Abstract
Summary
Using administrative data healthcare databases from five Canadian provinces, we compared prevalence estimates of diagnosed osteoporosis and incidence rates for related fractures in Canada. The algorithms adopted showed consistent age and sex patterns across all provinces and will be suitable for national surveillance and monitoring.
Purpose
This study aims to evaluate the feasibility of using provincial population-based administrative data to develop a national surveillance system of diagnosed osteoporosis and related fractures (forearm, humerus, vertebra, pelvis, and hip) in Canada.
Methods
Linked healthcare databases from five provinces representing approximately 85 % of the Canadian population were used. Multiple algorithms combining hospitalizations, physician visits, and osteoporosis prescription drug dispensations were evaluated in each province. The adopted algorithms for diagnosed osteoporosis and incident fractures combined hospitalizations and physician visits based on 3 years and 1 year of data, respectively. Sex-specific age-standardized osteoporosis prevalence and fracture incidence rates were estimated for each province from 1995/1996 to 2007/2008.
Results
Age-standardized prevalence of diagnosed osteoporosis in those ≥50 years increased over the study period but stabilized in the most recent years. Using the adopted algorithm produced provincial estimates ranging from 5.6 to 10.5 % for 2007/2008, with consistent age and sex patterns across provinces. The use of osteoporosis drug data resulted in higher osteoporosis estimates compared with estimates without drug data. Age-standardized incidence of fractures in those ≥40 years showed similar age and sex patterns across all provinces. The highest level of agreement among provinces was for hip and humerus fracture rates, with wider provincial variation for forearm, vertebra, and pelvis fractures.
Conclusions
Our results are consistent with previous validation works and confirm that the algorithms adopted will be suitable for the national monitoring of diagnosed osteoporosis and related fractures. A similar approach may be applicable to other countries with high-quality administrative data.
Keywords: Surveillance, Osteoporosis, Fractures, Prevalence, Incidence, Administrative data
Introduction
Osteoporosis and related fractures affects a large proportion of the adult population and results in excess morbidity, mortality, and economic burden in Canada as well as internationally. In 2010, the overall cost of osteoporosis was estimated to be over $2.3 billion among Canadians 50 years of age and older [1]. Globally, in the year 2000, there were an estimated 9.0 million low trauma fractures of which 1.6 million were at the hip, 1.7 million at the forearm, and 1.4 million were clinical vertebral fractures, resulting in a loss of 5.8 million disability adjusted life years [2].
Given the personal and societal impacts, measuring and monitoring osteoporosis and related fractures is an important component of a population-based chronic disease surveillance system. Population-based administrative databases, used for health system management and physician remuneration, offer a relatively quick and inexpensive way of providing longitudinal epidemiological data on an entire jurisdiction and therefore, have shown to be useful in the national surveillance of common chronic conditions [3, 4].
Osteoporosis and fracture algorithms using diagnostic codes found in administrative databases have been constructed and validated for surveillance purposes [5–11]. Leslie et al. validated algorithms to identify cases of diagnosed osteoporosis using administrative data and demonstrated that a relatively simple algorithm which combined hospitalizations and physician visits could achieve an acceptable level of sensitivity, specificity, and accuracy [5]. In addition, Lix et al. validated algorithms using administrative data to estimate fracture incidence of the hip, wrist, humerus, and clinical vertebrae and found that administrative data are generally useful for the surveillance of osteoporosis-related fractures although the validity depends on the fracture site and features of the algorithm [6].
Building on previous validation work, the current study evaluated the feasibility of using administrative data for the national surveillance of diagnosed osteoporosis and osteoporosis-related fractures which ultimately will help to inform the implementation of a population-based surveillance program. While several countries have established project-specific osteoporosis and fracture registries [12–14], to our knowledge, these have not been used to develop a framework for surveillance of osteoporosis and related fractures at a national level in Canada.
Methods
Databases
We selected a convenience sample of five Canadian provinces (British Columbia, Alberta, Manitoba, Ontario, and Quebec) with ready access to the following administrative databases: health insurance registry containing dates of insurance coverage and demographic information; hospital discharge abstract containing separation dates, admission dates, and diagnoses; physician services covering all fee-for-service claims and containing service date, physician specialty and diagnoses; and prescription drug dispensation databases containing outpatient dispensations only. Given Canada’s universal healthcare, these administrative databases (excluding prescription drug dispensation) have near complete coverage of provincial populations. Exclusions include individuals covered by Federal jurisdiction such as First Nations living on reserve, full-time members of the Canadian Armed Forces and individuals in the Royal Canadian Mounted Police and Federal correctional facilities. Records from 1995/1996 to 2007/2008 fiscal years (a fiscal year extends from 1 April to 31 March) were included where possible. Data were depersonalized or aggregated for confidentiality reasons and linked using an anonymized unique lifetime identifier. Ethics and data access approval were obtained in each jurisdiction.
Algorithms
Multiple algorithms combining hospitalizations (H), physician visits (P), and osteoporosis prescription drug dispensations were tested. The algorithms selected were informed by results from recent validation studies [5–8] as well as recommendations from experts in ascertaining osteoporosis and fractures from administrative databases (Canadian Chronic Disease Surveillance System [CCDSS] Osteoporosis Working Group). International Classification of Diseases (ICD) 9th Revision Clinical Modification (ICD-9-CM) and 10th Revision Canadian version (ICD-10-CA) in addition to drug identification number codes were used for data extraction.
Table 1 lists the algorithms adopted by the CCDSS Osteoporosis Working Group for osteoporosis and osteoporosis-related fractures (and their corresponding ICD-9-CM and ICD-10-CA codes) for future testing. Working group members’ decision regarding which algorithms to adopt was based on results from this feasibility study as well as results from the aforementioned validation studies [5–8], published osteoporosis prevalence and fracture incidence estimates [15–17], and the availability/completeness of the administrative databases required.
Table 1.
Adopted algorithms for diagnosed osteoporosis and osteoporosis-related fractures
| Health event | Algorithm | ICD-9-CM | ICD-10-CA |
|---|---|---|---|
| Osteoporosis | 1H or 1P in 3 years | 733.x | M80 and M81 |
| Fractures | Hip: 1H 1st diagnosis (6-month episode period) in 1 year | 820.x | S72.0–.2 |
| Forearm: 1H 1st diagnosis or 2P within 3 months (6-month episode period) in 1 year | 813.x | S52.x | |
| Vertebra: 1H 1st diagnosis or 1P (6-month episode period) in 1 year | 805.x | S22.0–.1, S32.0, and S32.7–.8 | |
| Humerus: 1H 1st diagnosis or 2P within 3 months (6-month episode period) in 1 year | 812.0x and 812.1x | S42.2 | |
| Pelvis: 1H 1st diagnosis or 2P within 3 months (6-month episode period) in 1 year | 808.x | S32.1, S32.3–.5, and S32.7–.8 |
H hospitalization, P physician visit, 6-month episode period where any like fracture codes during this period were considered part of the same event
Regarding the adopted algorithm for diagnosed osteoporosis, a person was identified as a case if he or she had at least one hospitalization or one physician visit with a diagnosis in any diagnosis field with the following ICD-9-CM (ICD-10-CA) codes: 733.x (M80 and M81) within a 3-year period. The adopted algorithms for fractures were based on hospitalizations only (hip) or hospitalizations and physician visits using 1 year of data. All fracture algorithms included a 6-month episode period where any like fracture codes during this period were considered part of the same event. The date of the first fracture code of a fracture event was used to establish the end-point of the 6-month episode period. Pathologic fractures were included since they represent a small proportion of all fractures, and their exclusion can lead to underestimation of the fracture burden due to osteoporosis [18].
Analysis
Osteoporosis prevalence and fracture incidence estimates were age-standardized to the 1991 Canadian population. Estimates are reported separately by sex within each province from 1995/1996 to 2007/2008 (except for Quebec where estimates were reported from 1996/1997 to 2007/2008 due to a lack of data availability in the earliest study year).
Results
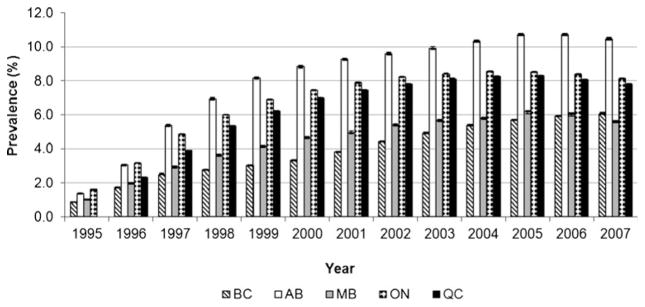
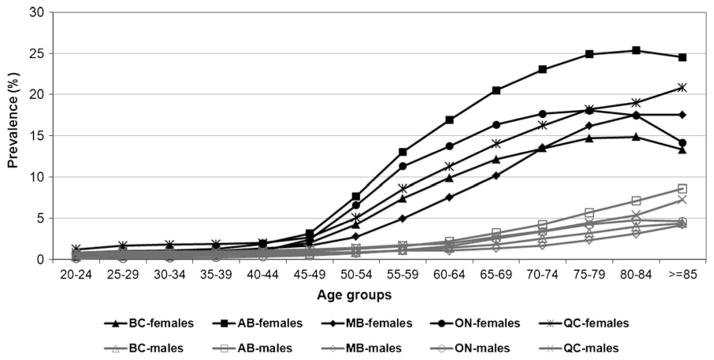
The age-standardized prevalence of diagnosed osteoporosis in those 50 years of age and older increased over the study period; however, rates stabilized in the most recent years (i.e., 2005/2006 to 2007/2008) (Fig. 1) The age-standardized prevalence estimates for women and men combined (age 50+) ranged from 5.6 (Manitoba) to 10.5 % (Alberta) in 2007/2008. All provinces demonstrated consistency in age and sex patterns with prevalence of diagnosed osteoporosis being higher in women and increasing with age (Fig. 2). Adding prescription drug dispensation data produced higher estimates of osteoporosis than the estimates produced without these data. For instance, with the addition of drug data, the adopted algorithm produced age-standardized prevalence estimates for women and men combined (age 65+) that ranged from 12.7 (Manitoba) to 23.4 % (Alberta) in 2007/2008 which are considerably higher than the total age-standardized prevalence estimates in those 65+ without drug data: 8.6 (Manitoba) and 14.6 % (Alberta), respectively.
Fig. 1.
Age-standardized prevalence of diagnosed osteoporosis (95 % CIs), both sexes, age 50 years and older, 1995/1996 to 2007/2008. BC British Columbia, AB Alberta, MB Manitoba, ON Ontario, QC Quebec
Fig. 2.
Age-specific prevalence of diagnosed osteoporosis for women (solid symbols) and men (open symbols), age 20 years and older, 2007/2008. BC British Columbia, AB Alberta, MB Manitoba, ON Ontario, QC Quebec
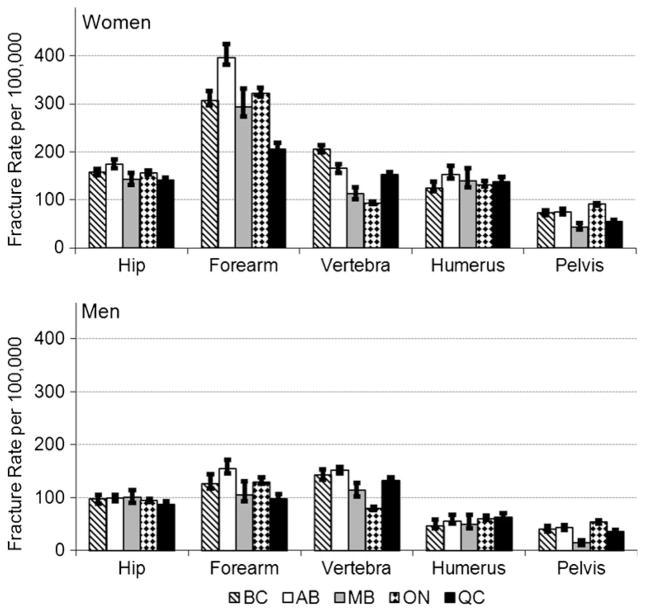
Age-standardized incidence of fractures in those 40 years of age and older were higher in women than men in all five provinces. The highest level of agreement across jurisdictions was for hip and humerus fracture rates with wider provincial variation for forearm, vertebra and pelvis fractures (Fig. 3). All fractures showed a consistent increase with older age (data not shown).
Fig. 3.
Age-standardized fracture rates (95 % CIs) per 100,000 population for women and men, age 40 years and older, 2007/2008. BC British Columbia, AB Alberta, MB Manitoba, ON Ontario, QC Quebec
Discussion
This study has shown the usefulness of using administrative data to measure diagnosed osteoporosis prevalence and osteoporosis-related fractures incidence.
Age-standardized prevalence of diagnosed osteoporosis in those 50 years of age and older increased over the study period but stabilized in the most recent years. The adopted algorithm for diagnosed osteoporosis prevalence (1H or 1P in 3 years) produced provincial estimates ranging from 5.6 to 10.5 % in 2007/2008 with consistent age and sex patterns across provinces. Age-standardized incidence of fractures in those 40 years of age and older showed similar sex and age patterns in all provinces. The highest level of agreement among provinces was among hip and humerus fracture with wider variation for forearm, vertebra, and pelvis fractures.
Receiving an osteoporosis diagnosis is largely dependent on an individual undergoing bone mineral density (BMD) testing therefore, differences in access to BMD testing likely contribute to the provincial variations observed in prevalence estimates. According to Osteoporosis Canada’s 2008 National Report Card, the number of publically funded BMD tests during the period of 1 April 2006 to 31 March 2007 showed Manitoba as having the lowest rate (59/1,000 population) and Alberta, the highest rate (335/1,000 population) [19]. Differences in features of physician services databases in both structure and content [20] may contribute to the provincial variations observed in the forearm, vertebra, and pelvis fracture rates given the highly consistent hip fracture rates which are derived primarily from hospitalization data.
Results are consistent with previous studies involving the validation of algorithms for osteoporosis and fracture surveillance using administrative data [5–8]. While results are slightly lower than self-reported estimates of diagnosed osteoporosis from the Canadian Community Health Survey (CCHS) which showed 11.9 % of Canadians 50 years and older reported they were diagnosed with osteoporosis by a health professional in 2009 [21]. However, it is important to bear in mind the inherent differences between administrative data and self-reported data. The prevalence estimates derived from administrative data represent individuals diagnosed with osteoporosis in a specified period of time (3 years) within an overall 13-year window whereas, the CCHS captures the proportion of individuals having ever been diagnosed with osteoporosis by a health professional.
Despite the strengths of using population-based data to evaluate the algorithms across multiple provinces, this study has several limitations. Using a convenience sample of five provinces may limit the generalizability of results although those provinces with the largest population were deliberately chosen to participate in this feasibility study and collectively represent more than 85 % of the national population. The fracture algorithms may result in an under reporting of fracture events as any new fractures during the 6-month episode period would not have been captured. Similar to other studies, incident vertebral fractures are likely under-ascertained even when both hospital and physician data are used to construct the algorithm as health professionals in in- and out-patient settings do not reliably diagnose these type of fractures [6, 8, 10].
Furthermore, since administrative databases were designed for health system management and physician remuneration purposes, there are several inherent limitations with regards to their use for chronic disease surveillance. Firstly, these data likely underestimate the true prevalence/incidence since those individuals who do not seek services, those seen by salaried physicians who do not shadow bill (i.e., claims submitted by physicians on alternative payment plans for services they provide for administrative purposes only), and those who have sought privately funded care would not be captured. In addition, administrative data lack indicators of disease severity. Lastly, administrative data do not currently contain information on socioeconomic characteristics. However, linking administrative data with postal codes and census data would provide additional opportunities to explore differences within jurisdictions based on location (e.g., urban versus rural) and socioeconomic status [22].
In conclusion, this study supports the feasibility of using administrative data for the national surveillance of diagnosed osteoporosis and osteoporosis-related fractures. The Public Health Agency of Canada plans to use the methodology of this feasibility study to conduct a national pilot study which will include data from all provinces and territories. The national pilot will also include an examination of the post-fracture care gap (i.e., the proportion of individuals that were newly screened, diagnosed, and/or treated for osteoporosis 12 months post-fracture). Results from the national pilot will ultimately help inform the implementation of a population-based osteoporosis surveillance program. The methods used may have broader application for other countries where national fracture registries exist or can be created from high quality administrative databases. Ongoing surveillance of diagnosed osteoporosis, osteoporosis-related fractures, and the post-fracture care gap will provide information that could help inform the planning and provision of screening, prevention, and treatment resources.
Acknowledgments
CCDSS Osteoporosis Working Group: Jacques Brown, Susan Jaglal, Sonia Jean, William D. Leslie, Lisa M. Lix, Pat McCrea, Louise McRae, Suzanne Morin, Siobhan O’Donnell, Jay Onysko, Alexandra Papaioannou, Kim Reimer, Louis Rochette, Kerry Siminoski, and Larry Svenson. Working Group members assisted with the interpretation of data, revising the manuscript critically for important intellectual content, and approving its submission. The conclusions in this manuscript reflect the opinions of individual experts and not their affiliated organizations.
Footnotes
Conflicts of interest None.
References
- 1.Tarride JE, Hopkins RB, Leslie WD, et al. The burden of illness of osteoporosis in Canada. Osteoporosis Int. 2012;23:2591–2600. doi: 10.1007/s00198-012-1931-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Public Health Agency of Canada. Report from the National Diabetes Surveillance System: diabetes in Canada, 2009. 2009 Available from http://www.phac-aspc.gc.ca/publicat/2009/ndssdic-snsddac-09/index-eng.php.
- 4.Public Health Agency of Canada. Report from the Canadian Chronic Disease Surveillance System: hypertension in Canada, 2010. 2010 Available from http://www.phac-aspc.gc.ca/cd-mc/cvd-mcv/ccdss-snsmc-2010/index-eng.php. [PubMed]
- 5.Leslie WD, Lix LM, Yogendran MS. Validation of a case definition for osteoporosis disease surveillance. Osteoporosis Int. 2011;1(22):37–46. doi: 10.1007/s00198-010-1225-2. [DOI] [PubMed] [Google Scholar]
- 6.Lix LM, Azimaee M, Osman BA, et al. Osteoporosis related fracture case definitions for population-based administrative data. BMC Public Health. 2012;12:301. doi: 10.1186/1471-2458-12-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lix LM, Yogendran M, Leslie WD, Shaw SY, Baumgartner R, Bowman C, Metge C, Gumel A, James RC, Hux JF. Using multiple data features improved the validity of osteoporosis case ascertainment from administrative data. J ClinEpidemiol. 2008;61:1250–1260. doi: 10.1016/j.jclinepi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Jean S, Candas B, Belzile E, et al. Algorithms can be used to identify fragility fracture cases in physician claims databases. Osteoporosis Int. 2012;23:483–501. doi: 10.1007/s00198-011-1559-4. [DOI] [PubMed] [Google Scholar]
- 9.Sund R. Utilization of routinely collected administrative data in monitoring the incidence of aging dependent hip fracture. Epidemiol Perspect Innov. 2007;7(4):2. doi: 10.1186/1742-5573-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis JR, Mudano AS, Solomon DH, Xi J, Melton ME, Saag KG. Identification and validation of vertebral compression fractures using administrative claims data. Med Care. 2009;47(1):69–72. doi: 10.1097/MLR.0b013e3181808c05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narongroeknawin P, Patkar NM, Shakoory B, Jain A, Curtis JR, Delzell E, Lander PH, Lopez-Ben RR, Pitt MJ, Safford MM, Volgas DA, Saag KG. Validation of diagnostic codes for subtrochanteric, diaphyseal, and atypical femoral fractures using administrative claims data. J Clin Densitom. 2012;15(1):92–102. doi: 10.1016/j.jocd.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrahamsen B, Vestergaard P. Declining incidence of hip fractures and the extent of use of anti-osteoporotic therapy in Denmark 1997–2006. Osteoporos Int. 2010;21:373–380. doi: 10.1007/s00198-009-0957-3. [DOI] [PubMed] [Google Scholar]
- 13.Landfeldt E, Strom O, Robbins S, et al. Adherence to treatment of primary osteoporosis and its association to fractures-the Swedish Adherence Register Analysis (SARA) Osteoporos Int. 2012;23:433–443. doi: 10.1007/s00198-011-1549-6. [DOI] [PubMed] [Google Scholar]
- 14.Icks A, Haastert B, Wildner M, et al. Trend of hip fracture incidence in Germany 1995–2004: a population-based study. Osteoporos Int. 2008;19:1139–1145. doi: 10.1007/s00198-007-0534-6. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int. 2000;11:669–674. doi: 10.1007/s001980070064. [DOI] [PubMed] [Google Scholar]
- 16.Ettinger B, Black DM, Wson-Hughes B, et al. Updated fracture incidence rates for the US version of FRAX. Osteoporos Int. 2010;21:25–33. doi: 10.1007/s00198-009-1032-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leslie WD, Lix LM, Langsetmo L, et al. Construction of a FRAX® model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int. 2011;22:817–827. doi: 10.1007/s00198-010-1464-2. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JR, Taylor AJ, Matthews RS, Ray MN, Becker DJ, Gary LC, Kilgore ML, Morrisey MA, Saag KG, Warriner A, et al. “Pathologic” fractures: should these be included in epidemiologic studies of osteoporotic fractures? Osteoporos Int. 2009;20:1969–1972. doi: 10.1007/s00198-009-0840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osteoporosis Canada: Breaking Barriers, Not Bones: 2008 National Report Card on Osteoporosis Care. Toronto: Osteoporosis Canada; 2008. Available from http://www.osteoporosis.ca/multimedia/images/english/home/2008NationalReportCard_Eng.pdf. [Google Scholar]
- 20.Lix LM, Walker R, Quan H, Nesdole R, Yang J, Chen G for the CHEP-ORTF Hypertension Outcomes and Surveillance Team. Features of physician services databases in Canada. Chronic Diseases and Injuries in Canada. 2012;32(4):186–193. [PubMed] [Google Scholar]
- 21.Garriguet D. Bone health: osteoporosis, calcium and vitamin D. Health Rep (Statistics Canada catalogue No 82-003-X) 2011;22(3):7–14. Available from http://www.statcan.gc.ca/pub/82-003-x/2011003/article/11515-eng.htm. [PubMed] [Google Scholar]
- 22.Kisely S, Lin E, Lesage A, Gilbert C, Smith M, Campbell LA, Vasiliadis HM. Use of administrative data for the surveillance of mental disorders in 5 provinces. Can J Psychiatry. 2009;54(8):571–575. doi: 10.1177/070674370905400810. [DOI] [PubMed] [Google Scholar]