Abstract
Context
Calcium and vitamin D are recommended for bone health, but there are concerns about adverse risks. Some clinical studies suggest that calcium intake may be cardioprotective, whereas others report increased risk associated with calcium supplements. Both low and high serum levels of 25-hydroxyvitamin D have been associated with increased mortality.
Objective
The purpose of this study was to determine the association between total calcium and vitamin D intake and mortality and heterogeneity by source of intake.
Design
The Canadian Multicentre Osteoporosis Study cohort is a population-based longitudinal cohort with a 10-year follow-up (1995–2007).
Setting
This study included randomly selected community-dwelling men and women.
Participants
A total of 9033 participants with nonmissing calcium and vitamin D intake data and follow-up were studied.
Exposure
Total calcium intake (dairy, nondairy food, and supplements) and total vitamin D intake (milk, yogurt, and supplements) were recorded.
Outcome
The outcome variable was all-cause mortality.
Results
There were 1160 deaths during the 10-year period. For women only, we found a possible benefit of higher total calcium intake, with a hazard ratio of 0.95 (95% confidence interval, 0.89–1.01) per 500-mg increase in daily calcium intake and no evidence of heterogeneity by source; use of calcium supplements was also associated with reduced mortality, with hazard ratio of 0.78 (95% confidence interval, 0.66–0.92) for users vs nonusers with statistically significant reductions remaining among those with doses up to 1000 mg/d. These associations were not modified by levels of concurrent vitamin D intake. No definitive associations were found among men.
Conclusions
Calcium supplements, up to 1000 mg/d, and increased dietary intake of calcium may be associated with reduced risk of mortality in women. We found no evidence of mortality benefit or harm associated with vitamin D intake.
Calcium and vitamin D are essential nutrients for skeletal health and the mainstays of osteoporosis prevention and treatment (1). There is uncertainty over the risk-to-benefit profile of total calcium and vitamin D from food and supplements. For calcium, some studies found that excess supplement intake may be associated with increased risk of cardiovascular events (2–4), whereas others found no increased risk of either arterial calcification (5, 6) or cardiovascular events (7). For vitamin D, many studies have noted that low levels of serum 25-hydroxyvitamin D [25(OH)D], the major circulating metabolite of vitamin D, are associated with a higher risk of cardiovascular events (8, 9) or cardiovascular and/or all-cause mortality (10–12); however, high levels of vitamin D have also been implicated in increased mortality in women (13) but not men (14).
The Institute of Medicine (IOM) recently issued a report concerning dietary reference intakes for calcium and vitamin D (15), concluding that the evidence supporting the necessity of adequate intake of calcium and vitamin D for skeletal health and fracture prevention was clear and consistent. However, several areas in which existing evidence was sparse were also identified, including the potential benefit or harm associated with nonskeletal health outcomes. Mortality, without consideration of cause, is an important component in the overall risk-benefit question. Our objective was to determine, in a prospective population-based cohort, the association between total calcium and vitamin D intakes and 10-year mortality, the influence of the source of nutrient (food vs supplement) on this association, and possible interactions between calcium and vitamin D intake and mortality.
Materials and Methods
Setting
The Canadian Multicentre Osteoporosis Study (CaMos) cohort is a population-based longitudinal cohort initiated in 1995 with recruitment of 9423 men and women. Eligible participants were at least 25 years old, lived within a 50-km radius of 1 of 9 Canadian cities and were able to converse in English, French, or Chinese. Households were randomly selected from a list of residential phone numbers, and participants were randomly selected from eligible household members using an age- and sex-stratified design, which oversampled women and older groups to ensure sufficient sample size among those at highest risk of fracture. Of those selected, 42% agreed to participate and had a baseline interview. Ethics approval was granted through McGill University and each participating center.
Subjects
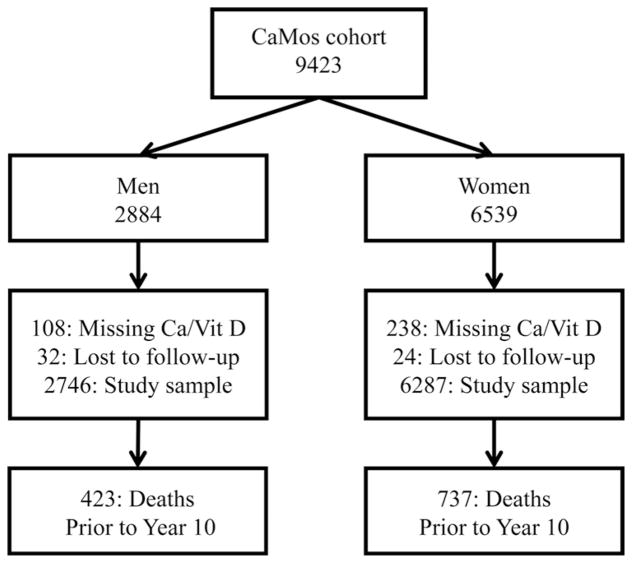
We included 9033 CaMos participants with nonmissing baseline total calcium and vitamin D intakes and follow-up data. The study exclusions and outcomes are detailed in Figure 1.
Figure 1.
Flowchart of participants in the CaMos, exclusions for missing data, loss to follow-up, and 10-year mortality. Ca, calcium; Vit D, vitamin D.
Data collection
All participants were given an interviewer-administered questionnaire and had height and weight measured at baseline (1995–1997) and year 5 (2000–2002). The questionnaire included demographics, general health, nutrition, medication use, and medical history. Information on dietary calcium and vitamin D intake was obtained from the abbreviated semiquantitative food frequency questionnaire (FFQ) included in the main questionnaire. The FFQ queried foods considered to be excellent sources of calcium and included milk to drink, milk products, and other calcium-containing foods (canned salmon, broccoli, dark leafy greens, dried peas or beans, whole wheat bread, white bread, and tofu). Vitamin D intake was based on milk and yogurt fortified with vitamin D; therefore, we did not consider other dietary sources of vitamin D such as fish. Calcium and vitamin D intake from nonfood sources was determined from the inventory of supplements and medications brought to the interview. A questionnaire concerning medication and fracture was mailed annually in all years except in those with a scheduled follow-up visit.
We have published a descriptive analysis of baseline calcium and vitamin D intakes in the CaMos cohort (16), and we have analyzed longitudinal changes in supplemental calcium and vitamin D intake and the associated changes in serum 25(OH)D and PTH (17). The distribution of vitamin D intake from supplements and fortified foods among CaMos participants was described previously (18). The reported 25(OH)D levels of CaMos participants were similar to those among Canadians reported from the Canadian Health Measures Survey (19).
Mortality
Participants were contacted annually throughout the study. Enrolled participants had alternate contact information. Obituaries were also screened for participants who could not be located. The relationship of fractures with mortality in CaMos was reported previously (20). The outcome variable for this analysis was all-cause mortality.
Statistical methods
We used a sex-stratified Cox proportional hazards model including person-time up until death, loss to follow-up, or year 10 interview. The covariates were considered as time-varying using the baseline covariates for the period from baseline to year 5 and then updating all covariates at year 5 for the period from year 5 to year 10, with separate strata for each period. We assessed the proportional hazards assumption and found only minor violations.
Total calcium intake and total vitamin D intake were considered as continuous variables without mutual adjustment for the other variable because of their collinearity (calcium and vitamin D from milk and supplements). To compare sources of calcium intake we constructed different variables to determine whether the source of exposure affected the association (supplements vs foods and dairy vs nondairy foods). Model diagnostics for vitamin D indicated that those with high vitamin D intake had undue influence on the linear relationship. Thus, we report results using categorical variables based on the IOM reference ranges (15). The moderate intake category was centered on the recommended dietary allowances, leaving high and low intakes as intakes substantially above or below the recommended dietary allowances. The potential interaction between calcium and vitamin D intake was also assessed using these categories.
We considered the following potential confounders: age, study center, education, body mass index (BMI), health status (SF-36 physical component summary [PCS] score), cigarette smoking, alcohol intake, physical activity, sun exposure, self-reported comorbidity (in men and women: hypertension, heart disease, stroke, type 2 diabetes, chronic obstructive pulmonary disease [COPD], and kidney stones; in women only: osteoporosis, thyroid disease, inflammatory bowel disease [IBD], breast cancer, and uterine cancer; in men only: prostate cancer), and medication (aspirin use or other nonsteroid anti-inflammatory drugs [NSAIDs]). We included all of the a priori specified potential confounders in the main reported analysis. In post hoc, analysis we also tested confounding by history of low-trauma fracture after age 50 years (men and women) and menopausal hormone use (women only), and the results were nearly identical. Statistical significance was set at P = .05.
Analysis was performed using Stata version 12.0 (StataCorp, College Station, Texas).
Results
The study sample consisted of 9033 men and women with 77,558 person-years and 1160 deaths accrued over the 10-year study period. There were 2746 men at baseline, among whom 201 (7.3%) were using calcium supplements alone, 120 (4.4%) were using vitamin D supplements alone, and 423 (15.4%) were using both calcium and vitamin D supplements. There were 6287 women at baseline, among whom 957 (15.2%) were using calcium supplements alone, 235 (3.7%) were using vitamin D supplements alone, and 1820 (29.0%) were using both calcium and vitamin D supplements.
Calcium intake
Table 1 shows the baseline characteristics of the study population stratified by sex and daily total calcium intake. There were statistically significant bivariate associations between calcium intake and education, alcohol intake, total physical activity, and total vitamin D intake in men and between calcium intake and age, education, SF-36 PCS score, total physical activity, total vitamin D intake, smoking, aspirin use, hypertension, osteoporosis, and thyroid disease in women.
Table 1.
Baseline Characteristics and 10-Year All-Cause Mortality Stratified by Sex and Category of Calcium Intake From Food and Supplements in the CaMos
| Men (n = 2746)
|
Women (n = 6287)
|
|||||
|---|---|---|---|---|---|---|
| Low, <800 mg (n = 1439) | Moderate, 800–1200 mg (n = 683) | High, ≥1200 mg (n = 624) | Low, <800 (n = 2558) | Moderate, 800–1200 mg (n = 1642) | High ≥1200 mg (n = 2087) | |
| Age, y | 60.6 ± 14.3 | 60.2 ± 14.5 | 60.1 ± 14.7 | 63.1 ± 13.4 | 63.5 ± 12.4 | 64.0 ± 12.0 |
| BMI, kg/m2 | 26.9 ± 4.1 | 27.0 ± 4.1 | 27.2 ± 3.9 | 27.0 ± 5.3 | 27.0 ± 5.1 | 26.8 ± 4.9 |
| SF-36 PCS score | 49.1 ± 9.2 | 48.8 ± 9.5 | 48.8 ± 9.7 | 46.6 ± 10.4 | 47.4 ± 10.1 | 45.6 ± 11.0 |
| Physical activity, 1000 MET · m/d | 2.47 ± 0.42 | 2.52 ± 0.44 | 2.55 ± 0.46 | 2.48 ± 0.34 | 2.52 ± 0.34 | 2.54 ± 0.35 |
| Vitamin D intake, μg | 2.74 ± 7.77 | 6.01 ± 20.8 | 10.2 ± 6.85 | 2.81 ± 9.40 | 6.27 ± 10.9 | 12.9 ± 23.7 |
| Deaths | 224 (15.6) | 111 (16.3) | 88 (14.1) | 334 (13.1) | 179 (10.9) | 224 (10.7) |
| Current smoker | 256 (17.8) | 132 (19.3) | 116 (18.6) | 404 (15.8) | 218 (13.3) | 269 (12.9) |
| High alcohol | 114 (8.0) | 35 (5.1) | 53 (8.7) | 135 (5.3) | 89 (5.4) | 99 (4.8) |
| Regular sun exposure | 373 (16.9) | 84 (18.7) | 14 (15.4) | 420 (9.1) | 119 (8.8) | 21 (7.1) |
| No high school diploma | 513 (35.6) | 198 (29.0) | 184 (29.5) | 1124 (43.9) | 586 (35.7) | 745 (35.7) |
| Aspirin use | 304 (21.1) | 147 (21.5) | 122 (19.6) | 387 (15.1) | 290 (17.7) | 359 (17.2) |
| Other NSAIDs | 107 (7.4) | 56 (8.2) | 55 (8.8) | 262 (10.2) | 175 (10.7) | 239 (11.5) |
| Heart disease | 143 (9.9) | 68 (9.9) | 49 (7.9) | 134 (5.2) | 81 (4.9) | 90 (4.3) |
| Type 2 diabetes | 88 (6.1) | 49 (7.2) | 43 (6.9) | 159 (6.2) | 92 (5.6) | 100 (4.8) |
| Hypertension | 352 (24.5) | 177 (25.9) | 149 (23.9) | 798 (31.2) | 507 (30.9) | 575 (27.6) |
| Stroke | 64 (4.5) | 26 (3.8) | 27 (4.3) | 84 (3.3) | 69 (4.2) | 76 (3.6) |
| COPD | 90 (6.3) | 40 (5.9) | 52 (8.3) | 224 (8.8) | 134 (8.2) | 187 (9.0) |
| Kidney stones | 157 (10.9) | 68 (10.0) | 54 (8.6) | 166 (6.5) | 103 (6.3) | 122 (5.9) |
| Osteoporosis | —a | — | — | 149 (5.8) | 159 (9.7) | 356 (17.1) |
| Thyroid disease | — | — | — | 417 (16.3) | 299 (18.2) | 402 (19.3) |
| IBD | — | — | — | 142 (5.6) | 99 (6.0) | 147 (7.0) |
| Breast cancer | — | — | — | 99 (3.9) | 64 (3.9) | 93 (4.5) |
| Uterine cancer | NA | NA | NA | 67 (2.6) | 33 (2.0) | 35 (1.7) |
| Prostate cancer | 40 (2.8) | 22 (3.2) | 21 (3.4) | NA | NA | NA |
Abbreviations: MET, metabolic equivalent; NA, not applicable. Data shown are means ± SD or n (%). Bivariate associations with P ≤ .05 are indicated in bold and those with P > .05 in italics.
—, low prevalence, not used in model.
The association between total calcium intake and mortality after adjustment for potential confounders is shown in Table 2. The results were inconclusive (ie, the 95% confidence interval [CI] included both clinically meaningful and null results) for both men and women. Consideration of the point estimates showed a beneficial trend among women with hazard ratio (HR) = 0.95 (95% CI, 0.89–1.01) per 500-mg increase in daily calcium intake. For men, the results were inconclusive with HR = 0.99 (95% CI, 0.91–1.08) per 500-mg increase in daily calcium intake; moreover, the CI included HRs associated with modest benefit or harm.
Table 2.
Association Between Calcium Intake (Total and by Source) and All-Cause Mortality Among Men and Women in the CaMos
| Calcium Intake | Hazard Ratio (95% CI)a
|
|
|---|---|---|
| Men | Women | |
| Total intake (per 500 mg) | 0.99 (0.91, 1.08) | 0.95 (0.89, 1.01) |
| Dietary | ||
| Food only (per 500 mg) | 1.00 (0.90, 1.10) | 0.95 (0.87, 1.03) |
| Dairy only (per 500 mg) | 0.98 (0.89, 1.09) | 0.95 (0.87, 1.04) |
| Nondairy only (per 500 mg) | 1.23 (0.77, 1.96) | 0.83 (0.47, 1.48) |
| Supplemental | ||
| Supplement nonuser | 1.00 (referent) | 1.00 (referent) |
| Supplement user | 1.05 (0.83, 1.32) | 0.78 (0.66, 0.92) |
| Low dose, <500 mg | 0.98 (0.75, 1.28) | 0.77 (0.63, 0.93) |
| Medium dose, 500–1000 mg | 1.32 (0.92, 1.91) | 0.75 (0.59, 0.96) |
| High dose, ≥1000 mg | 0.83 (0.40, 1.72) | 0.88 (0.65, 1.18) |
Not adjusted for concurrent vitamin D intake due to collinearity. Adjusted for confounders: age, study center, education, BMI, health status (SF-36 PCS score), cigarette smoking, alcohol intake, physical activity, sun exposure, self-reported comorbidity (in men and women: hypertension, heart disease, stroke, type 2 diabetes, COPD, and kidney stones; in women only: osteoporosis, thyroid disease, IBD, breast cancer, and uterine cancer; in men only: prostate cancer), and medication (aspirin use or other NSAIDs).
Whereas dietary source–specific associations (Table 2) were all nonsignificant, they were consistent in direction and magnitude with the associations for total calcium in women, and point estimates remained close to the null value in men, ie, no effect. We further addressed the association between calcium supplement intake and mortality by dose. Among women, calcium supplement users had a lower risk of mortality than nonusers with HR = 0.78 (95% CI, 0.66–0.92), but there was no dose-response effect noted among users. In fact, there was attenuation of the association, showing statistically significant lower mortality only for supplement users with a daily dose of <1000 mg.
Vitamin D intake
Table 3 shows the baseline characteristics of the study population stratified by sex and total daily vitamin D intake. There were statistically significant bivariate associations between vitamin D intake and calcium intake, education, smoking, and heart disease in men and between vitamin D intake and age, calcium intake, education, BMI, SF-36 PCS score, smoking, aspirin use, osteoporosis, and thyroid disease in women.
Table 3.
Baseline Characteristics and 10-Year All-Cause Mortality Stratified by Sex and Category of Vitamin D Intake From Food and Supplements in the CaMos
| Men (n = 2746)
|
Women (n = 6287)
|
|||||
|---|---|---|---|---|---|---|
| Low, <10 μg (n = 2206) | Moderate, 10–20 μg (n = 449) | High, ≥20 μg (n = 91) | Low, <10 μg (n = 4635) | Moderate, 10–20 μg (n = 1354) | High, ≥20 μg (n = 298) | |
| Age, y | 60.2 ± 14.5 | 61.3 ± 14.0 | 61.2 ± 14.1 | 63.2 ± 13.1 | 64.4 ± 11.6 | 65.4 ± 10.9 |
| BMI, kg/m2 | 27.0 ± 4.1 | 26.8 ± 4.0 | 27.7 ± 4.2 | 27.1 ± 5.2 | 26.6 ± 5.0 | 26.2 ± 4.7 |
| SF-36 PCS score | 49.1 ± 9.3 | 48.5 ± 9.7 | 48.3 ± 9.4 | 46.8 ± 10.4 | 45.9 ± 10.8 | 44.3 ± 11.4 |
| Physical activity, 1000 MET · m/d | 2.49 ± 0.44 | 2.52 ± 0.45 | 2.48 ± 0.43 | 2.51 ± 0.35 | 2.53 ± 0.34 | 2.50 ± 0.37 |
| Calcium, mg | 784 ± 459 | 1300 ± 641 | 1829 ± 1013 | 899 ± 519 | 1353 ± 637 | 1815 ± 765 |
| Deaths | 333 (15.1) | 74± (16.5) | 16 (17.6) | 537± (11.8) | 153± (11.3) | 37 (12.4) |
| Current smoker | 430 (19.5) | 65 (14.3) | 10 (11.0) | 678 (14.6) | 189 (14.0) | 24 (8.1) |
| High alcohol | 158 (7.2) | 37 (8.3) | 8 (8.8) | 234 (5.1) | 81 (6.0) | 8 (2.7) |
| Regular sun exposure | 373 (16.9) | 84 (18.7) | 14 (15.4) | 420 (9.1) | 119 (8.8) | 21 (7.1) |
| No high school diploma | 747 (33.8) | 122 (27.2) | 26 (28.6) | 1854 (40.0) | 484 (35.8) | 117 (39.3) |
| Aspirin use | 448 (20.3) | 106 (23.6) | 19 (20.9) | 732 (15.8) | 245 (18.1) | 59 (19.8) |
| Other NSAIDs | 107 (7.4) | 56 (8.2) | 55 (8.8) | 262 (10.2) | 175 (10.7) | 239 (11.5) |
| Heart disease | 228 (10.3) | 28 (6.2) | 4 (4.4) | 238 (5.1) | 59 (4.4) | 8 (2.4) |
| Type 2 diabetes | 140 (6.4) | 30 (6.7) | 10 (11.0) | 273 (5.9) | 67 (5.0) | 11 (3.7) |
| Hypertension | 546 (24.7) | 114 (25.4) | 18 (19.8) | 1419 (30.6) | 386 (28.5) | 75 (25.2) |
| Stroke | 94 (4.3) | 21 (4.7) | 2 (2.2) | 166 (3.6) | 53 (3.9) | 10 (3.4) |
| COPD | 142 (6.4) | 37 (8.2) | 3 (3.3) | 396 (8.5) | 128 (9.5) | 21 (7.1) |
| Kidney stones | 236 (10.7) | 35 (7.8) | 8 (8.8) | 283 (6.1) | 96 (7.1) | 14 (4.7) |
| Osteoporosis | —a | — | — | 392 (8.5) | 184 (13.6) | 88 (29.5) |
| Thyroid disease | — | — | — | 785 (16.9) | 267 (19.7) | 66 (22.2) |
| IBD | — | — | — | 278 (6.0) | 88 (6.5) | 22 (7.4) |
| Breast cancer | — | — | — | 186 (4.0) | 50 (3.7) | 20 (6.7) |
| Uterine cancer | NA | NA | NA | 94 (2.0) | 34 (2.5) | 7 (2.4) |
| Prostate cancer | 60 (2.7) | 18 (4.0) | 5 (5.5) | NA | NA | NA |
Abbreviations: MET, metabolic equivalent; NA, not applicable. Data shown are means ± SD or n (%). Bivariate associations with P ≤ .05 are indicated in bold and those with P > .05 in italics.
—, low prevalence, not used in model.
Overall, the associations between total vitamin D intake and mortality were also inconclusive. The cubic spline models all had 95% CI overlapping the null value over the entire range of vitamin D intake. Table 4 shows the estimates based on the IOM reference ranges. The adjusted HRs comparing low intakes (<400 IU or 10 μg daily) and high intakes (>800 IU or 20 μg daily) vs moderate intakes (400–800 IU or 10–20 μg) were inconclusive in both men and women.
Table 4.
Association Between Vitamin D Intake (Total and by Source) and All-Cause Mortality Among Men and Women in the CaMos
| Vitamin D Intake | Hazard Ratio (95% CI)a
|
|
|---|---|---|
| Men | Women | |
| Total | ||
| Moderate intake, 400–800 IU, 10–20 μg | 1 (referent) | 1 (referent) |
| Low intake, <400 IU, <10 μg | 0.97 (0.74–1.26) | 1.08 (0.90–1.31) |
| High intake, ≥800 IU, ≥20 μg | 1.02 (0.60–1.73) | 0.87 (0.63–1.21) |
| Dietary | ||
| Milk only (per 5 μg) | 0.98 (0.84–1.14) | 0.99 (0.87–1.14) |
| Supplemental | ||
| Supplement nonuser | 1.00 (referent) | 1.00 (referent) |
| Supplement user | 1.17 (0.92–1.48) | 0.84 (0.71–0.99) |
| Low dose, <400 IU, <10 μg | 1.17 (0.78–1.75) | 0.83 (0.64–1.08) |
| Moderate dose, 400–800 IU, 10–20 μg | 1.15 (0.87–1.52) | 0.83 (0.69–1.01) |
| High dose, ≥800 IU, ≥20 μg | 1.24 (0.63–2.48) | 0.91 (0.62–1.32) |
Not adjusted for concurrent calcium intake due to collinearity. Adjusted for confounders: age, study center, education, BMI, health status (SF-36 PCS score), cigarette smoking, alcohol intake, physical activity, sun exposure, self-reported comorbidity (in men and women: hypertension, heart disease, stroke, type 2 diabetes, COPD, and kidney stones; in women only: osteoporosis, thyroid disease, IBD, breast cancer, and uterine cancer; in men only: prostate cancer), and medication (aspirin use or other NSAIDs).
In further analysis, we considered possible variations in effect by source of intake (Table 4). The association between milk intake and mortality was inconclusive for both men and women. The association between vitamin D supplement use and mortality was inconclusive in men. Among women, vitamin D supplement users had a lower risk of mortality than nonusers with HR = 0.84 (95% CI, 0.71–0.99), but there was no dose-response effect noted among users.
Interaction of calcium and vitamin D
The previous analyses considered calcium intake and vitamin D intake separately. It is not possible to completely determine the independent effects of these 2 nutrients because 2 common sources (milk and multivitamins) contain both. However, we did consider global categories of intake and supplements to assess possible effect modification, confounding, and interaction. We found no statistical interaction between the categories of total vitamin D intake, total calcium intake, and mortality. In fact, the linear association between total calcium intake and mortality among women was similar for those with low-to-moderate and high total vitamin D intake. We also found no significant interaction between categories of vitamin D supplement use, calcium supplement use, and mortality. Moreover, with regard to supplement use, among women, there was a lower mortality risk among those who used calcium supplements alone (HR = 0.79; 95% CI, 0.62–1.00) or calcium and vitamin D supplements combined (HR = 0.77, 95% CI, 0.64–0.93) compared with those who did not use either calcium or vitamin D supplements. There were no conclusive results comparing those who used vitamin D supplements alone were compared with those who did not use either calcium or vitamin D supplements (HR = 0.94; 95% CI, 0.64–1.38). Thus, the significant effect observed for vitamin D supplement use in women noted in the previous section and in Table 4 is attributable to the concurrent intake of calcium supplements.
Discussion
We assessed the association between total calcium intake, total vitamin D intake, and all-cause mortality, including possible heterogeneity by the nutrient source and effect modification by the other nutrient. Our analysis showed that total calcium intake among women was more likely to be beneficial than harmful and that the same was true of calcium intake from dairy sources, nondairy sources, and supplements. In fact, we observed that supplemental calcium intake up to 1000 mg/d among women was associated with statistically significant decreased mortality, although the results were inconclusive for supplement intake exceeding 1000 mg/d. The lack of a demonstrable benefit observed for higher doses may be a threshold effect or a failure to determine effective intake, which is determined by variables that we did not measure (frequency, time of day, timing relative to meals, and formulation). Consequently, we cannot at this time advocate intakes in excess of this amount. We also found that the relationship between calcium and mortality was relatively consistent by strata of total vitamin D. Thus, in our data, there is no evidence of increased mortality risk associated specifically with calcium supplements at the typical intake levels of Canadian women.
Our results are concordant with findings from the Iowa Women’s Health Study in which use of calcium supplements was associated with a lower risk of mortality in older women (21). In a subgroup analysis of the Women’s Health Initiative (WHI), calcium supplementation was associated with increased risk of myocardial infarction (MI) and/or cardiovascular mortality among those not already taking personal calcium supplements at baseline; however, calcium supplementation was not associated with increased all-cause mortality in the same group (22). Furthermore, all-cause mortality was in fact lower in those receiving active treatment vs that in those receiving placebo among participants reporting personal calcium use, and, indeed, the original WHI report concluded that supplements may reduce mortality rates in postmenopausal women (23), consistent with results in the present study. A recent analysis of the WHI shows possible early risk for MI and coronary heart disease in selected subsets of participants, but, again, this was not associated with early mortality (24). Our results are partially consistent with results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg prospective cohort study in that moderately high dietary calcium intake was associated with a lower risk of MI, and the association differed by sex with attenuation of the protective effect in men (25). However, in contrast to our study, use of calcium supplements was associated with increased risk of MI (25).
Most recently, Michaelsson et al (26) found a U-shaped relationship between dietary calcium intake and mortality and between total intake and mortality, consistent with our finding that low calcium intake increases the risk of mortality. Our results differ with respect to high intake; a possible reason for the discrepancy is the different distribution of calcium intake, in particular with regard to dairy intake and supplement use. The strong attenuation of effect noted for their model using only baseline covariates could also be attributable to exposure misclassification, because their study lasted more than 20 years and there were time trends in calcium intake.
There are several plausible causal mechanisms for an apparent protective association. Supplemental calcium has been associated with lower plasma lipoprotein levels, a better lipid profile, and a lower risk of hypertension (27). Supplemental calcium will also protect against the potential adverse effects of low calcium intake, such as secondary hyperparathyroidism (28) and high serum PTH levels, which may accelerate bone turnover and mobilize bone calcium, resulting in bone loss, cause cardiovascular effects due to calcification of blood vessels (29), and be associated with increased mortality risk (30, 31). Calcium may also bind fatty acids in the colon and thus inhibit epithelial proliferation, and calcium supplementation has been associated with a lower risk of recurrent colorectal adenomatous polyps (32). Potential noncausal explanations include the indirect association of calcium intake from food with a healthy diet, including dairy products, whole grains, fish, and legumes, all of which are considered nutrient-dense foods and may have health effects unrelated to their calcium content (33, 34). Similarly, dietary calcium intake is related to total energy intake. Low energy intake may be a surrogate marker for frailty in women and associated with increased mortality. Some attenuation of real effects might also occur because of various calcium-containing compounds, dose distribution over the day, and cointake of nutrients interfering with calcium absorption (eg, oxalates and phytates). Finally, use of calcium supplements may be associated with unmeasured risk factors such as socioeconomic status and thus there may be residual confounding.
The relationship between vitamin D intake for milk and supplements and mortality was inconclusive in both men and women. We considered the main source of dietary vitamin D variation as well as a crude assessment of sunlight exposure, but there are certainly other exogenous and endogenous sources affecting overall vitamin D status that were not assessed. We found that after adjustment for all risk factors including concurrent use of calcium supplements, vitamin D supplements were not associated with reduced mortality risk in women. Thus, neither major vitamin source studied (milk and supplements) was associated with mortality. This result is consistent with a recent meta-analysis of calcium and vitamin D supplement trials, which found reduced mortality with calcium and vitamin D supplements combined but not with vitamin D supplements alone (35).
Both higher (15) and lower (36) levels of serum 25(OH)D (a clinical correlate of low vitamin D intake) have been associated with increased mortality. If the observed associations between 25(OH)D levels and mortality are attributable to variations in intake, then lower and higher intakes ought to be associated with higher mortality, which we did not observe. It is possible that, with regard to milk intake as a source of vitamin D, other milk constituents may dilute the association attributable to vitamin D, leading to a null association. Furthermore, the prevalence of inadequate vitamin D intake may not match the associated prevalence of inadequate 25(OH)D (37), thus demonstrating the potential importance of cutaneous sources of vitamin D, as we found previously (18) and the likelihood that this source may counteract the effects of low intake. In addition, serum 25(OH)D is also influenced by other factors, such as PTH, BMI, and age-related decline in renal function (17). Finally, the association between 25(OH)D and mortality may not in fact be causal at all, but due to an unidentified pathophysiologic factor that influences both 25(OH)D concentrations and mortality independently.
The US Preventive Services Task Force recently released recommendations against daily supplementation with 400 IU or less of vitamin D3 and 1000 mg or less of calcium for the primary prevention of fractures in noninstitutionalized postmenopausal women on the basis of evidence of no net benefit (38). The IOM found that calcium and vitamin D are necessary for skeletal growth and maintenance and recommended dietary reference intakes based on this premise (15). If calcium and vitamin D supplementation are indeed required to ensure adequate intake levels for bone health, then our study provides assurance that, in community-dwelling individuals, there is no increased mortality associated with ingestion of modest amounts of supplemental calcium and vitamin D and that there may in fact be a mortality benefit.
The strengths of the present analysis include assessment of calcium and vitamin D intake from food and supplements in a large population-based sample. In particular, our assessment of calcium and vitamin D supplement use was derived from a complete inventory of all medications and supplements and does not rely on recall to determine ingredients and/or doses. The baseline interviewer-administered questionnaire had detailed lifestyle and demographic information and we used measured height and weight to determine BMI.
Limitations of the study include a low response rate (42%), which could result in selection bias. CaMos investigators have previously addressed potential selection bias and found limited nonresponse bias, except perhaps in the oldest age groups (≥80 years) (39). We used an abbreviated FFQ, which captures most calcium intake at 2 discrete time points, but only included foods supplemented with vitamin D. Potential causal pathways to all-cause mortality could only be hypothesized because of the observational nature of the study and lack of information concerning the cause of death. The cohort size and mortality rate may have limited our ability to detect significant relationships between calcium or vitamin D intake and mortality. Finally, death ascertainment may be incomplete and although we used a time-to-event analysis, it assumes that the censoring mechanism is noninformative.
In summary, we found that use of calcium supplements up to 1000 mg/d in women was associated with reduced mortality. This association was consistent with potential associations between overall calcium intake and mortality as well as calcium intake from dairy and nondairy food and mortality. We did not find any significant independent association between vitamin D intake and mortality nor did vitamin D intake affect the association noted between calcium and mortality. Thus, our recommendation is to assess dietary intake to meet calcium and vitamin D requirements for bone health and to consider supplementation necessary to meet the requirements.
Acknowledgments
We thank all the participants in the CaMos who made this study possible and members of the CaMos research group, who were instrumental in the ongoing success of the CaMos cohort. In addition, we thank Suzette Poliquin, who performed the first analysis of calcium and vitamin D intakes, and Susan Barr, who devised the initial CaMos FFQ portion of the CaMos questionnaire.
Abbreviations
- BMI
body mass index
- CaMos
Canadian Multicentre Osteoporosis Study
- CI
confidence interval
- FFQ
food frequency questionnaire
- HR
hazard ratio
- 25(OH)D
25-hydroxyvitamin D
- IOM
Institute of Medicine
- MI
myocardial infarction
- WHI
Women’s Health Initiative
Footnotes
The members of the CaMos Research Group are the following: David Goltzman (coprincipal investigator, McGill University, Montreal), Nancy Kreiger (coprincipal investigator, University of Toronto, Toronto), and Alan Tenenhouse (principal investigator emeritus, Toronto); CaMos Coordinating Centre, McGill University, Montreal, Quebec, Suzanne Godmaire (research assistant), Silvia Dumont (research assistant), Claudie Berger (statistician), and Lisa Langsetmo (Fellow); Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (codirector), and Emma Sheppard (coordinator); Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (codirectors), and Barbara Stanfield (coordinator); Laval University, Quebec City, Quebec: Jacques P Brown (director), Louis Bessette (codirector), and Jeanette Dumont (coordinator); Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (codirector), and Barbara Matthews (coordinator); University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie Jamal (codirector), Tim Murray (past director), and Barbara Gardner, Bray (coordinator); McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (codirector), and Laura Pickard (coordinator); University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (codirector), and Jola Thingvold (coordinator); University of Calgary, Calgary, Alberta: David A Hanley (director) and Jane Allan (coordinator); University of British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Millan Patel (codirector), Brian Lentle (radiologist), and Nerkeza Andjelic (coordinator).
Disclosure Summary: C.S.K. was an advisory board member or consultant or received speaker fees from Amgen, Danone, Eli Lilly, Merck Frosst, and Novartis. D.A.H. was an advisory board member or consultant or received speaker fees from Amgen, Eli Lilly, Merck, Novartis, and NPS Pharmaceuticals. S.A.J. was an advisory board member or consultant or received speaker fees from Novartis, Amgen, Warner, Chilcott, Genzyme, Shire, and Cytochroma. S.J.W. disclosures are General Mills (Yoplait) and Abbott. S.N.M. holds an unrestricted research grant from Amgen and is a consultant for Novartis, Amgen, Eli Lilly, and Merck Frosst. A.H. was a board member or consultant or received speaker fees from Novartis, Amgen, and Shire. R.G.J. was an advisory board member for Novartis, Merck Frosst, Amgen, and Eli Lilly. A.P. was a consultant or received speaker fees from Amgen, Eli Lilly, Merck Frosst, and Novartis. D.G. was an advisory board member or consultant for Amgen, Eli Lilly, Merck Frosst, and Novartis. The other authors have nothing to disclose.
The Canadian Multicenter Osteoporosis Study is currently funded by the Canadian Institutes of Health Research, Amgen, Merck Frosst Canada Ltd, the Dairy Farmers of Canada, Novartis, and Eli Lilly and Company.
References
- 1.Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolland MJ, Barber PA, Doughty RN, et al. Vascular events in healthy older women receiving calcium supplementation: randomised controlled trial. BMJ. 2008;336:262–266. doi: 10.1136/bmj.39440.525752.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pentti K, Tuppurainen MT, Honkanen R, et al. Use of calcium supplements and the risk of coronary heart disease in 52–62-year-old women: the Kuopio Osteoporosis Risk Factor and Prevention Study. Maturitas. 2009;63:73–78. doi: 10.1016/j.maturitas.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang TK, Bolland MJ, van Pelt NC, et al. Relationships between vascular calcification, calcium metabolism, bone density, and fractures. J Bone Miner Res. 2010;25:2777–2785. doi: 10.1002/jbmr.183. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Allison MA, Carr JJ, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 2010;17:683–691. doi: 10.1097/gme.0b013e3181d683b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis JR, Calver J, Zhu K, Flicker L, Prince RL. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res. 2011;26:35–41. doi: 10.1002/jbmr.176. [DOI] [PubMed] [Google Scholar]
- 8.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–260. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Melamed ML, Michos ED, Post W, Astor B. 25-Hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiscella K, Franks P. Vitamin D, race, and cardiovascular mortality: findings from a national US sample. Ann Fam Med. 2010;8:11–18. doi: 10.1370/afm.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobnig H, Pilz S, Scharnagl H, et al. Independent association of low serum 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168:1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- 13.Ensrud KE, Ewing SK, Fredman L, et al. Circulating 25-hydroxyvitamin D levels and frailty status in older women. J Clin Endocrinol Metab. 2010;95:5266–5273. doi: 10.1210/jc.2010-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ensrud KE, Blackwell TL, Cauley JA, et al. Circulating 25-hydroxyvitamin D levels and frailty in older men: the osteoporotic fractures in men study. J Am Geriatr Soc. 2011;59:101–106. doi: 10.1111/j.1532-5415.2010.03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poliquin S, Joseph L, Gray-Donald K. Calcium and vitamin D intakes in an adult Canadian population. Can J Diet Pract Res. 2009;70:21–27. doi: 10.3148/70.1.2009.21. [DOI] [PubMed] [Google Scholar]
- 17.Berger C, Greene-Finestone LS, Langsetmo L, et al. Temporal trends and determinants of longitudinal change in 25-hydroxyvitamin D and parathyroid hormone levels. J Bone Miner Res. 2012;27:1381–1389. doi: 10.1002/jbmr.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene-Finestone LS, Berger C, de Groh, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22:1389–1399. doi: 10.1007/s00198-010-1362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langlois K, Greene-Finestone L, Little J, Hidiroglou N, Whiting S. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Health Rep. 2010;21:47–55. [PubMed] [Google Scholar]
- 20.Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181:265–271. doi: 10.1503/cmaj.081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mursu J, Robien K, Harnack LJ, Park K, Jacobs DR., Jr Dietary supplements and mortality rate in older women: the Iowa Women’s Health Study. Arch Intern Med. 2011;171:1625–1633. doi: 10.1001/archinternmed.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bolland MJ, Grey A, Gamble GD, Reid IR. Calcium and vitamin D supplements and health outcomes: a reanalysis of the Women’s Health Initiative (WHI) limited-access data set. Am J Clin Nutr. 2011;94:1144–1149. doi: 10.3945/ajcn.111.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaCroix AZ, Kotchen J, Anderson G, et al. Calcium plus vitamin D supplementation and mortality in postmenopausal women: the Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2009;64:559–567. doi: 10.1093/gerona/glp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women’s Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24:567–580. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li K, Kaaks R, Linseisen J, Rohrmann S. Associations of dietary calcium intake and calcium supplementation with myocardial infarction and stroke risk and overall cardiovascular mortality in the Heidelberg cohort of the European Prospective Investigation into Cancer and Nutrition study (EPIC-Heidelberg) Heart. 2012;98:920–925. doi: 10.1136/heartjnl-2011-301345. [DOI] [PubMed] [Google Scholar]
- 26.Michaelsson K, Melhus H, Warensjo LE, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ. 2013;346:f228. doi: 10.1136/bmj.f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid IR, Ames R, Mason B, et al. Effects of calcium supplementation on lipids, blood pressure, and body composition in healthy older men: a randomized controlled trial. Am J Clin Nutr. 2010;91:131–139. doi: 10.3945/ajcn.2009.28097. [DOI] [PubMed] [Google Scholar]
- 28.Paik JM, Curhan GC, Taylor EN. Calcium intake and risk of primary hyperparathyroidism in women: prospective cohort study. BMJ. 2012;345:e6390. doi: 10.1136/bmj.e6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aloia J, Bojadzievski T, Yusupov E, et al. The relative influence of calcium intake and vitamin D status on serum parathyroid hormone and bone turnover biomarkers in a double-blind, placebo-controlled parallel group, longitudinal factorial design. J Clin Endocrinol Metab. 2010;95:3216–3224. doi: 10.1210/jc.2009-1294. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone is associated with increased mortality independent of 25-hy-droxy vitamin D status, bone mass, and renal function in the frail and very old: a cohort study. J Clin Endocrinol Metab. 2004;89:5477–5481. doi: 10.1210/jc.2004-0307. [DOI] [PubMed] [Google Scholar]
- 31.Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: the CopD study. J Clin Endocrinol Metab. 2012;97:2644–2652. doi: 10.1210/jc.2012-1176. [DOI] [PubMed] [Google Scholar]
- 32.Weingarten MA, Zalmanovici A, Yaphe J. Dietary calcium supplementation for preventing colorectal cancer and adenomatous polyps. Cochrane Database Syst Rev. 2008;1:CD003548. doi: 10.1002/14651858.CD003548.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langsetmo L, Poliquin S, Hanley DA, et al. Dietary patterns in Canadian men and women ages 25 and older: relationship to demographics, body mass index, and bone mineral density. BMC Musculoskelet Disord. 2010;11:20. doi: 10.1186/1471-2474-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langsetmo L, Hanley DA, Prior JC, et al. Dietary patterns and incident low-trauma fractures in postmenopausal women and men aged ≥50 y: a population-based cohort study. Am J Clin Nutr. 2011;93:192–199. doi: 10.3945/ajcn.110.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rejnmark L, Avenell A, Masud T, et al. Vitamin D with calcium reduces mortality: patient level pooled analysis of 70,528 patients from eight major vitamin D trials. J Clin Endocrinol Metab. 2012;97:2670–2681. doi: 10.1210/jc.2011-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schottker B, Ball D, Gellert C, Brenner H. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev. doi: 10.1016/j.arr.2012.02.004. [published online ahead of print February 17, 2012] [DOI] [PubMed] [Google Scholar]
- 37.Whiting SJ, Langlois KA, Vatanparast H, Greene-Finestone LS. The vitamin D status of Canadians relative to the 2011 Dietary Reference Intakes: an examination in children and adults with and without supplement use. Am J Clin Nutr. 2011;94:128–135. doi: 10.3945/ajcn.111.013268. [DOI] [PubMed] [Google Scholar]
- 38.Moyer VA. Vitamin D and calcium supplementation to prevent fractures in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. doi: 10.7326/0003-4819-158-9-201305070-00603. [published online ahead of print February 26, 2013] [DOI] [PubMed] [Google Scholar]
- 39.Kmetic A, Joseph L, Berger C, Tenenhouse A. Multiple imputation to account for missing data in a survey: estimating the prevalence of osteoporosis. Epidemiology. 2002;13:437–444. doi: 10.1097/00001648-200207000-00012. [DOI] [PubMed] [Google Scholar]