To the Editor
Insufficient serum 25 hydroxyvitamin D (25 (OH)D) levels are associated with poor serum calcium absorption, which leads to high serum parathyroid hormone and high rates of bone resorption.1,2 Thus, serum 25 (OH)D level is an important risk factor for bone loss and fragility fractures.3
Vitamin D supplementation may prevent falls and reduce bone loss and fractures associated with osteoporosis by elevating serum 25(OH)D. Osteoporosis Canada4 and the National Osteoporosis Foundation5 have endorsed 75 nmol/L6,7 as the optimal level of serum 25(OH)D for bone health. The current study was designed to determine the level of vitamin D sufficiency (≥75nmol/L) in elderly long-term care (LTC) residents and to examine the association between vitamin D supplementation and subsequent serum 25(OH)D levels.
METHODS
One hundred two residents randomly selected from four LTC facilities in southern Ontario, Canada, participated in this cross-sectional study. The facilities belonged to a national chain (Revera Inc., Mississauga, Ontario, Canada) that, at a corporate level, was initiating an osteoporosis and fracture prevention program, but these four facilities were not part of the prevention program. Residents were eligible to participate if they were age 50 and older and were not receiving palliative care. The resident or their legal representative provided informed consent. A research ethics board approved the study.
Staff at each LTC facility collected demographic and vitamin D supplementation data from medical charts. The prevalence of vitamin D insufficiency was determined based on the laboratory reference ranges (0–75 nmol/L, deficiency or insufficiency; 75–250 nmol/L, sufficiency). Study serum vitamin D levels were determined in one centralized laboratory. Multivariable logistic regression analysis was performed to determine the relationship between vitamin D3 supplementation and serum levels. Vitamin D3 supplementation was categorized into four groups (0 IU/d (reference group), 1–400 IU/d, 401–800 IU/d, >800 IU/d). Covariates included in the analysis consisted of resident age (continuous variable), calcium supplementation dose (mg/d), and sex.
RESULTS
The mean age ± standard deviation of the residents was 83.2 ± 8.7. Of the 102 residents, 31.4% were men. Forty-five percent (45/100) and 49% (48/98) of residents were taking calcium and vitamin D3 supplements, respectively. Of the 48 individuals taking vitamin D3 supplementation, 37.5% (18) were taking 0–400 IU/d, 18.8%8 were taking 401–800 IU/d, and 43.8% (21) were taking more than 800 IU/d of vitamin D3. Mean serum 25(OH)D levels were 62.6 ± 27.5, 72.8 ± 22.2, 98.9 ± 26.3, and 96.0 ± 26.2 nmol/L for those taking no supplements, 1–400 IU/d, 401–800 IU/d, and more than 800 IU/d.
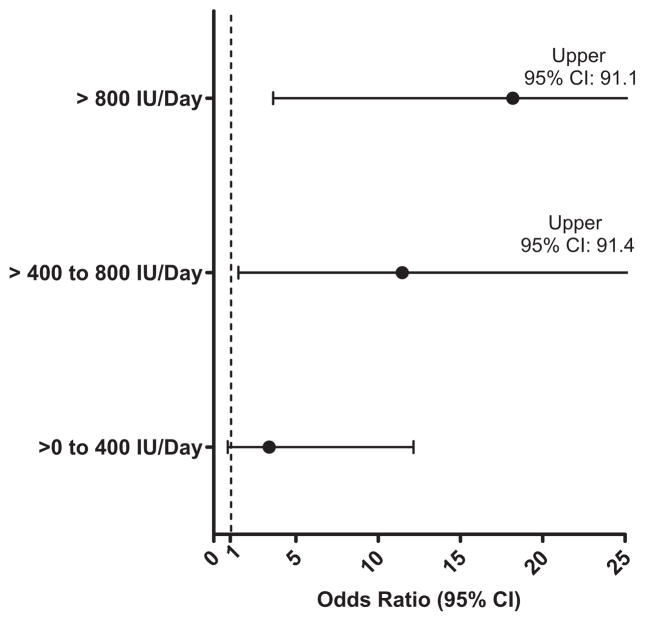
A dose effect was observed between vitamin D3 supplementation and serum 25(OH)D; 26.0% (13/50), 44.4% (8/ 18), 77.8% (7/9), and 91.0% (17/21) of residents taking no supplements, 1–400 IU/d, 401–800 IU/d, and more than 800 IU/d, respectively, exceeded the 75-nmol/L threshold. Individuals taking 401–800 IU/d and more than 800 IU/d had greater adjusted odds of exceeding the 75 nmol/L than those not taking vitamin D3 supplements (Figure 1).
Figure 1.
Adjusted odds ratios (95% confidence intervals (CIs)) according to vitamin dose for residents exceeding the 75-nmol/L threshold (reference level: no vitamin D3 supplementation).
DISCUSSION
These results indicate that individuals living in LTC facilities in Ontario, Canada, benefited from vitamin D3 supplementation and that the higher the vitamin D3 dose the higher the odds of exceeding the 75 nmol/L threshold. For instance, more than 90% of LTC residents taking the recommended dose of vitamin D3 had serum 25(OH)D levels in the target range. Greater emphasis needs to be placed on vitamin D3 supplementation at LTC facilities, given that 51% of individuals were not taking any vitamin D3 and that 54% of individuals were below the 75 nmol/L optimal threshold.
These findings suggest that strategies including mandatory vitamin D supplementation for residents of LTC facilities or higher daily doses of vitamin D (recommended daily dose) may be necessary to achieve optimal 25(OH)D levels. Attaining adequate 25(OH)D values is important from a therapeutic standpoint, given that these levels are needed to prevent falls and to ensure optimal effectiveness of bisphosphonates for the treatment of osteoporosis and osteoporotic fractures.8,9
In conclusion, most residents taking more than 400 IU/d of vitamin D3 achieve optimal levels of 25(OH)D. Nevertheless, although vitamin D supplementation appears to clinically increase serum 25(OH)D levels, some residents in LTC homes are not taking adequate vitamin D supplementation and are not reaching the therapeutic target. Thus, knowledge translation initiatives may be needed to reduce the gap between knowledge and practice and to increase the effective use of vitamin D supplementation in LTC residents.
Acknowledgments
Conflict of Interest: Funded by the Ontario Long-term Care Osteoporosis Strategy and in-kind support from Revera, Inc. A Papaioannou has been a consultant and speaker for Amgen, Lily, Merck, Novartis, Procter & Gamble, sanofiaventis, Servier, and Warner Chilcot and has conducted clinical trials for Lilly, Merck, Procter & Gamble, sanofiaventis, and Warner Chilcot.
Author Contributions: G. Ioannidis conducted all data analysis, interpreted the data analyses, and drafted the letter. C.C. Kennedy J. Dykeman, S. Dudziak, and A. Papaioannou developed the study concept and critically appraised the letter.
Sponsor’s Role: Employees of the sponsor helped in developing the study concept and reviewing the letter.
Contributor Information
George Ioannidis, McMaster University, Hamilton, Ontario, Canada.
Courtney C. Kennedy, McMaster University, Hamilton, Ontario, Canada.
Alexandra Papaioannou, McMaster University, Hamilton, Ontario, Canada.
Joanne Dykeman, Revera Inc, Mississauga, Ontario, Canada.
Sandra Dudziak, Revera Inc, Mississauga, Ontario, Canada.
References
- 1.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 2.Bronner F. Recent developments in intestinal calcium absorption. Nutr Rev. 2009;67:109–113. doi: 10.1111/j.1753-4887.2008.00147.x. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF, Chen TC. Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 4.Hanley DA, Cranney A, Jones G, et al. for the Guidelines Committee of the Scientific Advisory Council of Osteoporosis Canada. Vitamin D in adult health and disease: A review and guideline statement from osteoporosis Canada. Can Med Assoc J. 2010;182:E610–E618. doi: 10.1503/cmaj.091062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010. [Accessed September 8, 2011]. Available at: http://www.nof.org/professionals/clinical-guidelines. [Google Scholar]
- 6.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: A meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 8.Mastaglia SR, Pellegrini GG, Mandalunis PM, et al. Vitamin D insufficiency reduces the protective effect of bisphosphonate on ovariectomy-induced bone loss in rats. Bone. 2006;39:837–844. doi: 10.1016/j.bone.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Adami S, Giannini S, Bianchi G, et al. Vitamin D status and response to treatment in post-menopausal osteoporosis. Osteoporos Int. 2009;20:239–244. doi: 10.1007/s00198-008-0650-y. [DOI] [PubMed] [Google Scholar]