Abstract
Age-standardized rates of hip fracture in Canada declined during the period 1985 to 2005. We investigated whether this incidence pattern is explained by period effects, cohort effects, or both. All hospitalizations during the study period with primary diagnosis of hip fracture were identified. Age- and sex-specific hip fracture rates were calculated for nineteen 5-year age groups and four 5-year calendar periods, resulting in 20 birth cohorts. The effect of age, calendar period, and birth cohort on hip fracture rates was assessed using age-period-cohort models as proposed by Clayton and Schiffers. From 1985 to 2005, a total of 570,872 hospitalizations for hip fracture were identified. Age-standardized rates for hip fracture have progressively declined for females and males. The annual linear decrease in rates per 5-year period were 12% for females and 7% for males (both p < 0.0001). Significant birth cohort effects were also observed for both sexes (p < 0.0001). Cohorts born before 1950 had a higher risk of hip fracture, whereas those born after 1954 had a lower risk. After adjusting for age and constant annual linear change (drift term common to both period and cohort effects), we observed a significant nonlinear birth cohort effect for males (p = 0.0126) but not for females (p = 0.9960). In contrast, the nonlinear period effect, after adjustment for age and drift term, was significant for females (p = 0.0373) but not for males (p = 0.2515). For males, we observed no additional nonlinear period effect after adjusting for age and birth cohort, whereas for females, we observed no additional nonlinear birth cohort effect after adjusting for age and period. Although hip fracture rates decreased in both sexes, different factors may explain these changes. In addition to the constant annual linear decrease, nonlinear birth cohort effects were identified for males, and calendar period effects were identified for females as possible explanations.
Keywords: INCIDENCE RATE, HIP FRACTURES, TRENDS, AGE-PERIOD-COHORT ANALYSIS, OSTEOPOROSIS
Introduction
Long-term trend studies of hip fracture rates can provide useful information for quantifying the burden of osteoporosis and evaluating the potential impact of prevention efforts. Over the last decades, several countries have observed declining age-adjusted hip fracture rates.(1–5) However, few studies have attempted to assess if these declining rates can be attributed to changes occurring over a specific calendar period (period effect) or to changes affecting individuals born at a specific time (cohort effect).(6–9) Period effects reflect changes occurring at a specific time and affect all individuals regardless of age. For example, the application of a new diagnostic technique or treatment for osteoporosis in a population might lead to a decrease in hip fracture rates explained by a period effect. Conversely, cohort effects reflect changes applicable to individuals born at a specific time. For example, if younger generations adopt a lifestyle improvement (e.g., an increase in physical activity), this might lead to a decrease in hip fracture rates explained by a cohort effect. Age-period-cohort analysis aims to model the effects of these factors on rates.
Recently, the Osteoporosis Surveillance Expert Working Group reported the declining trends in age-adjusted hip fracture rates in Canada.(1) The current study explores whether these observed trends can be explained by a period or a cohort effect in order to narrow in on the factors underlying these changes.
Materials and Methods
Data collection
We used data from the Hospital Morbidity Database (HMDB), which provides data on hospital discharges in Canada and is housed at the Canadian Institute for Health information (CIHI). For each hospital inpatient event, the HMDB contains administrative, clinical, and demographic information and allows the generation of national discharge statistics by diagnoses and procedures. To ensure high-quality information, the CIHI uses a data quality enhancement program.(10) The data for this study were obtained through the Public Health Agency of Canada (PHAC), and the study was reviewed and approved by the PHAC through the approvals process for peer-reviewed publications.
All hospitalizations from January 1, 1985 to December 31, 2005 in which the primary reason for hospitalization was hip fracture were extracted from the HMDB. Hip fractures were identified by ICD-9-CM diagnosis 820.x or by ICD-10-CM diagnosis S72.0-S72.2. Hospitalizations resulting from complications or revisions of hip fracture surgery were excluded. The numbers of hip fractures were stratified by calendar year, sex, and age (5-year interval). Estimates of the population size using a similar stratification were obtained from national census data performed every 5 years with interpolation for between-census years. Data from northern territories (Northwest Territories, Yukon, and Nunavut), representing 0.3% of the Canadian population, were not available for all years, and therefore hip fracture cases and population size from these regions were excluded from all analyses. Age- and sex-specific hip fracture rates (per 100,000 person-years) were calculated for 19 age groups (from 0–4 to ≥85 years, 5-year intervals) and four calendar periods from 1985 to 2004 (5-year intervals). This stratification resulted in 20 birth cohorts from 1905–09 through 2000–04 (5-year intervals).
Age-period-cohort analysis
To evaluate the effects of age, period, and birth cohort on hip fracture rates, the age-period-cohort approach proposed by Clayton and Schiffers was used.(11) This method aims to solve the problem that age, period, and cohort effects cannot simply be estimated in the same model because they are linearly dependent on each other. To address this problem, these effects are sequentially modeled and the deviation of each of the three factors from linearity is examined. More specifically, this method uses several models to split the period and cohort effects into three terms: a linear drift term, which represents the constant annual linear change in hip fracture rates that are common to both period and cohort effects, and two nonlinear terms specifically attributed to period or cohort effect. Therefore, sequential generalized linear models were fitted with a negative-binomial distribution and log link function and specific population used as the offset variable.
The first model considered the effect of age alone and did not include terms for temporal change. The second model fitted age and a drift term representing a constant annual linear change in the incidence rate attributed equally to period and birth cohort effects. Comparison of these first two models provides a test for the drift term. A third model fitted age-period effects and a fourth model fitted age-cohort effects. The latter two models were compared with the second model to test for nonlinearities that may be attributable to period or cohort effects. A final fifth model was fitted to the full age-period-cohort effects. To test the statistical significance of each factor, the deviances of each model were compared. Males and females were modeled separately. SAS software (version 9.1, SAS Institute, Cary, NC, USA) was used to perform all statistical analysis. The statistical significance level was defined as p ≤ 0.05.
Results
Descriptive analysis
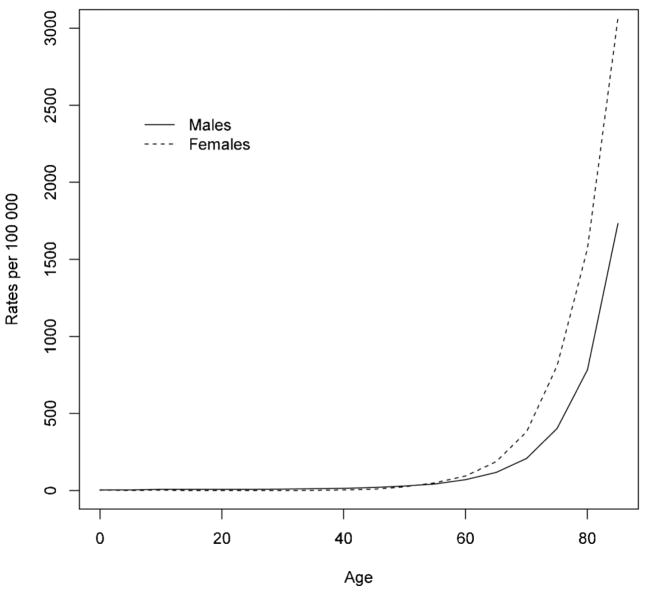
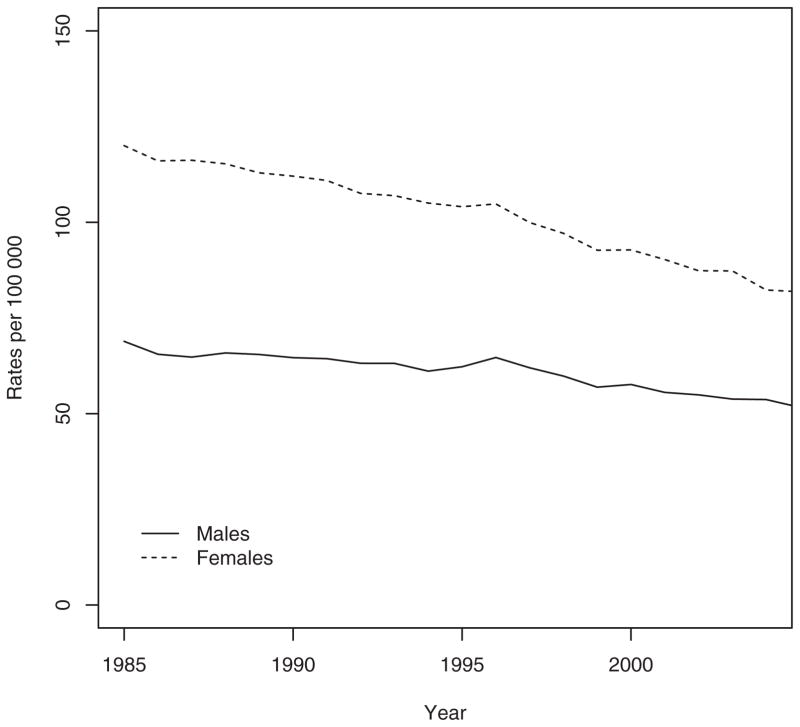
During the study period (1985 to 2005), a total of 570,872 hospitalizations for hip fracture (413,019 [72.3%] among females and 157,853 [27.7%] among males) were identified. Fig. 1 presents the sex-specific incidence rates by age. Incidence rates in females and males below age 50 years were low with an exponential increase after age 50. For females, the incidence rates per 100,000 person-years increased from 3.74 for age 0–4 years to 3066.11 for age ≥85 years. Likewise, the incidence rates per 100,000 person-years for males increased from 1.79 to 1730.09 for age 0–4 and ≥85 years, respectively. For females, age-adjusted hip fracture rates per 100,000 person-years (adjusted to the age structure of the 1991 Canadian population) decreased by 31.8% from 118.6 (95% confidence interval [CI] 115.9 to 121.4) in 1985 to 80.9 (95% CI 79.2 to 82.6) in 2005. For males, age-adjusted hip fracture rates per 100,000 person-years decreased by 25.0% from 68.2 (95% CI 65.6 to 70.8) in 1985 to 51.1 (95% CI 49.4 to 52.7) in 2005. Moreover, for males and females, Fig. 2 shows decreasing hip fracture rates with a change in slope around 1996 and greater slope reduction after 1996. This observation has been previously validated using joinpoint regression analysis that confirmed a statistical significant change in slope in 1996.(1)
Fig. 1.
Hip fracture rates by age (years) in males and females in Canada, 1985 to 2005.
Fig. 2.
Annual age-standardized rates of hip fractures per 100,000 person-years.
Period effects
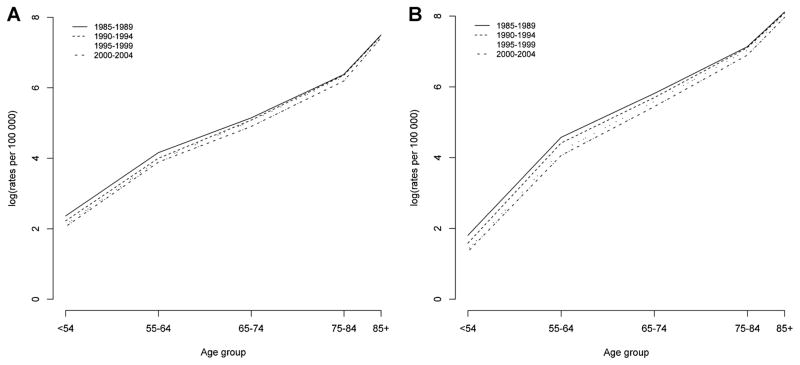
Fig. 3 shows hip fracture rates by age for males and females for four different calendar periods. Period effects are present if hip fracture rates at all age groups are higher or lower than those in other time periods. For females and males, Fig. 3 shows a decrease in hip fracture rates for each successive period across all age groups, indicating that a period effect is present.
Fig. 3.
Hip fracture rates by age in different calendar periods in (A) males and (B) females in Canada, 1985 to 2004.
Cohort effects
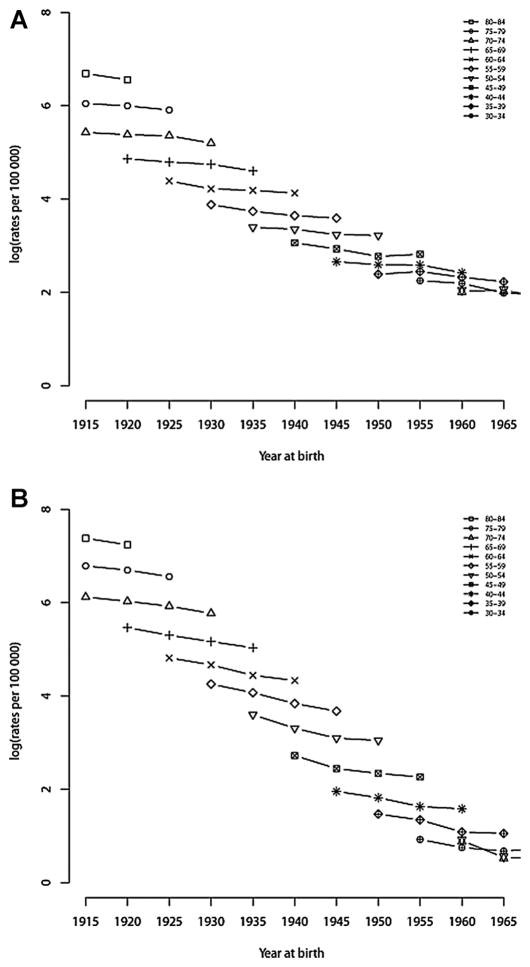
Fig. 4 shows hip fracture rates by age and birth cohort. For males and females in every 5-year age interval, hip fracture rates showed a slight decrease in each successive birth cohort, indicating that a cohort effect is present.
Fig. 4.
Hip fracture rates in successive 5-year birth cohorts at different ages in (A) males and (B) females in Canada, 1985 to 2004.
The age-period-cohort models
Table 1 shows the incidence rate ratios (IRR) adjusted for age in the period models and birth cohort models for males and females. Significant period effects were observed in both males and females (p < 0.0001). Compared with the incidence rates in 1985–1989, hip fracture rates in 2000–2004 decreased by 21% and 32% in males and females, respectively. Significant cohort effects were also observed for males and females (p < 0.0001). After controlling for age effects, cohorts born before 1950 had a higher risk for hip fracture, whereas those born after 1954 had a lower risk of hip fracture. The drift parameter, which represents the linear trend in incidence owing to either a period or a cohort effect, was significant for both sexes (p < 0.0001). For males, there was a 7% decrease in the incidence rates per 5-year period or birth cohort, whereas for females, this decrease was 12% per 5-year period or birth cohort.
Table 1.
Incidence Rate Ratios (IRR) and 95% Confidence Intervals (CI) for the Period and Cohort Effects Adjusted for Age in Canada, 1985 to 2004
| Males | Females | |
|---|---|---|
| Period | ||
| 1985–1989 | 1.0 | 1.0 |
| 1990–1994 | 0.92 (0.90–0.95) | 0.88 (0.85–0.91) |
| 1995–1999 | 0.87 (0.84–0.89) | 0.77 (0.74–0.79) |
| 2000–2004 | 0.79 (0.77–0.81) | 0.68 (0.66–0.71) |
| Birth cohort | ||
| 2000–2004 | 0.21 (0.15–0.28) | 0.15 (0.11–0.22) |
| 1995–1999 | 0.30 (0.25–0.37) | 0.25 (0.19–0.33) |
| 1990–1994 | 0.41 (0.35–0.48) | 0.31 (0.24–0.39) |
| 1985–1989 | 0.48 (0.42–0.55) | 0.40 (0.32–0.51) |
| 1980–1984 | 0.49 (0.43–0.55) | 0.45 (0.36–0.55) |
| 1975–1979 | 0.54 (0.48–0.60) | 0.52 (0.43–0.64) |
| 1970–1974 | 0.72 (0.66–0.80) | 0.64 (0.54–0.76) |
| 1965–1969 | 0.83 (0.76–0.90) | 0.67 (0.58–0.76) |
| 1960–1964 | 0.88 (0.82–0.94) | 0.76 (0.68–0.84) |
| 1955–1959 | 1.01 (0.95–1.07) | 0.87 (0.80–0.95) |
| 1950–1954 | 1.0 | 1.0 |
| 1945–1949 | 1.09 (0.97–1.15) | 1.10 (1.03–1.18) |
| 1940–1944 | 1.21 (1.14–1.29) | 1.37 (1.28–1.47) |
| 1935–1939 | 1.28 (1.19–1.36) | 1.68 (1.56–1.80) |
| 1930–1934 | 1.41 (1.32–1.52) | 1.99 (1.84–2.14) |
| 1925–1929 | 1.58 (1.46–1.70) | 2.29 (2.12–2.47) |
| 1920–1924 | 1.65 (1.53–1.78) | 2.60 (2.39–2.81) |
| 1915–1919 | 1.79 (1.65–1.94) | 2.87 (2.65–3.12) |
| 1910–1914 | 1.91 (1.75–2.07) | 3.06 (2.81–3.33) |
| 1905–1909 | 1.90 (1.73–2.08) | 3.16 (2.88–3.47) |
| Drift, IRR per 5 years | 0.93 (0.92–0.93) | 0.88 (0.87–0.89) |
Table 2 shows the results for the age-period-cohort analysis. In males and females, after adjustment for age, we observed a significant constant linear decrease (age-drift model) common to both period and cohort effects. The nonlinear birth cohort effect, after adjustment for age and drift (age-cohort model), was significant for males (p = 0.0126) but not for females (p = 0.9960). In contrast, the nonlinear period effect, after adjustment for age and drift (age-period model), was significant for females (p = 0.0373) but not for males (p = 0.2515). For males, there was no evidence of an additional period effect after adjusting for age and birth cohort. For females, there was no evidence of an additional birth cohort effect after adjusting for age and period. Therefore, the age-cohort model was preferred for males and the age-period model was preferred for females.
Table 2.
Results for the Age-Period-Cohort Effects Modeling
| Sex | Model | Deviance (df) | ΔD (Δdf) | p Value |
|---|---|---|---|---|
| Males | Age (A) | 469.3012 (361) | Ref | Ref |
| Age-drift (AD) | 461.1777 (360) | 8.1235 (1) | 0.0044 | |
| Age-period (AP) | 463.9386 (358) | 2.7609 (2) | 0.2515 | |
| Age-cohort (AC) | 401.0704 (322) | 60.1073 (38) | 0.0126 | |
| Age-period-cohort (APC) | 401.6855 (319) | 0.6151 (3) | 0.8930 | |
| Females | Age (A) | 422.3466 (361) | Ref | Ref |
| Age-drift (AD) | 409.7177 (360) | 12.6289 (1) | 0.0004 | |
| Age-period (AP) | 403.1398 (358) | 6.5779 (2) | 0.0373 | |
| Age-cohort (AC) | 390.8632 (322) | 18.8545 (38) | 0.9960 | |
| Age-period-cohort (APC) | 381.7467 (319) | 21.3931 (39) | 0.9901 |
Discussion
In this study, we investigated whether declining hip fracture rates observed in Canada(1) were explained by period effect, cohort effect, or both. Our results suggest differences between males and females. First, the annual linear decrease (drift term, common to both period and cohort effects) in hip fracture rates is greater in females (12% decrease per 5-year period) compared with males (7% decrease per 5-year period). Second, in males, we observed a significant nonlinear decrease in rates that can be explained mainly by a cohort effect, whereas in females, the nonlinear decrease can be explained mainly by a period effect. These results are consistent with our previous study,(1) where joinpoint regression analysis identified a change in linear slope around 1996 for both males and females, with a rate of decrease greater in females than in males both before and after 1996. This inflexion point accounts for the significant nonlinear decrease observed in the current study.
The observation of a nonlinear period effect is consistent with the introduction of new diagnostic and treatment approaches and introduction of preventive action over time. It is not surprising to observe a significant nonlinear period effect for females and not for males because it is well recognized that osteoporosis is underdiagnosed and undertreated in males compared with females.(2,12–14) Moreover, a recent study evaluating trends in bone mineral density (BMD) testing and bone-sparing medications in the province of Ontario, Canada, showed that BMD testing increased between 1992 and 2001 among females and males; however, testing rates were 10 times greater in females.(2) In addition, the authors reported a steady increase in antiresorptive bone-sparing medications from 1996 to 2003 in both males and females >65 years of age, but the total number of filled prescriptions was 10 times greater in females compared with males. These results are consistent with our previous study demonstrating a greater decline in hip fractures since 1996, which could be related to a more widespread use of BMD testing and treatment for osteoporosis.(1) Although there has been an increase in both sexes in BMD testing and osteoporosis treatment, the much lower frequency among males may explain why a nonlinear period effect was not detected. Therefore, in males, the effect of these factors is probably quite weak and is observed through the drift term common to both period and cohort effects. However, these factors do not explain completely the decrease observed before the widespread use of BMD testing and introduction of osteoporosis treatment. A recent report from the Oslo Health Study suggests that the reduction observed in hip fracture rates in females can be explained by a 22-fold increase in the use of hormone replacement therapy (HRT) from 1979 to 1999.(15) However, since the reported adverse effects of HRT, there has been a substantial decline in its use.(16) They also observed a significant increase in the use of bisphosphonate prescriptions, which coincided with a more marked reduction in hip fracture rates.(17) Fall-prevention programs have also been suggested as a contributing factor to explain the observed decreases in incidence of hip fracture.(5) However, there is little evidence that the introduction of fall-prevention programs is effective in reducing the number of fall-related injuries at the population level.(18) Moreover, a recent study observed that despite significant improvements in fall prevention, the rates of fall-related hospitalizations among older people are still increasing.(19) In this study, between 1998 and 2009, different trends were observed according to injury type. The rates for nonfracture-related injuries increased, whereas rates for fracture injuries declined. Therefore, it is difficult to correlate a decrease in observed hip fracture rates with fall-prevention efforts.
A healthy cohort effect reflecting changes in factors such as BMI, smoking, or activity level has also been suggested to explain the decline in hip fracture rates.(1,7) Consistent with this hypothesis, we found a decline in hip fracture rates in all successive birth cohorts for males and females. Among females, the cohort effect was only present in the linear annual decrease as measured by the drift term. However, among males, we observed an additional significant nonlinear birth cohort effect. This sex-specific difference may be related to the drift term being much weaker in males compared with females and therefore less likely to obscure a birth cohort effect. Different assumptions can also support the observation of this cohort effect. First, similar to other industrialized populations, the prevalence of obesity in Canadians has increased greatly over the last two decades, especially among men and children.(20,21) A recent meta-analysis has shown that the rates of any osteoporotic fracture including hip fracture are lower in those with higher BMI independent of age and sex.(22) Therefore, the epidemic of obesity might be contributing to the observed reduction in hip fracture rates. Second, from 1985 to 2001, the prevalence of current smoking (i.e., daily smokers and occasional smokers) declined significantly in adults aged 15 years and older with a larger decline between 1991 and 2001, and a larger decrease in males’ current smoking prevalence compared with females’ prevalence.(23) Because it is well known that smoking is a risk factor for hip fracture,(24) the decline in smoking prevalence, particularly in males, may explain, in part, the reduction in hip fracture rates. Finally, earlier age at menarche and later age of menopause have also been suggested as contributing to the reduction in osteoporotic fractures among women.(25)
To our knowledge, only a few studies have evaluated the effect of birth cohort and/or period on hip fracture rates.(6–9) Effects of birth cohort on hip fracture rates have been estimated by Samelson and colleagues and by Evens and colleagues, and, unlike our study, their results suggest that risk of hip fracture increases for each successive birth cohort.(6,8) Langley and colleagues have observed as in our study that later cohorts have lower risk of hip fracture, but in contrast to our study, the period effects showed a steady increase in risk.(7) These authors explain the decline observed in later cohorts by increasing health and improvement in physical status of older people since the last century and explain the steady increase observed in period effect by an increased survival in very frail individuals. Moreover, a recent study in Switzerland showed that the decrease observed in women was attributed to a decrease in the incidence of hip fracture among institution-dwelling elderly women.(26) Finally, similar to our study, the more recent work of Rosengren and colleagues showed that period and cohort effects are more marked among women than men, with a significant reduction in hip fracture incidence in each subsequent birth cohort or period.(9)
The first strength of this study is the population-based data representing the Canadian population, thereby eliminating selection biases related to sample-based studies. Second, this study used discharge diagnoses to identify hip fractures, and a previous study has reported a very high coding reliability of hip fractures.(27) However, our study also has some potential limitations. In the hospital discharge data set used, we were not able to differentiate across all years of the study a second fracture from interhospital transfer; therefore, some duplication of cases is possible. Moreover, during the study period, changes in hospital diagnosis coding from ICD-9-CM to ICD-10-CA and the dates of implementation differed among the different provinces, but this change does not explain the decrease in hip fracture rates present before any changes in coding classification occurred. Another limitation of this study is the lack of clinical information that could be implicated in the change of hip fracture risks. Unfortunately, information on residential/institutional status, BMD testing, or treatments was not available in our data set, limiting the assessment of the effect of these factors.
In conclusion, although hip fracture rates have decreased in males and females over the years in Canada, a combination of factors may be contributing to these changes. In recent years, there have been more widespread preventive measures, diagnosis, and treatment for osteoporosis. In addition to the linear decrease in rates common to both period and cohort effects, there is evidence of significant period effects in females and birth cohort effects in males. However, the causal factors underlying these changes remain uncertain and further study is required to identify possible causes behind these changes.
Acknowledgments
We acknowledge members of the Osteoporosis Surveillance Expert Working Group for their contribution to the study. This research was supported by Public Health Agency of Canada.
The role of the Osteoporosis Surveillance Expert Working Group is to provide expert advice and guidance on indicators, data sources, and approaches to national osteoporosis surveillance in Canada to the Public Health Agency of Canada. The members include: Jacques Brown, MD, Laval University, Quebec City, Quebec, Canada; Ann Cranney, MD, MSc, University of Ottawa, Ottawa, Ontario, Canada; David A Hanley, MD, University of Calgary, Calgary, Alberta, Canada; Susan Jaglal, PhD, University of Toronto, Toronto, Ontario, Canada; Sonia Jean, PhD, Institut National de Santé Publique du Québec, Quebec City, Quebec, Canada; Famida Jiwa, MHSC, DC, Osteoporosis Canada, Toronto, Ontario, Canada; Stephanie Kaiser, MD, Dalhousie University, Halifax, Nova Scotia, Canada; David L Kendler, MD, Prohealth Clinical Research Centre, Vancouver, British Columbia, Canada; William D Leslie, MD, MSc, University of Manitoba, Winnipeg, Canada; Suzanne Morin, MD, MSc, McGill University, Montreal, Quebec, Canada; Alexandra Papaioannou, MD, MSc, McMaster University, Hamilton, Ontario, Canada; and Kerry Siminoski, MD, University of Alberta, Edmonton, Alberta, Canada.
Authors’ roles: Study design: SJ, SO, and WL; study conduct: SJ; acquisition of data: SO, CL, PW, CB, and WL; data analysis: SJ and WL; data interpretation: SJ, SO, CL, PW, CB, JB, SM, AP, SJ, and WL; drafting the manuscript: SJ, SO, and WD; revising manuscript and approving the final version of the manuscript: all authors. The analyses and conclusions in this report reflect the opinions of individual experts and not their affiliated organizations.
Footnotes
Disclosures
All authors state that they have no conflicts of interest. All authors had no restrictions on access to raw data or statistical analyses.
References
- 1.Leslie WD, O’Donnell S, Jean S, Lagacé C, Walsh P, Bancej C, Morin S, Hanley DA, Papaioannou A. Trends in hip fracture rates in Canada. JAMA. 2009;302(8):883–9. doi: 10.1001/jama.2009.1231. [DOI] [PubMed] [Google Scholar]
- 2.Jaglal SB, Weller I, Mamdani M, Hawker G, Kreder H, Jaakkimainen L, Adachi JD. Population trends in BMD testing, treatment, and hip fracture rates: are hip fracture projections wrong? J Bone Miner Res. 2005;20(6):898–05. doi: 10.1359/JBMR.041231. [DOI] [PubMed] [Google Scholar]
- 3.Lefaivre KA, Levy AR, Sobolev B, Cheng SY, Kuramoto L, Guy P. Changes in first hip fracture rates in British Columbia Canada, 1990–2004. Osteoporos Int. 2011;22(11):2817–27. doi: 10.1007/s00198-010-1488-7. [DOI] [PubMed] [Google Scholar]
- 4.Bergström U, Jonsson H, Gustafson Y, Pettersson U, Stenlund H, Svensson O. The hip fracture incidence curve is shifting to the right. Acta Orthopaedica. 2009;80(5):520–4. doi: 10.3109/17453670903278282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chevalley T, Guilley E, Herrmann FR, Hoffmeyer P, Rapin CH, Rizzoli R. Incidence of hip fracture over a 10-year period (1991–2000): reversal of a secular trend. Bone. 2007;40(5):1284–9. doi: 10.1016/j.bone.2006.12.063. [DOI] [PubMed] [Google Scholar]
- 6.Samelson EJ, Zhang Y, Kiel DP, Hannan MT, Felson DT. Effect of birth cohort on risk of hip fracture: age-specific incidence rates in the Framingham Study. Am J Public Health. 2002;92(5):858–62. doi: 10.2105/ajph.92.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley J, Samaranayaka A, Davie G, Campbell AJ. Age, cohort and period effects on hip fracture incidence: analysis and predictions from New Zealand data 1974–2007. Osteoporos Int. 2011;22(1):105–11. doi: 10.1007/s00198-010-1205-6. [DOI] [PubMed] [Google Scholar]
- 8.Evans JG, Seagroatt V, Goldacre MJ. Secular trends in proximal femoral fracture, Oxford record linkage study area and England 1968–86. J Epidemiol Community Health. 1997;51:424–9. doi: 10.1136/jech.51.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosengren BE, Ahlborg HG, Mellström D, Nilsson JA, Björk J, Karlsson MK. Secular trends in Swedish hip fractures 1987–2002: birth cohort and period effects. Epidemiology. 2012;23(4):623–30. doi: 10.1097/EDE.0b013e318256982a. [DOI] [PubMed] [Google Scholar]
- 10.Richards J, Brown A, Homan C. Proceedings of Statistics Canada Symposium 2001: achieving data quality in a statistical agency [Internet] Ottawa: Statistics Canada; The data quality study of the Canadian discharge abstract database: a methodological perspective. [cited 2009 Jun 6]. Available from: http://secure.cihi.ca/cihiweb/en/downloads/quality_dadconfpaper_e.pdf. [Google Scholar]
- 11.Clayton D, Schiffers E. Models for temporal variation in cancer rates II: age-period-cohort models. Stat Med. 1987;6(4):469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 12.Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006;35(5):293–305. doi: 10.1016/j.semarthrit.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Papaioannou A, Kennedy CC, Ioannidis G, Gao Y, Sawka AM, Goltzman D, Tenenhouse A, Pickard L, Olsynski WP, Davison KS, Kaiser S, Josse RG, Kreiger N, Hanley DA, Prior JC, Brown JP, Anastassiades T, Adachi JD CaMos Research Group. The osteoporosis care gap in men with fragility fractures: the Canadian Multicentre Osteoporosis Study. Osteoporos Int. 2008;19(4):581–7. doi: 10.1007/s00198-007-0483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldstein AC, Nichols G, Orwoll E, Elmer PJ, Smith DH, Herson M, Aickin M. The near absence of osteoporosis treatment in older men with fractures. Osteoporos Int. 2005;16(8):953–62. doi: 10.1007/s00198-005-1950-0. [DOI] [PubMed] [Google Scholar]
- 15.Meyer HE, Lofthus CM, Sogaard AJ, Falch JA. Change in the use of hormone replacement therapy and the incidence of fracture in Oslo. Osteoporos Int. 2009;20:827–30. doi: 10.1007/s00198-008-0679-y. [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Jonhson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Fisher AA, O’Brien ED, Davis MW. Trends in hip fracture epidemiology in Australia: possible impact of bisphosphonates and hormone replacement therapy. Bone. 2009;45(2):246–53. doi: 10.1016/j.bone.2009.04.244. [DOI] [PubMed] [Google Scholar]
- 18.Gates S, Fisher JD, Cooke MW, Carter YH, Lamb SE. Multifactorial assessment and targeted intervention for preventing falls and injuries among older people in community and emergency care settings: systematic review and meta-analysis. BMJ. 2008;336(7636):130–3. doi: 10.1136/bmj.39412.525243.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson WL, Mitchell R. Conflicting trends in fall-related injury hospitalizations among older people: variations by injury type. Osteoporos Int. 2011;22(10):2623–31. doi: 10.1007/s00198-010-1511-z. [DOI] [PubMed] [Google Scholar]
- 20.Katzmarzyk PT. The Canadian obesity epidemic, 1985–1998. CMAJ. 2002;166(8):1039–40. [PMC free article] [PubMed] [Google Scholar]
- 21.Tremblay MS, Katzmarzyk PT, Willms JD. Temporal trends in overweight and obesity in Canada, 1981–1996. Int J Obes Relat Metab Disord. 2002;26(4):538–43. [PubMed] [Google Scholar]
- 22.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, Eisman JA, Kroger H, Fujiwara S, Garnero P, McCloskey EV, Mellstrom D, Melton LJ, 3rd, Meunier PJ, Pols HA, Reeve J, Silman A, Tenenhouse A. Body mass index as a predictor of fracture risk: a meta analysis. Osteoporos Int. 2005;16(2):1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 23.Gilmore J. Report on smoking in Canada, 1985 to 2001 (catalogue 82F0077XIE) Ottawa: Statistics Canada; 2002. [Google Scholar]
- 24.Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–62. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 25.Icks A, Haastert B, Wildmer M, Becker C, Meyer G. Trends of hip fracture incidence in Germany 1995–2004: a population-based study. Osteoporos Int. 2008;19:1139–45. doi: 10.1007/s00198-007-0534-6. [DOI] [PubMed] [Google Scholar]
- 26.Guilley E, Chevalley T, Herrmann F, Baccino D, Hoffmeyer P, Rapin C-H, Rizzoli R. Reversal of the hip fracture secular trend is related to a decrease in the incidence in institution-dwelling elderly women. Osteoporos Int. 2008;19(12):1741–7. doi: 10.1007/s00198-008-0610-6. [DOI] [PubMed] [Google Scholar]
- 27.Levy AR, Mayo NE, Grimard G. Rates of transcervical and pertrochanteric hip fractures in province of Quebec, Canada 1981–1992. Am J Epidemiol. 1995;142(4):428–36. doi: 10.1093/oxfordjournals.aje.a117651. [DOI] [PubMed] [Google Scholar]