wade CE, Holcomb JB. Combat wounds in operation Iraqi freedom and operation enduring freedom. Journal of Trauma-Injury Infection and Critical Care. 2008;64:295–299. doi: 10.1097/TA.0b013e318163b875. [DOI] [PubMed] [Google Scholar]
- 3.Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P. Wound healing in the 21st century. Journal of the American Academy of Dermatology. 2010;63:866–881. doi: 10.1016/j.jaad.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 4.Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicologic Pathology. 2007;35:767–779. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- 5.Lee SB, Kalluri R. Mechanistic connection between inflammation and fibrosis. Kidney International. 2010;78:S22–S26. doi: 10.1038/ki.2010.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. Diabetic Neuropathic Foot Ulcers: The association of wound size, wound duration, and wound grade on healing. Diabetes Care. 2002;25:1835–1839. doi: 10.2337/diacare.25.10.1835. [DOI] [PubMed] [Google Scholar]
- 7.Cierny GI, Byrd HS, Jones RE. Primary versus Delayed Soft Tissue Coverage for Severe Open Tibial Fractures: A Comparison of Results. Clinical Orthopaedics and Related Research. 1983;178:54–63. [PubMed] [Google Scholar]
- 8.Gute DC, Ishida T, Yarimizu K, Korthuis RJ. Inflammatory responses to ischemia and reperfusion in skeletal muscle. Molecular and cellular biochemistry. 1998;179:169–187. doi: 10.1023/a:1006832207864. [DOI] [PubMed] [Google Scholar]
- 9.Guo S, DiPietro LA. Factors Affecting Wound Healing. Journal of Dental Research. 2010;89:219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YS, Lew DH, Tark KC, Rah DK, Hong JP. Effect of Recombinant Human Epidermal Growth Factor Against Cutaneous Scar Formation in Murine Full-thickness Wound Healing. Journal of Korean Medical Science. 2010;25:589–596. doi: 10.3346/jkms.2010.25.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.M. Abercrombie MHF, James DW. Collagen Formation and Wound Contracture during Repair of Small Excised Wounds in the Skin of Rats. J Embryol exp Morph. 1954;2:264–274. [Google Scholar]
- 12.Kwan P, Hori K, Ding J, Tredget EE. Scar and Contracture: Biological Principles. Hand Clinics. 2009;25:511-+. doi: 10.1016/j.hcl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 14.Gomathi K, Gopinath D, Ahmed MR, Jayakumar R. Quercetin incorporated collagen matrices for dermal wound healing processes in rat. Biomaterials. 2003;24:2767–2772. doi: 10.1016/s0142-9612(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 15.Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. International journal of nanomedicine. 2011;6:1621–1630. doi: 10.2147/IJN.S22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vicentini FTMdC, Georgetti SR, Bentley MVLB, Fonseca MJV. Assessment of in vitro methodologies to determine topical and transdermal delivery of the flavonoid quercetin. Brazilian Journal of Pharmaceutical Sciences. 2009;45:357–364. [Google Scholar]
- 17.Cotran RS. Robbins Pathologic Basis of Disease. 5th. Philadelphia: Saunders; 1994. [Google Scholar]
- 18.Hewitson TD, Kelynack KJ, Tait MG, et al. Pirfenidone reduces in vitro rat renal fibroblast activation and mitogenesis. Journal of Nephrology. 2001;14:453–460. [PubMed] [Google Scholar]
- 19.Richeldi L, Yasothan U, Kirkpatrick P. Pirfenidone. Nat Rev Drug Discov. 2011;10:489–490. doi: 10.1038/nrd3495. [DOI] [PubMed] [Google Scholar]
- 20.Card JW, Racz WJ, Brien JF, Margolin SB, Massey TE. Differential Effects of Pirfenidone on Acute Pulmonary Injury and Ensuing Fibrosis in the Hamster Model of Amiodarone-Induced Pulmonary Toxicity. Toxicological Sciences. 2003;75:169–180. doi: 10.1093/toxsci/kfg167. [DOI] [PubMed] [Google Scholar]
- 21.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of Pirfenidone on Procollagen Gene Expression at the Transcriptional Level in Bleomycin Hamster Model of Lung Fibrosis. Journal of Pharmacology and Experimental Therapeutics. 1999;289:211–218. [PubMed] [Google Scholar]
- 22.Kaneko M, Inoue H, Nakazawa R, et al. Pirfenidone induces intercellular adhesion molecule-1 (ICAM-1) down-regulation on cultured human synovial fibroblasts. Clinical and Experimental Immunology. 1998;113:72–76. doi: 10.1046/j.1365-2249.1998.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Lee PI. Programmable Drug-Delivery From an Erodible Association Poylmer System. Pharm Res. 1993;10:1144–1152. doi: 10.1023/a:1018960016756. [DOI] [PubMed] [Google Scholar]
- 24.Jeon JH, Thomas MV, Puleo DA. Bioerodible devices for intermittent release of simvastatin acid. International Journal of Pharmaceutics. 2007;340:6–12. doi: 10.1016/j.ijpharm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeon JH, Puleo DA. Alternating release of different bioactive molecules from a complexation polymer system. Biomaterials. 2008;29:3591–3598. doi: 10.1016/j.biomaterials.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundararaj SC, Thomas MV, Peyyala R, Dziubla TD, Puleo DA. Design of a multiple drug delivery system directed at periodontitis. Biomaterials. 2013;34:8835–8842. doi: 10.1016/j.biomaterials.2013.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabek CVSR, Dziubla TD, Puleo DA. The Effect of Plasticizers on the Erosion and Mechanical Properties of Polymer Films. Journal of Biomaterials Applications. 2014;28:779–789. doi: 10.1177/0885328213480979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric Systems for Controlled Drug Release. Chemical Reviews. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 29.Grassi M, Grassi G, Lapasin R, Colombo I. Understanding Drug Release and Absorption Mechanisms: A Physical and Mathematical Approach. Francis: Taylor; 2006. [Google Scholar]
- 30.Sundararaj SC, Thomas MV, Dziubla TD, Puleo DA. Bioerodible system for sequential release of multiple drugs. Acta Biomaterialia. 2014;10:115–125. doi: 10.1016/j.actbio.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chamarthy SP, Pinal R. Plasticizer concentration and the performance of a diffusion-controlled polymeric drug delivery system. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2008;331:25–30. [Google Scholar]
- 32.Medlicott NJ, Tucker IG, Rathbone MJ, Holborow DW, Jones DS. Chlorhexidine release from poly(epsilon-caprolactone) films prepared by solvent evaporation. International Journal of Pharmaceutics. 1996;143:25–35. [Google Scholar]
- 33.Lei L, Liu X, Guo S, Tang M, Cheng L, Tian L. 5-Fluorouracil-loaded multilayered films for drug controlled releasing stent application: Drug release, microstructure, and ex vivo permeation behaviors. Journal of Controlled Release. 2010;146:45–53. doi: 10.1016/j.jconrel.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Shin SC. Controlled release of atenolol from the ethylene-vinyl acetate matrix. Int J Pharm. 2004;273:23–27. doi: 10.1016/j.ijpharm.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Govender T, Stolnik S, Garnett MC, Illum L, Davis SS. PLGA nanoparticles prepared by nanoprecipitation: drug loading and release studies of a water soluble drug. Journal of Controlled Release. 1999;57:171–185. doi: 10.1016/s0168-3659(98)00116-3. [DOI] [PubMed] [Google Scholar]
- 36.Anderson JM. Biological responses to materials. Annual Review of Materials Research. 2001;31:81–110. [Google Scholar]
- 37.Sundararaj SC, Dziubla TD, Al-Sabbagh M, Rabek CL, Thomas MV, Puleo DA. Comparison of Sequential Drug Release In Vitro and In Vivo. Journal of Biomedical Materials Research Part B. Applied Biomaterials. 2015 doi: 10.1002/jbm.b.33472. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heller J. Controlled drug release from poly(ortho esters) — A surface eroding polymer. Journal of Controlled Release. 1985;2:167–177. [Google Scholar]
- 39.Heller J. Development of poly(ortho esters): a historical overview. Biomaterials. 1990;11:659–665. doi: 10.1016/0142-9612(90)90024-k. [DOI] [PubMed] [Google Scholar]
- 40.Okarter TU, Singla K. The effects of plasticizers on the release of metoprolol tartrate from granules coated with a polymethacrylate film. Drug Development and Industrial Pharmacy. 2000;26:323–329. doi: 10.1081/ddc-100100360. [DOI] [PubMed] [Google Scholar]
- 41.Kibria G, Roni M, Absar M, Jalil R-U. Effect of plasticizer on release kinetics of diclofenac sodium pellets coated with Eudragit RS 30 D. AAPS Pharmscitech. 2008;9:1240–1246. doi: 10.1208/s12249-008-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platzer N, Sears, Darby The Technology of Plasticizers. Journal of Polymer Science: Polymer Letters Edition. 1982;20:459-. [Google Scholar]
- 43.Dittrich ESaM. Pharmaceutically Used Plasticizers. In: Luqman M, editor. Recent Advances in Plasticizers. Tech; 2012. pp. 45–68. [Google Scholar]
- 44.Administration FaD, editor. 2011. Quality by Design for ANDAs: An Example for Modified Release Dosage Forms. [Google Scholar]
- 45.Gutiérrez-Rocca J, McGinity JW. Influence of water soluble and insoluble plasticizers on the physical and mechanical properties of acrylic resin copolymers. International Journal of Pharmaceutics. 1994;103:293–301. [Google Scholar]
- 46.Triethyl Citrate [database on the Internet] 2014 Available from: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf. [Google Scholar]
- 47.Tributyl Citrate [database on the Internet] 2014 Available from: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf. [Google Scholar]
- 48.Pirfenidone [database on the Internet] 2014 Available from: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf. [Google Scholar]
- 49.Quercetin [database on the Internet] 2014 Available from: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf. [Google Scholar]
- 50.Walker EH, Pacold ME, Perisic O, et al. Structural Determinants of Phosphoinositide 3-Kinase Inhibition by Wortmannin, LY294002, Quercetin, Myricetin, and Staurosporine. Molecular Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- 51.Huang CL, Steele TWJ, Widjaja E, Boey FYC, Venkatraman SS, Loo JSC. The influence of additives in modulating drug delivery and degradation of PLGA thin films. NPG Asia Mater. 2013;5:e54. [Google Scholar]
- 52.Harte I, Birkinshaw C, Jones E, Kennedy J, DeBarra E. The effect of citrate ester plasticizers on the thermal and mechanical properties of poly(DL-lactide) Journal of Applied Polymer Science. 2013;127:1997–2003. [Google Scholar]
- 53.Jackson WJ, Caldwell JR. Antiplasticization. II. Characteristics of antiplasticizers. Journal of Applied Polymer Science. 1967;11:211–226. [Google Scholar]
- 54.Siepmann F, Le Brun V, Siepmann J. Drugs acting as plasticizers in polymeric systems: A quantitative treatment. Journal of Controlled Release. 2006;115:298–306. doi: 10.1016/j.jconrel.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Kunze C, Freier T, Kramer S, Schmitz KP. Anti-inflammatory prodrugs as plasticizers for biodegradable implant materials based on poly(3-hydroxybutyrate) Journal of Materials Science-Materials in Medicine. 2002;13:1051–1055. doi: 10.1023/a:1020392606225. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, Pang H, Guo Z, et al. Interactions between drugs and polymers influencing hot melt extrusion. Journal of Pharmacy and Pharmacology. 2014;66:148–166. doi: 10.1111/jphp.12183. [DOI] [PubMed] [Google Scholar]
- 57.Pongjanyakul T, Puttipipatkhachorn S. Alginate-magnesium aluminum silicate films: Effect of plasticizers on film properties, drug permeation and drug release from coated tablets. International Journal of Pharmaceutics. 2007;333:34–44. doi: 10.1016/j.ijpharm.2006.09.046. [DOI] [PubMed] [Google Scholar]