Abstract
Purpose
The purpose of this study was to determine whether trabecular bone mineralization differed in adults with type 2 diabetes compared to adults without type 2 diabetes.
Methods
Proximal femur specimens were obtained following a total hip replacement procedure from men and women ≥65 years of age with and without type 2 diabetes. A scanning electron microscope was used for quantitative backscattered electron imaging (qBEI) analysis of trabecular bone samples from the femoral neck. Gray scale images (pixel size=5.6 μm2) were uploaded to ImageJ software and gray level (GL) values were converted to calcium concentrations (weight [wt] % calcium [Ca]) using data obtained with energy dispersive X-ray spectrometry. The following bone mineralization density distribution (BMDD) outcomes were collected: the weighted mean bone calcium concentration (CaMEAN), the most frequently occurring bone calcium concentration (CaPEAK) and mineralization heterogeneity (CaWIDTH). Differences between groups were assessed using the Student’s t-test for normally distributed data and Mann–Whitney U-test for non-normally distributed data. An alpha value of <0.05 was considered significant.
Results
Thirty-five Caucasian participants were recruited (mean [standard deviation, SD] age, 75.5 [6.5] years): 14 adults with type 2 diabetes (years since type 2 diabetes diagnosis, 13.5 [7.4] years) and 21 adults without type 2 diabetes. In the adults with type 2 diabetes, bone CaMEAN was 4.9% greater (20.36 [0.98] wt.% Ca versus 19.40 [1.07] wt.% Ca, p=0.015) and CaWIDTH was 9.4% lower (median [interquartile range] 3.55 [2.99–4.12] wt.% Ca versus 3.95 [0.71] wt.% Ca, p<0.001) compared to controls. There was no between-group difference in CaPEAK (21.12 [0.97] wt.% Ca for type 2 diabetes versus 20.44 [1.30] wt.% Ca for controls, p=0.121).
Conclusion
The combination of elevated mean calcium concentration in bone and lower mineralization heterogeneity in adults with type 2 diabetes may have deleterious effects on the biomechanical properties of bone. These microscopic alterations in bone mineralization, which may be mediated by suppressed bone remodeling, further elucidate higher fracture risk in adults with type 2 diabetes.
Keywords: Bone mineralization density distribution (BMDD), Type 2 diabetes, Osteoporosis Quantitative backscattered electron imaging, Bone quality
Introduction
Adults with type 2 diabetes have an elevated risk of hip fracture compared to those without diabetes [1,2] despite higher bone mineral density (BMD) [3]. When used in fracture risk prediction models, dual X-ray absorptiometry (DXA)-derived BMD measurements improve fracture discrimination in non-diabetic populations compared to not using BMD [4,5]. However, fracture risk prediction models incorporating BMD for adults with type 2 diabetes could be potentially misleading; there is evidence that the risk of fracture is increased in adults with type 2 diabetes even after controlling for BMD and other risk factors [6]. Areal BMD can be confounded by bone size [7], degenerative changes in the spine [8], and overlying fat [9]. Therefore using BMD as a measure of bone health in adults with type 2 diabetes may not be predictive of fracture in this group. Although BMD does contribute to bone strength, fracture susceptibility is also influenced by microscopic tissue qualities such as bone mineralization, which may play a greater role in bone strength in those with type 2 diabetes.
The bone remodeling process dictates bone mineralization. Suppressed bone remodeling allows the progression of secondary mineralization and mineral maturation [10,11]. Histomorphometric measures of bone remodeling are strongly correlated with the degree of bone mineralization [12,13]. In adults with type 2 diabetes, bone remodeling is suppressed compared to their non-diabetic counterparts [14–19], which could lead to delayed removal of bone packets, elevated bone mineralization and a reduction in mineralization heterogeneity.
Quantitative backscattered electron imaging (qBEI) is used for quantifying local mineralization variations in bone [20–22]. The qBEI signal strength, reflected in gray level steps of an image is dependent on the elements present in a specimen [23]. Therefore this method is useful for distinguishing between samples with differing average atomic number [24]. When applied to bone samples, gray level is linearly related to calcium concentration (expressed as weight [wt] percent calcium [Ca], wt.% Ca), where brighter regions of gray scale images of bone represent higher mineral concentration and darker regions represent lower mineral concentration [22,25–27]. This local variation in mineralization has been described as the bone mineralization density distribution (BMDD), from which the following key outcomes are derived: weighted mean calcium concentration (CaMEAN), most frequently occurring calcium concentration (CaPEAK), and the mineralization heterogeneity (CaWIDTH) [28]. Variations in these BMDD variables in bone samples from populations with elevated fracture risk have been reported, suggesting the utility of this technique in quantifying meaningful variability in bone mineralization [12,29–32]. Furthermore, the use of qBEI to quantify mineral content in bone samples is both valid [25,27] and reproducible [28].
The objective of this ex-vivo study was to determine whether bone mineralization assessed by qBEI is different in excised femoral neck trabecular bone samples from adults with type 2 diabetes compared to controls without diabetes. We hypothesized that CaMEAN and CaPEAK would be greater and CaWIDTH would be lower in adults with type 2 diabetes compared to controls without diabetes.
Materials and methods
Study participants
Participants were recruited from Hamilton Health Sciences Orthopedic Program at Juravinski Hospital in Hamilton, Canada to make up the convenience sample for this ex-vivo study. Recruitment occurred between August 2010 and December 2011, and groups were divided based on a diagnosis of type 2 diabetes [33]. Participants were not matched based on age and gender as previous research suggests that these factors do not influence BMDD outcomes [34]. Eligible participants were men and women who were undergoing total hip replacement due to osteoarthritis and were ≥65 years of age at the time of surgery. Total hip replacement procedure is indicated for patients who have severe radiographic joint degeneration and have not had adequate pain relief and improvement in function from non-pharmacologic and pharmacologic treatments [35]. Potential participants were excluded from the study if they: 1) were currently taking or had taken osteoporosis-related medication (bisphosphonates, hormone therapy, selective estrogen receptor modulator, calcitonin, parathyroid hormone or denosumab) in the past 24 months; 2) had a history of metastatic cancer in the past 10 years 3); were currently taking systemic glucocorticoids for 3 months at a dose of >2.5 mg/day; or 4) had a diagnosis of severe renal disease (creatinine clearance<30 mL/min) [36], hyperparathyroidism, hypoparathyroidism, Paget’s disease, Cushing’s Syndrome, or osteogenesis imperfecta. The study protocol was approved by the McMaster University Faculty of Health Sciences/Hamilton Health Sciences Research Ethics Board.
Descriptive data
The following information was collected for descriptive purposes: demographics, use of walking aids, history of an osteoporotic fracture (i.e., non-vertebral or vertebral non-traumatic fracture [37]), number of years since menopause, smoking status, presence of diseases included in the Charlson Index [38], and number of years since type 2 diabetes diagnosis. Chart abstraction was performed to collect diabetes-related medication, daily calcium and vitamin D supplement intake (including multivitamins) and non-steroidal anti-inflammatory drug (NSAID) intake. NSAIDs captured in this study included: celecoxib, diclofenac, difunisal, etodolac, fenoprofen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, mefenamic acid, meloxicam, nabumetone, naproxen, oxaprozin, piroxicam, sulindac and tolmetin. Medication and supplement intakes were verified in the pre-operative participant interview. The Physical Activity Scale for the Elderly (PASE) was administered to estimate participation in activities over the past 7 days [39]. Participants’ height and weight were obtained from pre-operative consultation notes and BMI (kg/m2) was calculated. Clinic staff ordered pre-operative random glucose and albumin, and post-operative creatinine. The Cockcroft-Gault equation was used to calculate creatinine clearance [36].
Sample preparation
Immediately following surgical excision, each proximal femur was wrapped in saline soaked gauze for transportation. A 5 mm thick sagittal section of the femoral neck was cut at the most distal end of the sample using a handsaw at an orientation expected to be approximately perpendicular to the Haversian canals in the cortical bone (Fig. 1). Conventional methods were used for preparing the samples scanning electron microscopy [40]. The anterior section was fixed in 0.2 M glutaraldehyde (2% v/v) in 0.1 M sodium cacodylate buffer pH 7.4 [41]. Following fixation for 24 h, samples were degreased with a series of methanol and chloroform washes and agitated for 30 min in an ultrasonic bath (Branson Ultrasonic Cleaner, Emerson Industrial Automation, St. Louis, MO, USA) to remove excess marrow. The samples were dehydrated in 70%, 80%, 90%, 96% and 100% ethanol washes [40] and dried at 60 °C for 4 h.
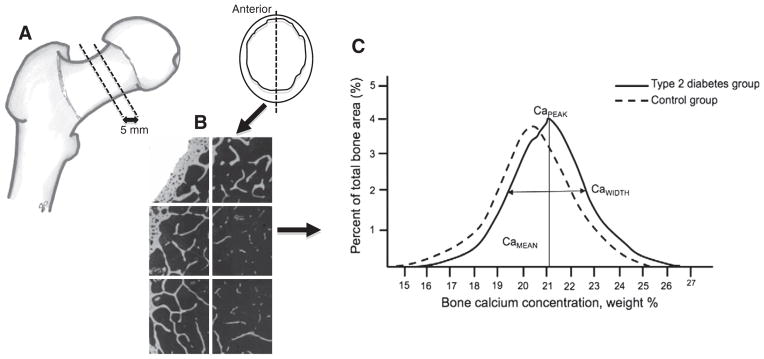
Fig. 1.
A. Schematic diagram representing a proximal femur and a typical section of femoral neck used for qBEI (anterior section). B. Example of the frames captured of a bone sample using qBEI and BMDD outcomes. C. Schematic of a BMDD histogram and outcome measurements for adults with type 2 diabetes and controls, including the weighted mean calcium concentration (CaMEAN), the most frequently occurring calcium concentration (CaPEAK) and full width at half maximum of the histogram peak reflecting mineralization heterogeneity (CaWIDTH).
Quantitative Backscattered Electron Imaging (qBEI)
The anterior section was embedded in resin (15 parts EpoFix Resin and 2 parts Epoar EpoFix Hardener, Struers Ltd. Mississauga, ON, Canada) using evacuation (Struers Epovac, Struers Ltd. Mississauga, ON, Canada). Blocks with planoparallel surfaces were carefully prepared using 180 grit silicon carbide paper on a rotary wheel and a sequence of lapping disks (30 μm, 9 μm, 3 μm, 1 μm aluminum oxide particle size) (Allied High Tech Products Rancho Dominguez, CA, USA) with an aqueous suspension of 0.5 μm colloidal silica. Samples were rinsed with distilled water to remove residual colloidal silica and viewed under a dissecting microscope to ensure that there were no topographical artifacts [42]. Samples were sputter-coated (Precision Etching Coating System, Model 682, Gatan Inc. Warrendale, PA, USA) with amorphous carbon, outlined with silver paint (High Purity Silver Paint, SPI Supplies, Structure Probe Inc. Westchester, PA, USA) and mounted on a stub using carbon conductive tape. The thickness of each sample was measured using a digital micrometer (Digital Absolute Micrometer, Mitutoyo Canada Mississauga, ON, Canada).
A scanning electron microscope (SEM, Vega II LSU, Tescan USA Inc. Cranberry Township, PA, USA) equipped with a tungsten filament and an annular mono-crystal scintillator backscattered electron detector was used for qBEI. The electron beam accelerating voltage was 20 kV and probe current 110 pA. The emission current, which was set in a Faraday cup at the beginning of each imaging session, was checked at the end of each imaging session to ensure that major fluctuation (>1 pA) did not occur [28]. A working distance of 15 mm was kept constant by adjusting the stage based on the thickness of the standards and each bone sample (mean [standard deviation, SD] thickness=3.73 [0.41] mm). At a magnification of 50× and a scan speed of 48.6 μs/pixel, 1024×1024 qBEI grayscale images were generated with a pixel size of 5.6 μm2. The detector contrast (gain) and brightness (black) were set to 49.5% and 89.0%, respectively.
Given that qBEI intensity and gray level are proportional to average atomic number of a material, we scanned standards of known average atomic number (Z) (Carbon [C], Z=6; magnesium oxide [MgO], Z=10.41; aluminum [Al], Z=13) before and after scanning each bone sample for standardization purposes. If the difference between the gray level values of the standard scanned before and after the bone sample was greater than 4 gray level steps (intra-assay technical variance of 0.27%) [28], the data from that imaging session were removed from the analysis. The entire area of the bone sample was captured in separate frames (5.7 mm2), with the number of frames captured being dependent on bone sample size (Fig. 1). An average of 5 frames per sample were captured, to follow the methods used in other studies [12].
Image analysis
Images of the standards and bone samples were uploaded to ImageJ (version 1.44o, National Institutes of Health, Bethesda, MD, USA) [24,28]. The gray scale images were composed of pixels with values between 0 and 255, where gray level=0 corresponded to a pixel with zero backscattered electron intensity (black) and gray level=255 corresponded to a pixel with full backscattered electron intensity (white). Using the ‘rectangle’ selection tool, a consistent location was selected for gray level analysis for C, MgO and Al. For analysis of bone samples, a standard threshold level, GLt, was used for image sets to differentiate between bone and marrow space, and the pixels associated with the marrow space were excluded from analysis. This standard threshold was set at a gray level of 72 (average atomic number=7.74) and was selected using the Otsu method for binarizing the images [43]. The same standard threshold was used for analysis of all bone samples. The maximum number of selections of trabecular bone was made in each frame using the ‘wand’ and ‘polygon’ selection tools, with the exception of areas containing artifact (i.e., “bone dust” from the preparation process or organic matter). Cortical bone analysis was excluded from this study because cortical bone is composed of osteons, which undergo successive stages of Haversian remodeling [44]. Because of the relatively slow rate of cortical remodeling compared to hemiosteonal remodeling of trabecular bone [45], the mineralization of cortical osteons may not entirely reflective of altered bone metabolism in adults with type 2 diabetes. That is, a significant fraction of cortical osteons could have formed and not yet been replaced prior to the onset or during the first years of diabetes. In addition, microcracks, and not bone remodeling kinetics, are believed to be the strongest stimulus for bone resorption in cortical bone [46,47]. Therefore, we focused on the more rapidly remodeling trabecular bone, which is more likely to reflect bone mineralization as a result of type 2 diabetes.
Gray level frequency histograms were exported to Microsoft Excel (Microsoft Excel for Mac 2011, version 14.1.3, Microsoft Canada Co. Mississauga, ON, Canada) where the weighted mean gray level (WMGL) was determined according to the formula [24,48,49]:
| (1) |
where Ai is the number of pixels with the ith gray level value, GLi is the ith gray level>GLt, and At is the total number of pixels of bone. In addition, the gray level values for the peak (mode) of the distribution and the full width at half maximum (FWHM) were determined [28] (Fig. 1). A researcher who was blinded to group allocation completed image analyses.
Gray level standardization
The standards were used to graph the relationship between average atomic number and gray level, similar to the methods described by others [24,28,48]. For each imaging session of a bone sample, a standard trend-line (Li) was generated, and average y-intercept (b) and slope (m) were calculated for all sessions, yielding an average standard trend-line (Lμ) (y=0.031x+5.505, R2 =0.99). Using a linear transformation, gray level values of bone were standardized in order for gray level values from independent imaging sessions to be compared. The following formulae was used:
| (2) |
where GLstd is the standardized gray level value for the bone used in analysis; GLi is the non-standardized gray level for the bone; mi and bi are the slope and y-intercept, respectively of Li; and mμ and bμ are the slope and y-intercept, respectively of Lμ.
Conversion of gray levels to calcium concentration
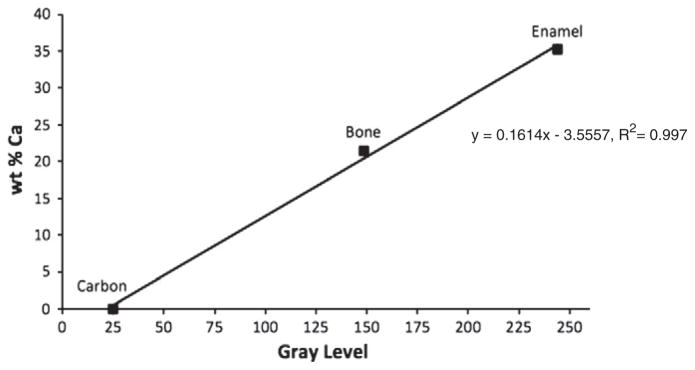
The WMGL, peak and FWHM gray level values were converted to bone sample calcium (Ca) concentration, expressed as wt.% Ca [26–28]. Using the qBEI parameters and image analysis protocol described above, we determined the mean gray level of 10 points on a homogeneous area of tooth enamel (36.1 wt.% Ca [50]) and carbon (0 wt.% Ca). We also randomly selected a bone sample and determined the mean gray level of 10 points within a uniform area of trabecular bone. Immediately following qBEI, energy dispersive x-ray spectrometry (INCA X-max microanalysis system, Oxford Instruments, Tubney Woods, Abingdon, Oxfordshire, UK) was used to determine the wt.% Ca of the points analyzed with qBEI. The probe current and working distance remained at 20 kV and 15 mm, respectively as these are the optimal settings for the x-ray detector, and magnification was set at 300×. To quantify the wt.% Ca in each sample, a pure sample of calcite (CaCO3) was used as a reference standard. Differences in matrix effects between calcite and bone are corrected for by ZAF software. Calcite is widely used as a Ca standard in X-ray analysis [49]. The mean GL value and wt.% Ca were plotted against each other (Fig. 2).
Fig. 2.
Standardization of gray level values to calcium concentrations (wt % Ca) based on the linear relationship between GL and wt.% Ca.
Using the linear relationship (y=0.1614x−3.5557, R2=0.997), the WMGL, mode gray level and FWHM gray level values were converted to wt.% Ca. To assess the reproducibility of the qBEI and image analysis technique, the standards and one bone sample were scanned on 8 different days. The coefficient of variation (CV%) for the qBEI technique and image analysis was 1.8% for CaMEAN, 1.6% for CaPEAK, and 3.6% for CaWIDTH.
Statistical analysis
Normality of the data was tested using the Kolmogorov–Smirnov test [51]. Data are presented as mean (SD) for normally distributed data, and median (interquartile range) for non-normally distributed data. Data for CaMEAN, CaPEAK, and CaWIDTH were compared between the group of adults with type 2 diabetes and the control group without type 2 diabetes using an independent Student’s t-test for normally distributed data, and Mann–Whitney U-test for non-normally distributed data. The sample size was estimated using data on bone mineralization in patients with mild primary hyperparathyroidism [12]. As CaMEAN is the primary outcome for the present study, the sample size was based on the data for this variable. The difference in CaMEAN between adults with and without mild primary hyperparathyroidism was 0.57 wt.% Ca with an average standard deviation of 0.53 wt.% Ca [12]. Using a power of 80% and an alpha level of 0.05, the estimated sample size was 14 participants per group. Analyses were completed using SPSS version 20.0 for Macintosh (IBM Corporation. Markham, ON, Canada). The criterion for statistical significance was set at alpha level <0.05.
Results
The study participants included 14 adults with type 2 diabetes and 21 adults without diabetes (Table 1). The mean (SD) age of all participants was 75.5 (6.5) years. All study participants were Caucasian, and the average time since menopause for the female participants was 30 (7) years. The majority of participants with and without type 2 diabetes used a walking aid (9/14 [64.3%] and 13/21 [61.9%] respectively, p=0.736) and had similar physical activity levels according to the PASE questionnaire results (Table 1). The average time since diagnosis of type 2 diabetes was 13.5 (7.4) years. In the type 2 diabetes group, three participants (3/14 [21.4%]) were diet-controlled, 7 participants (7/14 [50.0%]) were taking a biguanide or insulin secretagogue sulfonylurea, and 4 participants (4/14 [28.6%]) were on insulin therapy.
Table 1.
Descriptive characteristics of participants enrolled in the study.
| Participants with type 2 diabetes
|
Control
|
Difference between groups
|
|
|---|---|---|---|
| n=14 | n=21 | p-value | |
| Age, years | 73.8 (6.0) | 76.4 (6.8) | 0.245 |
| Female, n (%) | 6 (42.9) | 14 (66.7) | 0.221 |
| History of non-traumatic osteoporotic fracturea, n (%) | 3 (21.4) | 3 (14.3) | 0.541 |
| BMI, kg/mb | 30.7 (7.4) | 28.9 (5.4) | 0.462 |
| Time since menopauseb, years | 27.6 (5.8) | 31.0 (7.2) | 0.348 |
| Current smoker, n (%) | 2 (14.3) | 3 (14.3) | 0.956 |
| Number of prescribed medications | 5.8 (2.9) | 5.1 (1.6) | 0.376 |
| Age-adjusted Charlson Index | 4.4 (0.8) | 2.2 (2.4) | <0.001 |
| Calcium intake from supplementsc, mg/day | 463 (560) | 343 (462) | 0.511 |
| Vitamin D3 intake from supplementsc, IU/day | 785 (1224) | 559 (651) | 0.547 |
| Currently prescribed NSAID, n (%) | 5 (35.7) | 6 (28.6) | 0.287 |
| PASE score | 85.6 (37.8) | 82.3 (82.7) | 0.884 |
| Serum biochemistry | |||
| Random glucose, mmol/L | 8.3 (2.1) | 5.6 (1.1) | <0.001 |
| Creatinine, μmol/L | 75.6 (20.4) | 75.4 (19.9) | 0.978 |
| Cockcroft-gault value, mL/min | 96.2 (31.4) | 86.2 (24.4) | 0.319 |
| Albumin, g/L | 32.9 (3.5) | 33.9 (4.1) | 0.562 |
Values are mean (SD), unless indicated otherwise.
Abbreviations: BMI, body mass index; NSAID, non-steroidal anti-inflammatory drug; PASE, Physical Activity Scale for the Elderly; IU, international units.
Non-traumatic osteoporotic fracture includes hip, wrist, spine or proximal humerus fracture occurring after age 40.
Number of years since menopause reported from female participants only.
Including amount from multivitamin, if applicable.
Bone mineralization: qBEI
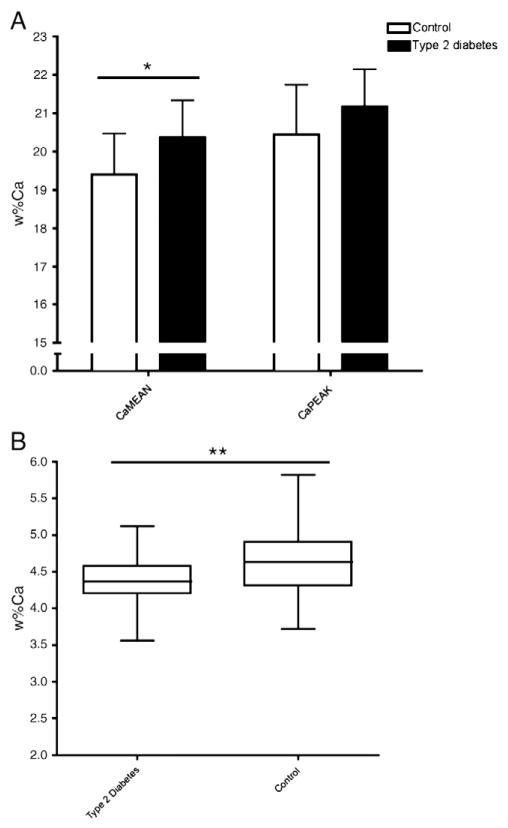
After removing the invalid data sets,13 samples from adults with type 2 diabetes and 19 samples from adults without diabetes were analyzed. Between-group comparison of the bone mineralization outcomes, shown in Fig. 3, revealed that CaMEAN was 4.9% greater in the group of adults with type 2 diabetes (20.36 [0.98] wt.% Ca versus 19.40 [1.07] wt.% Ca, p=0.015). CaWIDTH, which reflects the mineralization heterogeneity, was 9.4% lower in adults with type 2 diabetes compared to controls (3.55 [2.99–4.12] wt.% Ca versus 3.95 [0.71] wt.% Ca, p<0.001). There was no difference in CaPEAK between groups (21.12 [0.97] wt.% Ca for type 2 diabetes versus 20.44 [1.30] wt.% Ca for controls, p=0.121).
Fig. 3.
Comparison of CaMEAN, CaPEAK, (A) and CaWIDTH (B) between adults with type 2 diabetes and adults without type 2 diabetes.
Between-group difference, * p<0.05, ** p<0.001.
Abbreviations: Ca, calcium; wt.%Ca, calcium concentration; weighted mean calcium concentration (CaMEAN); the most frequently occurring calcium concentration (CaPEAK); full width at half maximum of the histogram peak reflecting mineralization heterogeneity (CaWIDTH).
Discussion
Our key findings were that the mean calcium concentration was higher and mineralization less heterogeneous in femoral neck trabecular bone samples from adults with type 2 diabetes compared to controls without diabetes. Given that these bone qualities can influence bone strength, our findings may provide some insight into increased bone fragility that has been observed in adults with type 2 diabetes.
Elevated bone mineralization in adults with type 2 diabetes may result in bone brittleness, lower bending strength, reduced fracture toughness and poor energy absorption [52–57]. Microcracks are also more likely to form in highly mineralized cortical bone samples from the femoral neck [58] and in highly mineralized regions of trabecular bone in samples from patients with a history of an osteoporotic fracture [54]. The potential mechanisms causing elevated bone calcium concentration in adults with type 2 diabetes are suppressed bone remodeling and/or accumulation of advanced glycation end-products in the bone. When bone remodeling is reduced, the secondary mineralization phase is lengthened. This causes an increase in mineral crystal size and mineral content, which may explain our finding of elevated bone calcium concentration in adults with type 2 diabetes [59,60]. Advanced glycation end-products formed by the Maillard reaction [61] are found in the urine and serum of adults with type 2 diabetes [62,63] and can bind to the nitrogen sites of type 1 collagen in bone. The binding of advanced glycation end-products increases the number of carboxyl groups on the surface of the collagen fibrils, which serve as nucleation sites for hydroxyapatite formation [64]. Regardless of the mechanism causing elevated mineralization in bone specimens from adults with type 2 diabetes, an imbalance in mineral and collagen in bone could lead to a less ductile and more brittle material that requires less energy to fracture [56].
Suppressed bone remodeling may also explain the finding of reduced mineralization heterogeneity in adults with type 2 diabetes. Suppressed bone remodeling leads to a greater amount of time between bone packet resorption and formation, resulting in a more uniform bone material comprised of bone packets of a similar calcium concentration [65]. The dependence of bone calcium content and mineralization heterogeneity on the rate of bone remodeling have been shown in patients who experience an increase in bone remodeling with teriparatide (human parathyroid hormone [1–34]) [66] and in patients who experience a reduction in bone remodeling with bisphosphonates [30,67]. In postmenopausal women treated with teriparatide for one year, a 10% increase in mineralization heterogeneity is observed due to the formation of new bone packets [66]. In bisphosphonate treated patients, an increase of approximately 3–5% in mean bone calcium concentration occurs concomitantly with a 20–30% reduction in mineralization heterogeneity, caused by the antiresorptive action of bisphosphonates [67,68]. Of note, increases in bone volume and improvements in bone microarchitecture often accompany changes in bone mineralization in bisphosphonate-treated patients, which may also contribute to the fracture prevention efficacy of bisphosphonates [67,69]. Whether mineralization heterogeneity is important to overall bone fragility is not clear. One study reported no impact of mineralization heterogeneity on bone mechanical properties [70], while others suggest that a more heterogeneously mineralized tissue results in better defense against microcrack propagation [71]. In adults with type 2 diabetes, it may be that bone calcium concentration is elevated to a point that is pathological to bone health (i.e., higher than bisphosphonate-elevated bone calcium concentration). The contribution of reduced mineralization heterogeneity to bone fragility is not clear and requires further research.
Our findings in humans are in contrast to what has been observed in in a rodent model of type 2 diabetes [72]. Hamann and colleagues reported no diabetes-attributable difference in mean calcium concentration in distal femur bone specimens, but did report an elevation in mineralization heterogeneity the rodent model of type 2 diabetes compared to wildtype [34]. However, the skeletal phenotypes of type 2 diabetes in the rodent model and in humans are different, in that lower BMD and higher bone resorption is observed in rodents with diabetes [72], compared to higher BMD and chronic low bone remodeling in adults with longer-standing type 2 diabetes [3,14–19].
There are several study limitations to acknowledge. In this study, we standardized the gray level values of the standards in order to compare bone gray levels between imaging sessions. Ideally, the gray levels of the standards should be maintained at the same value for each imaging session by adjusting the contrast and brightness [28], however, fine control of contrast and brightness was not possible with the instrument that we used. Also, this was a cross-sectional study of patients who were undergoing total hip replacement, therefore caution must be taken when generalizing of our findings to adults without osteoarthritis. Bone mineralization may be elevated in patients with osteoarthritis due to osteophyte production, however our samples were obtained from the femoral neck where osteophytes were not evident. There are also no muscle insertion sites along the femoral neck ruling out the possibility that elevated mineralization was an artifact due to calcified fibrocartilaginous areas [57]. While it is not known whether bone mineralization is impacted by osteoarthritis at the femoral neck site that was assessed in the present study, contradictory evidence exists for other sites. In one study using bone samples from the intertrochanteric region of the proximal femur, no difference in mean bone calcium concentration was detected between adults with end-stage osteoarthritis and controls [73]. In contrast, in a study investigating the impact of osteoarthritis on bone mineralization, samples with evidence of calcified fibrocartilage were more highly mineralized compared to samples from controls without osteoarthritis [74]. Given that the samples from the present study were taken from the femoral neck, a site that is unlikely to be affected by calcified fibrocartilage, and osteoarthritis was a common diagnosis between adults with and without type 2 diabetes, we are confident that a diagnosis of osteoarthritis did not influence our conclusion that bone mineralization is elevated and less heterogeneous in adults with type 2 diabetes. Another limitation was that the amount of femoral neck on the excised specimen was dependent on the level at which the surgeon resected the femoral neck to accommodate the prosthesis. Therefore, the 5 mm section of bone was not from a uniform location on the femoral neck, although there is no evidence suggesting that this would influence the BMDD outcomes. Study participants with type 2 diabetes were on various types of medication for diabetes control. It was beyond the scope of the present study to investigate the influence of different medications on bone mineralization, but certain medications can modify bone remodeling [75] and potentially BMDD outcomes. We did not assess bone turnover markers, as a much larger sample size would have been required. Numerous well-powered studies have found that men and women with longer-standing type 2 diabetes have lower concentrations of bone turnover markers compared to controls without diabetes [14–19]. Assessing measures of trabecular bone microarchitecture or levels of advanced glycation end-products in the bone was beyond the scope of the research, but are suggested to be different in adults with type 2 diabetes [76,77].
Conclusion
This study revealed that there are microscopic differences in bone mineralization in older adults with type 2 diabetes compared to controls. The mean bone calcium concentration was elevated and there was less mineralization heterogeneity, possibly explained by suppressed bone remodeling in adults with type 2 diabetes. These differences in mineralization may contribute to bone brittleness and reduced defense against microcrack propagation. The findings from our study emphasize a need for future research on osteoporosis-related medications and bone mineralization in adults with type 2 diabetes. Also, determining whether advanced glycation end-products contribute to elevated bone mineralization would be clinically important given that the formation of advanced glycation end-products could be prevented with better glycemic control or pharmacotherapy. Suppressing bone remodeling further with bisphosphonates in adults with type 2 diabetes [78] might further increase bone calcium concentration and bone brittleness. The impact of other interventions on bone quality and fracture prevention in adults with type 2 diabetes should be explored.
Acknowledgments
We would like to thank Vezna Relic, Hayley McCormack and Michelle Ball who assisted in participant recruitment. We are grateful for the cooperation and assistance from all clinical staff in the Juravinski Hospital Operating Room, Martin Knyf for his technical expertise with sample preparation, Marcia Reid and Ernie Spitzer of the Electron Microscopy Department at McMaster University, Gianluigi Botton, Fred Pearson, Andy Duft and Steve Koprich of the Canadian Centre for Electron Microscopy. This study was funded by an unrestricted grant from Amgen Canada Inc. Salary support was provided by the Ontario Graduate Scholarship and a grant to HPS from the Natural Sciences and Engineering Research Council of Canada. None of the sponsors had any role in obtaining data, analyzing data or writing the manuscript.
References
- 1.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes — a meta-analysis. Osteoporos Int. 2007;18(4):427–44. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 2.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 3.Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–32. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18(8):1033–46. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 5.Fraser LA, Langsetmo L, Berger C, Ioannidis G, Goltzman D, Adachi JD, et al. Fracture prediction and calibration of a Canadian FRAX tool: a population-based report from CaMos. Osteoporos Int. 2011;22(3):829–37. doi: 10.1007/s00198-010-1465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giangregorio LM, Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, et al. FRAX underestimates fracture risk in patients with diabetes. J Bone Miner Res. 2012;27(2):301–8. doi: 10.1002/jbmr.556. [DOI] [PubMed] [Google Scholar]
- 7.Faulkner RA, McCulloch RG, Fyke SL, De Coteau WE, McKay HA, Bailey DA, et al. Comparison of areal and estimated volumetric bone mineral density values between older men and women. Osteoporos Int. 1995;5(4):271–5. doi: 10.1007/BF01774017. [DOI] [PubMed] [Google Scholar]
- 8.Guglielmi G, Floriani I, Torri V, Li J, van Kuijk C, Genant HK, et al. Effect of spinal degenerative changes on volumetric bone mineral density of the central skeleton as measured by quantitative computed tomography. Acta Radiol. 2005;46(3):269–75. doi: 10.1080/02841850510012661. [DOI] [PubMed] [Google Scholar]
- 9.Binkley N, Krueger D, Vallarta-Ast N. An overlying fat panniculus affects femur bone mass measurement. J Clin Densitom. 2003;6(3):199–204. doi: 10.1385/jcd:6:3:199. [DOI] [PubMed] [Google Scholar]
- 10.Boivin G, Meunier PJ. Methodological considerations in measurement of bone mineral content. Osteoporos Int. 2003;14(Suppl 5):S22–7. doi: 10.1007/s00198-003-1469-1. discussion S27–8. [DOI] [PubMed] [Google Scholar]
- 11.Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42(3):456–66. doi: 10.1016/j.bone.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Roschger P, Dempster DW, Zhou H, Paschalis EP, Silverberg SJ, Shane E, et al. New observations on bone quality in mild primary hyperparathyroidism as determined by quantitative backscattered electron imaging. J Bone Miner Res. 2007;22(5):717–23. doi: 10.1359/jbmr.070120. [DOI] [PubMed] [Google Scholar]
- 13.Misof BM, Roschger P, Cosman F, Kurland ES, Tesch W, Messmer P, et al. Effects of intermittent parathyroid hormone administration on bone mineralization density in iliac crest biopsies from patients with osteoporosis: a paired study before and after treatment. J Clin Endocrinol Metab. 2003;88(3):1150–6. doi: 10.1210/jc.2002-021988. [DOI] [PubMed] [Google Scholar]
- 14.Reyes-Garcia R, Rozas-Moreno P, Lopez-Gallardo G, Garcia-Martin A, Varsavsky M, Aviles-Perez MD, et al. Serum levels of bone resorption markers are decreased in patients with type 2 diabetes. Acta Diabetol. 2011 Nov 1; doi: 10.1007/s00592-011-0347-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Gerdhem P, Isaksson A, Akesson K, Obrant KJ. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int. 2005;16(12):1506–12. doi: 10.1007/s00198-005-1877-5. [DOI] [PubMed] [Google Scholar]
- 16.Akin O, Gol K, Akturk M, Erkaya S. Evaluation of bone turnover in postmenopausal patients with type 2 diabetes mellitus using biochemical markers and bone mineral density measurements. Gynecol Endocrinol. 2003;17(1):19–29. [PubMed] [Google Scholar]
- 17.Inaba M, Nishizawa Y, Mita K, Kumeda Y, Emoto M, Kawagishi T, et al. Poor glycemic control impairs the response of biochemical parameters of bone formation and resorption to exogenous 1,25-dihydroxyvitamin D3 in patients with type 2 diabetes. Osteoporos Int. 1999;9(6):525–31. doi: 10.1007/s001980050180. [DOI] [PubMed] [Google Scholar]
- 18.Isaia GC, Ardissone P, Di Stefano M, Ferrari D, Martina V, Porta M, et al. Bone metabolism in type 2 diabetes mellitus. Acta Diabetol. 1999;36(1–2):35–8. doi: 10.1007/s005920050142. [DOI] [PubMed] [Google Scholar]
- 19.Bouillon R. Diabetic bone disease. Low turnover osteoporosis related to decreased IGF-I production. Verh K Acad Geneeskd Belg. 1992;54(4):365–91. discussion 391–2. [PubMed] [Google Scholar]
- 20.Boyde A, Jones SJ. Back-scattered electron imaging of skeletal tissues. Metab Bone Dis Relat Res. 1983–1984;5(3):145–50. doi: 10.1016/0221-8747(83)90016-4. [DOI] [PubMed] [Google Scholar]
- 21.Boyde A, Reid SA. A new method of scanning electron microscopy for imaging biological tissues. Nature. 1983;302(5908):522–3. doi: 10.1038/302522a0. [DOI] [PubMed] [Google Scholar]
- 22.Reid SA, Boyde A. Changes in the mineral density distribution in human bone with age: image analysis using backscattered electrons in the SEM. J Bone Miner Res. 1987;2(1):13–22. doi: 10.1002/jbmr.5650020104. [DOI] [PubMed] [Google Scholar]
- 23.Ball MD, McCartney DG. The measurement of atomic number and composition in an SEM using backscattered detectors. J Microsc. 1981;124(1):57–68. [Google Scholar]
- 24.Skedros JG, Bloebaum RD, Bachus KN, Boyce TM. The meaning of graylevels in backscattered electron images of bone. J Biomed Mater Res. 1993;27(1):47–56. doi: 10.1002/jbm.820270107. [DOI] [PubMed] [Google Scholar]
- 25.Skedros JG, Bloebaum RD, Bachus KN, Boyce TM, Constantz B. Influence of mineral content and composition on graylevels in backscattered electron images of bone. J Biomed Mater Res. 1993;27(1):57–64. doi: 10.1002/jbm.820270108. [DOI] [PubMed] [Google Scholar]
- 26.Roschger P, Plenk H, Jr, Klaushofer K, Eschberger J. A new scanning electron microscopy approach to the quantification of bone mineral distribution: backscattered electron image grey-levels correlated to calcium K alpha-line intensities. Scanning Microsc. 1995;9(1):75–86. discussion 86–8. [PubMed] [Google Scholar]
- 27.Vajda EG, Bloebaum RD, Skedros JG. Validation of energy dispersive x-ray spectrometry as a method to standardize backscattered electron images of bone. Cells Mater. 1996;6:79–92. [Google Scholar]
- 28.Roschger P, Fratzl P, Eschberger J, Klaushofer K. Validation of quantitative backscattered electron imaging for the measurement of mineral density distribution in human bone biopsies. Bone. 1998;23(4):319–26. doi: 10.1016/s8756-3282(98)00112-4. [DOI] [PubMed] [Google Scholar]
- 29.Misof BM, Bodingbauer M, Roschger P, Wekerle T, Pakrah B, Haas M, et al. Short-term effects of high-dose zoledronic acid treatment on bone mineralization density distribution after orthotopic liver transplantation. Calcif Tissue Int. 2008;83(3):167–75. doi: 10.1007/s00223-008-9161-2. [DOI] [PubMed] [Google Scholar]
- 30.Hofstaetter JG, Hofstaetter SG, Nawrot-Wawrzyniak K, Hiertz H, Grohs JG, Trieb K, et al. Mineralization pattern of vertebral bone material following fragility fracture of the spine. J Orthop Res. 2012;30(7):1089–94. doi: 10.1002/jor.22026. [DOI] [PubMed] [Google Scholar]
- 31.Hofstaetter JG, Roetzer KM, Krepler P, Nawrot-Wawrzyniak K, Schwarzbraun T, Klaushofer K, et al. Altered bone matrix mineralization in a patient with Rett syndrome. Bone. 2010;47(3):701–5. doi: 10.1016/j.bone.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Hofstaetter JG, Roschger P, Klaushofer K, Kim HK. Increased matrix mineralization in the immature femoral head following ischemic osteonecrosis. Bone. 2010;46(2):379–85. doi: 10.1016/j.bone.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Canadian Diabetes Association Clinical Practice Guidelines Committee. Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2008;32:S1–S201. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Roschger P, Gupta HS, Berzlanovich A, Ittner G, Dempster DW, Fratzl P, et al. Constant mineralization density distribution in cancellous human bone. Bone. 2003;32(3):316–23. doi: 10.1016/s8756-3282(02)00973-0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 37.Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int. 2001;12(5):417–27. doi: 10.1007/s001980170112. [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 40.Boyde A. Scanning electron microscopy of bone. Methods Mol Biol. 2012;816:365–400. doi: 10.1007/978-1-61779-415-5_24. [DOI] [PubMed] [Google Scholar]
- 41.Hayat MA. Fixation for electron microscopy. New York, NY: Academic Press; 1981. [Google Scholar]
- 42.Vajda EG, Humphrey S, Skedros JG, Bloebaum RD. Influence of topography and specimen preparation on backscattered electron images of bone. Scanning. 1999;21(6):379–87. doi: 10.1002/sca.4950210604. [DOI] [PubMed] [Google Scholar]
- 43.Otsu NA. A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern. 1979;9:62–6. [Google Scholar]
- 44.Frost HM. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res. 1969;3(3):211–37. doi: 10.1007/BF02058664. [DOI] [PubMed] [Google Scholar]
- 45.Parfitt AM. Skeletal heterogeneity and the purpose of bone remodeling: implications for understanding osteoporosis. In: Marcus R, Feldman D, Nelson D, Rosen CJ, editors. Fundamentals of osteoporosis. 3. New York, NY: Academic Press; 2009. [Google Scholar]
- 46.Burr DB. Remodeling and the repair of fatigue damage. Calcif Tissue Int. 1993;53(Suppl 1):S75–80. doi: 10.1007/BF01673407. discussion S80–1. [DOI] [PubMed] [Google Scholar]
- 47.Burr DB, Martin RB. Calculating the probability that microcracks initiate resorption spaces. J Biomech. 1993;26(4–5):613–6. doi: 10.1016/0021-9290(93)90023-8. [DOI] [PubMed] [Google Scholar]
- 48.Boyce TM, Bloebaum RD, Bachus KN, Skedros JG. Reproducible methods for calibrating the backscattered electron signal for quantitative assessment of mineral content in bone. Scanning Microsc. 1990;4:591–600. discussion 600–3. [PubMed] [Google Scholar]
- 49.Bloebaum RD, Skedros JG, Vajda EG, Bachus KN, Constantz BR. Determining mineral content variations in bone using backscattered electron imaging. Bone. 1997;20(5):485–90. doi: 10.1016/s8756-3282(97)00015-x. [DOI] [PubMed] [Google Scholar]
- 50.Elliot JC. Calcium phosphate biominerals. In: Kohn MJ, Rakovan JF, Hughes JM, editors. Geochemical, geobiological and materials importance. Washington, DC: Mineralogical Society of America; 2002. pp. 427–53. [Google Scholar]
- 51.Smirnov NV. Table for estimating the goodness of fit of empirical distributions. Ann Math Stat. 1948;19(2):279–81. [Google Scholar]
- 52.Currey JD. Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci. 1984;304(1121):509–18. doi: 10.1098/rstb.1984.0042. [DOI] [PubMed] [Google Scholar]
- 53.McCormack J, Stover SM, Gibeling JC, Fyhrie DP. Effects of mineral content on the fracture properties of equine cortical bone in double-notched beams. Bone. 2012;50(6):1275–80. doi: 10.1016/j.bone.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busse B, Hahn M, Soltau M, Zustin J, Puschel K, Duda GN, et al. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: mineralization, morphology and biomechanics of human single trabeculae. Bone. 2009;45(6):1034–43. doi: 10.1016/j.bone.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Currey JD. Changes in the impact energy absorption of bone with age. J Biomech. 1979;12(6):459–69. doi: 10.1016/0021-9290(79)90031-9. [DOI] [PubMed] [Google Scholar]
- 56.Currey JD, Brear K, Zioupos P. The effects of ageing and changes in mineral content in degrading the toughness of human femora. J Biomech. 1996;29:257–60. doi: 10.1016/0021-9290(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 57.Vajda EG, Bloebaum RD. Age-related hypermineralization in the female proximal human femur. Anat Rec. 1999;255(2):202–11. doi: 10.1002/(SICI)1097-0185(19990601)255:2<202::AID-AR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 58.Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14(5):769–78. doi: 10.1016/8756-3282(93)90209-s. [DOI] [PubMed] [Google Scholar]
- 59.Ascenzi A, Bonucci E, Ostrowski K, Sliwowski A, Dziedzic-Goclawska A, Stachowicz W, et al. Initial studies on the crystallinity of the mineral fraction and ash content of isolated human and bovine osteons differing in the their degree of calcification. Calcif Tissue Res. 1977;23(1):7–11. doi: 10.1007/BF02012760. [DOI] [PubMed] [Google Scholar]
- 60.Paschalis E, DiCarlo E, Betts F, Sherman P, Mendelsohn R, Boskey AL. FTIR microspectroscopic analysis of human osteonal bone. Calcif Tissue Int. 1996;59(6):480–7. doi: 10.1007/BF00369214. [DOI] [PubMed] [Google Scholar]
- 61.Dyer DG, Blackledge JA, Katz BM, Hull CJ, Adkisson HD, Thorpe SR, et al. The Maillard reaction in vivo. Z Ernahrungswiss. 1991;30(1):29–45. doi: 10.1007/BF01910730. [DOI] [PubMed] [Google Scholar]
- 62.Boehm BO, Schilling S, Rosinger S, Lang GE, Lang GK, Kientsch-Engel R, et al. Elevated serum levels of N(epsilon)-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47(8):1376–9. doi: 10.1007/s00125-004-1455-y. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–6. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrlich H, Hanke T, Simon P, Born R, Fischer C, Frolov A, et al. Carboxymethylation of the fibrillar collagen with respect to the formation of hydroxyapatite. J Biomed Mater Res B Appl Biomater. 2010;92B:542–51. doi: 10.1002/jbm.b.31551. [DOI] [PubMed] [Google Scholar]
- 65.Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40(5):1308–19. doi: 10.1016/j.bone.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 66.Misof BM, Paschalis EP, Blouin S, Fratzl-Zelman N, Klaushofer K, Roschger P. Effects of 1 year of daily teriparatide treatment on iliacal bone mineralization density distribution (BMDD) in postmenopausal osteoporotic women previously treated with alendronate or risedronate. J Bone Miner Res. 2010;25(11):2297–303. doi: 10.1002/jbmr.198. [DOI] [PubMed] [Google Scholar]
- 67.Roschger P, Rinnerthaler S, Yates J, Rodan GA, Fratzl P, Klaushofer K. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29(2):185–91. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- 68.Zoehrer R, Roschger P, Paschalis EP, Hofstaetter JG, Durchschlag E, Fratzl P, et al. Effects of 3- and 5-year treatment with risedronate on bone mineralization density distribution in triple biopsies of the iliac crest in postmenopausal women. J Bone Miner Res. 2006;21(7):1106–12. doi: 10.1359/jbmr.060401. [DOI] [PubMed] [Google Scholar]
- 69.Recker R, Masarachai P, Santora A, Howard T, Chavassieux P, Arlot M, et al. Trabecular bone microarchitecture after alendronate treatment of osteoporotic women. Curr Med Res Opin. 2005;21(2):185–94. doi: 10.1185/030079904X20259. [DOI] [PubMed] [Google Scholar]
- 70.Follet H, Viguet-Carrin S, Burt-Pichat B, Depalle B, Bala Y, Gineyts E, et al. Effects of preexisting microdamage, collagen cross-links, degree of mineralization, age, and architecture on compressive mechanical properties of elderly human vertebral trabecular bone. J Orthop Res. 2011;29(4):481–8. doi: 10.1002/jor.21275. [DOI] [PubMed] [Google Scholar]
- 71.Burr D. Microdamage and bone strength. Osteoporos Int. 2003;14(Suppl 5):S67–72. doi: 10.1007/s00198-003-1476-2. [DOI] [PubMed] [Google Scholar]
- 72.Hamann C, Goettsch C, Mettelsiefen J, Henkenjohann V, Rauner M, Hempel U, et al. Delayed bone regeneration and low bone mass in a rat model of insulin-resistant type 2 diabetes mellitus is due to impaired osteoblast function. Am J Physiol Endocrinol Metab. 2011;301(6):E1220–8. doi: 10.1152/ajpendo.00378.2011. [DOI] [PubMed] [Google Scholar]
- 73.Carpentier VT, Wong J, Yeap Y, Gan C, Sutton-Smith P, Badiei A, et al. Increased proportion of hypermineralized osteocyte lacunae in osteoporotic and osteoarthritic human trabecular bone: implications for bone remodeling. Bone. 2012;50:688–94. doi: 10.1016/j.bone.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 74.Boyde A, Jones SJ, Aerssens J, Dequeker J. Mineral density quantitation of the human cortical iliac crest by backscattered electron image analysis: variations with age, sex, and degree of osteoarthritis. Bone. 1995;16:619–27. doi: 10.1016/8756-3282(95)00119-x. [DOI] [PubMed] [Google Scholar]
- 75.Cornish J, Callon KE, Reid IR. Insulin increases histomorphometric indices of bone formation In vivo. Calcif Tissue Int. 1996;59(6):492–5. doi: 10.1007/BF00369216. [DOI] [PubMed] [Google Scholar]
- 76.Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17:1514–23. doi: 10.1007/s00198-006-0155-5. [DOI] [PubMed] [Google Scholar]
- 77.Pritchard JM, Giangregorio LM, Atkinson SA, Beattie KA, Inglis D, Ioannidis G, et al. Association of larger holes in the trabecular bone at the distal radius in postmenopausal women with type 2 diabetes mellitus compared to controls. Arthritis Care Res. 2012;64:83–91. doi: 10.1002/acr.20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27(7):1547–53. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]