Abstract
The WHO fracture risk assessment tool (FRAX®) estimates an individual’s 10-yr major osteoporotic and hip fracture probabilities. When bone mineral density (BMD) is included in the FRAX calculation, only the femoral neck measurement can be used. Recently, a procedure was reported for adjusting major osteoporotic fracture probability from FRAX with femoral neck BMD based on the difference (offset) between the lumbar spine and the femoral neck T-score values. The objective of the current analysis was to independently evaluate this algorithm in a population-based cohort of 4575 women and 1813 men aged 50 yr and older from the Canadian Multicentre Osteoporosis Study. For women and men combined, there was a 15% (95% confidence interval 7–24%) increase in major osteoporotic fracture risk for each offset T-score after adjusting for FRAX probability calculated with femoral neck BMD. The effect was stronger in women than men, but a significant sex interaction was not detected. Among the full cohort, 5.5% had their risk category reclassified after using the offset adjustment. Sex- and age-dependent offsets (equivalent to an offset based on Z-scores) showed improved risk classification among individuals designated to be at moderate risk with the conventional FRAX probability measurement. In summary, the T-score difference between the lumbar spine and femoral neck is an independent risk factor for major osteoporotic fractures that is independent of the FRAX probability calculated with femoral neck BMD.
Keywords: Fracture prediction models, bone mineral density, dual-energy X-ray absorptiometry, FRAX, osteoporosis
Introduction
In 2008, the WHO Collaborating Center for Metabolic Bone Diseases released a fracture risk assessment tool (FRAX®) for estimation of individualized 10-yr probability of hip and osteoporotic fracture (composite of hip, clinical spine, distal forearm, and proximal humerus) with and without bone mineral density (BMD) (1). FRAX integrates 7 clinical risk factors (CRFs, prior fragility fracture, a parental history of hip fracture, smoking, use of systemic glucocorticoids, high alcohol intake, body mass index [BMI], and rheumatoid arthritis), which, in addition to age and sex, contribute to fracture risk independently of BMD (2,3).
When BMD is included in the FRAX calculation, the femoral neck measurement must be used. The FRAX algorithm was calibrated for use of femoral neck BMD derived from DXA based on the strength of the association with subsequent fractures (particularly hip fractures), large representation among the FRAX derivation cohorts, and availability of a reference standard database for BMD normalization (NHANES III white female) (4–7). Although other BMD measurement sites can also be used for fracture risk assessment and osteoporosis diagnosis (7), they are not currently a component of FRAX. In part, this reflects incomplete data for the FRAX derivation cohorts and lack of an international reference standard for BMD normalization. Some sites, such as the lumbar spine, are also prone to measurement artifact (e.g., degenerative change, compression fracture, vascular calcification).
It is not uncommon to find situations where T-scores from the lumbar spine and femoral neck show “discordance” given the modest correlation in BMD between these 2 sites (typically R =0.6–0.7) (8,9). Previous work has demonstrated the feasibility and incremental improvement in fracture risk prediction using lumbar spine BMD in addition to femoral neck BMD (10). More recently, a simple procedure for adjusting major osteoporotic fracture probability (from FRAX with femoral neck BMD) based on the T-score difference (termed “offset”) between the lumbar spine and femoral neck was reported (11). The lumbar spine-femoral neck “offset” was calculated as the numeric difference in the respective T-scores (lumbar spine minus femoral neck). A negative offset indicated a lumbar spine T-score less than the femoral neck T-score, whereas a positive offset indicated a lumbar spine T-score greater than the femoral neck T-score. The following rule was formulated: “Increase/decrease FRAX estimate for a major fracture by one-tenth for each rounded T-score difference between lumbar spine and femoral neck.” The offset adjustment was found to reclassify fracture probability in a relatively small proportion overall (less than 10%), but re-classified a larger number (1 in 4) of those with moderate risk when the offset exceeded 1 standard deviation (SD). The objective of the current analysis was to evaluate this algorithm in an independent population-based population.
Methods
Patient Population
The performance characteristics of the Canadian FRAX tool were studied in a sample selected from participants in an on-going population-based longitudinal cohort study, the Canadian Multicentre Osteoporosis Study (CaMos). We included all CaMos participants, with follow-up data, aged ≥50 yr at study entry. The methodological details of CaMos have been described elsewhere (12). Briefly, eligible participants were at least 25 yr old at the start of the study, lived within a 50-km radius of 1 of 9 Canadian cities (St John’s, Halifax, Quebec City, Toronto, Hamilton, Kingston, Saskatoon, Calgary, and Vancouver) and were able to converse in English, French, or Chinese (Toronto and Vancouver). Households were randomly selected from a list of residential phone numbers and participants were randomly selected from eligible household members using a standard protocol. Of those selected, 43% agreed to participate and had a baseline interview. All study participants gave written informed consent in accordance with the Helsinki declaration. Ethics approval was granted through McGill University and the appropriate ethics review boards for each participating center.
Data Collection
Participants completed a standardized interviewer-administered questionnaire (CaMos questionnaire ©1995) at baseline, with assessment of demographics, general health, nutrition, medication use, and medical history. The questionnaire was designed to capture detailed information about risk factors for fractures, including information about prior fractures, and as such assessed: all previous fractures (fracture site, date, and circumstances), family history of osteoporosis/fracture, and falls in past month. Participants had a baseline clinical assessment that included measurement of height, weight, and BMD.
BMD Measurements
BMD was measured at the lumbar spine (L1–L4) and proximal femur. Seven centers used Hologic densitometers and 2 used GE Lunar densitometers. All Lunar measurements were converted to equivalent Hologic values using standard reference formulas (13,14).
All densitometers were cross calibrated using a European spine phantom circulated between study centers. A more detailed description of BMD quality control appears elsewhere (15). Femoral neck T-scores were calculated in both men and women using the NHANES III white female reference values as recommended by the WHO Collaborating Center. Lumbar spine T-scores for L1–L4 were calculated using the manufacturer White female reference values, which did not differ significantly from reference values based on our cohort (16). For the primary analysis, obviously abnormal vertebrae were excluded from the analysis (e.g., compression fractures, overlying artifacts, previous surgical intervention, and grossly abnormal anatomy). Secondary analyses were performed using an additional automated algorithm for vertebral exclusions (17).
Other FRAX Variables
Self-reports of rheumatoid arthritis may not be reliable because of confusion with osteoarthritis; therefore, we derived a variable for rheumatoid arthritis based on self-report diagnosis plus treatment using the drug codes for methotrexate, hydroxychloroquine, or corticosteroids. Corticosteroid use was based on the drug codes for oral or intravenous glucocorticoids. History of osteoporotic fracture after the age of 50 yr was assessed using the baseline questionnaire, and excluded fractures of the head, hands, ankle, or feet, and those because of high trauma. All FRAX estimates were based on baseline CRFs with the exception of parental hip fracture, which was a composite; history of parental hip fracture was used for everyone with year 5 data, whereas history of any parental osteoporotic fracturewas used from the baseline questionnaire for those without year 5 data.
Fracture Probability
The WHO Coordinating Center used the Canadian FRAX tool calibrated using national hip fracture and mortality data along with the FRAX predictor variables from CaMos to calculate 10-yr fracture probability. Discrimination (“How well did the model perform in terms of risk stratification?”) and calibration (“Was the observed fracture risk consistent with the predicted fracture risk?”) of the Canadian FRAX tool has been established in 2 independent Canadian cohorts including CaMos (18,19). Fracture probabilities for hip and major osteoporotic sites were calculated using CRF and femoral neck BMD. The WHO Coordinating Center was blinded to fracture outcomes in CaMos. In the primary analysis, the procedure for adjusting major osteoporotic fracture probability based on the T-score offset between the lumbar spine and femoral neck was then applied using the adjustment factor of one-tenth for each rounded T-score difference (11). In a secondary analysis, we adjusted for sex- and age-dependent differences in the offset (equivalent to an offset based on Z-scores).
Fracture Assessment
Self-reported incident clinical fractures were identified by yearly postal questionnaire or at the scheduled interval for in-person reassessment (3rd, 5th, and 10th year after study entry). Timing of the 10-yr visit was not exactly 10 yr from the baseline visit in all the participants—in some it was slightly less and in some slightly more. We included all incident fractures reported at the 10-yr visit in the analysis. When fractures were identified by yearly postal questionnaire, confirmation and further information concerning the fracture was gathered using a structured interview that included date, fracture site, circumstances leading to fracture, and medical treatment. Participants who reported fractures were asked for consent to contact the treating physician or hospital for verification and for acquisition of further details. For the current analysis, we included incident fractures of hip, upper arm, forearm/wrist, or clinical spine, regardless of the degree of trauma involved. These are the same skeletal sites used by FRAX, but was not limited to fractures arising from low trauma (1).
Statistics
All results are reported as mean ± SD unless otherwise stated. Group comparisons for continuous data were conducted with the Student t-test and for categorical data using a Chi-square test of independence. A Cox proportional hazards model was used to study fracture risk as a function of the unrounded offset variable which was adjusted for the FRAX probability of major osteoporotic fracture (based on femoral neck BMD) as an additional covariate in the model. FRAX probability was log-transformed because of a skewed distribution. Death was modeled as a competing hazard. The FRAX predictions without adjustment for offset were compared with FRAX predictions adjusted for the rounded offset. Absolute 10-yr fracture probabilities using FRAX without and with the rounded offset adjustment were categorized as low risk (<10%), moderate risk (10–20%), and high risk (>20%) in accordance with Canadian reporting guidelines (20). Fracture discrimination was assessed using the area under the receiver operating characteristic curve and calibration was determined by comparing predicted with observed 10-yr fracture outcomes in linear regression models. The number of individuals in whom the rounded offset adjustment reclassified risk to a different category was determined according to the method of Janes et al (21). The numbers of fractures under the 2 systems were cross tabulated, and the linear trend in fractures after applying the rounded offset adjustment was assessed using the Cochran-Armitage test. As an additional global measure of incremental model prediction, we calculated the integrated discrimination index (IDI) as described by Pencina et al (22). Within each subgroup, fracture outcomes to 10 yr were estimated using the Kaplan-Meier method. Statistical analyses were performed with Statistica version 10.0 (StatSoft, Tulsa, OK) and SPSS for Windows version 18.0 (SPSS Inc., Chicago, IL).
Results
The characteristics of the 4575 women and 1813 men aged 50 yr or older at baseline included in the study cohort are summarized in Table 1. Age, BMI, and observation period were similar. Women had significantly lower mean BMD values at the femoral neck and lumbar spine (p <0.001) with significantly greater major osteoporotic fracture probability (p <0.001).
Table 1.
Baseline Characteristics of the Study Cohort
| Characteristic | Overall
|
Women
|
Men
|
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| N= | 6388 | 4575 | 1813 |
| Age (yr) | 65.6 ± 8.9 | 65.7 ± 8.8 | 65.1 ± 9.1 |
| Body mass index (kg/m2) | 27.1 ± 4.6 | 27.1 ± 4.9 | 27.2 ± 3.8 |
| FRAX major osteoporotic | 9.2 ± 7.2 | 10.8 ± 7.8 | 5.4 ± 3.2 |
| Lumbar spine (LS) T-score | −0.89 ± 1.63 | −1.23 ± 1.52 | −0.05 ± 1.57 |
| Femoral neck (FN) T-score | −1.21 ± 1.18 | −1.48 ± 1.09 | −0.55 ± 1.15 |
| Minimum T-score | −1.54 ± 1.22 | −1.83 ± 1.14 | −0.83 ± 1.14 |
| Minimum T-score osteoporotic | 1372 (21.5) | 1253 (27.4) | 119 (6.6) |
| Offset (LS minus FN, unrounded) | 0.32 ± 1.17 | 0.25 ± 1.12 | 0.50 ± 1.26 |
| Observation (yr) | 9.1 ± 2.1 | 9.2 ± 2.1 | 9.0 ± 2.3 |
Note: Data are mean ± SD or N (percent).
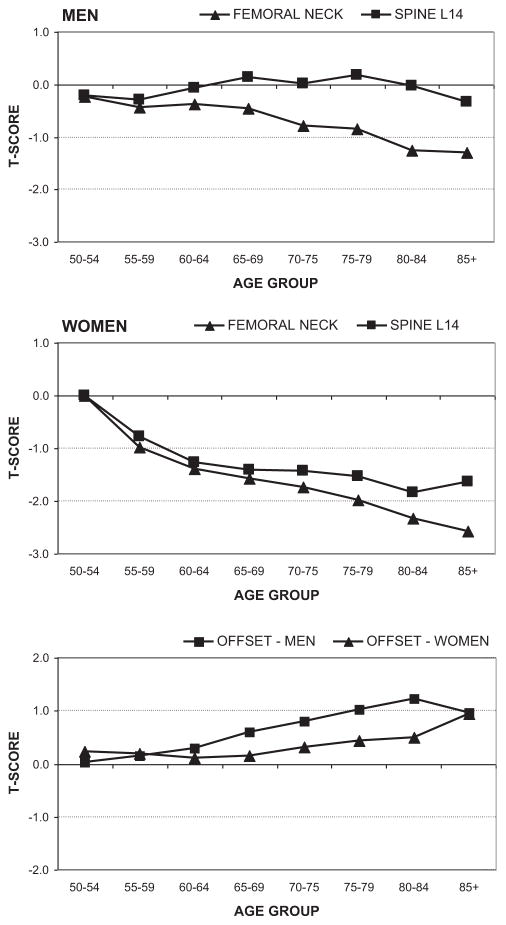
The average unrounded offset between the lumbar spine and femoral neck T-scores was less in women (0.2 ± 1.1) than men (0.5 ± 1.3, p < 0.001). There was a significant effect of increasing age on T-score measurements and the offset as shown in Fig. 1. Femoral neck T-score declined with older age in both women and men, but the decline was greater in women. Lumbar spine T-score declined with older age in women but showed no appreciable age-related change in men. These factors contributed to an age-related increase in the offset that was greater in men than women. Among women, there was a minimal increase in the offset until age 70 yr, whereas in men there was a progressive increase across the age spectrum.
Fig. 1.
Age-related trends in mean T-score for the femoral neck, lumbar spine, and unrounded offset for men and women.
The effect of the unrounded T-score offset between the lumbar spine and femoral neck on major osteoporotic fracture risk was assessed in Cox proportional hazards models adjusted for major osteoporotic fracture probability calculated with femoral neck BMD. For women and men combined, the hazard ratio (HR) was 1.15 per offset SD (95% CI: 1.07–1.24, p < 0.001). Similar results were obtained when vertebral exclusions were performed using an automated algorithm (HR 1.17 per offset SD, 95% CI: 1.08–1.26, p < 0.001) and when the offset was adjusted for sex- and age-dependent differences (HR 1.18 per offset SD, 95% CI: 1.08–1.27, p < 0.001). When stratified by sex, the offset effect was stronger among women (HR 1.17, 95% CI: 1.08–1.27, p < 0.001) than among men (HR 1.10, 95% CI: 0.93–1.29, p = 0.264) but a significant sex interaction was not detected (p-for-interaction =0.356) (Table 2).
Table 2.
Hazard Ratios (HRs) and 95% Confidence Intervals (CIs) for Major Osteoporotic Fracture Risk According to Each Unrounded Unit Increase in the Lumbar Spine-Femoral Neck T-Score Difference (Offset) Adjusted for FRAX Probability of Major Osteoporotic Fracture Probability
| Subgroup | HR | 95% CI | p Value |
|---|---|---|---|
| Both women and men | 1.15 | 1.07–1.24 | <0.001 |
| Women only | 1.17 | 1.08–1.27 | <0.001 |
| Men only | 1.10 | 0.93–1.29 | 0.264 |
Note: Data from Cox proportional hazards models.
p-For-sex-interaction = 0.356.
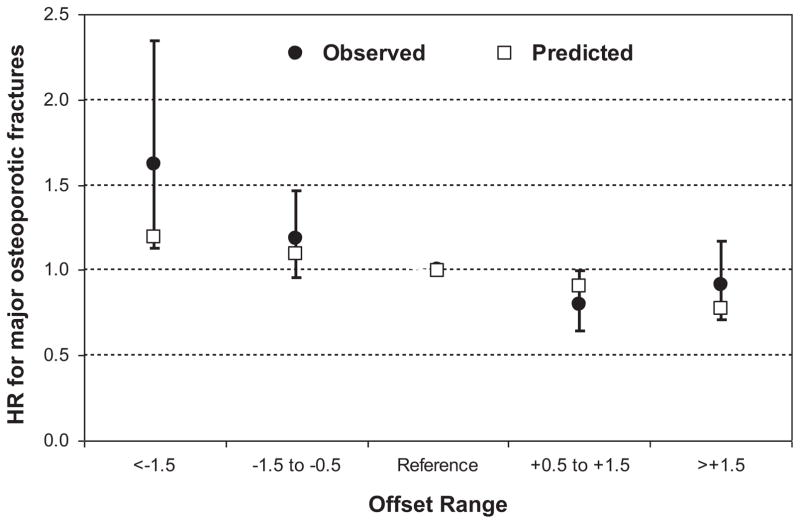
The frequency of different offset categories (below −1.5, from −1.5 to −0.5, from +0.5 to +1.5, greater than +1.5; reference category from −0.5 to +0.5) is shown in Table 3. Fig. 2 shows the effect of these categories on major osteoporotic fracture risk adjusted for FRAX probability. In all cases, the observed 95% CIs contain the predicted value.
Table 3.
Frequency Distribution of Unrounded Spine-Hip T-Score Offset Categories Without and Sex- and Age-Specific Adjustments
| Offset category | N | % | Mean offset | SD |
|---|---|---|---|---|
| Unadjusted spine-hip T-score difference | ||||
| Below −1.5 | 261 | 4.1 | −1.93 | 0.42 |
| −1.5 to −0.5 | 1270 | 19.9 | −0.92 | 0.27 |
| −0.5 to +0.5 (Reference) | 2263 | 35.4 | +0.01 | 0.29 |
| +0.5 to +1.5 | 1644 | 25.7 | +0.94 | 0.28 |
| Greater than +1.5 | 950 | 14.9 | +2.28 | 0.72 |
| Sex- and age-adjusted spine-hip T-score difference | ||||
| Below −1.5 | 497 | 7.8 | −1.97 | 0.45 |
| −1.5 to −0.5 | 1685 | 26.4 | −0.94 | 0.28 |
| −0.5 to +0.5 (Reference) | 2280 | 35.7 | 0.00 | 0.29 |
| +0.5 to +1.5 | 1323 | 20.7 | +0.93 | 0.28 |
| Greater than +1.5 | 603 | 9.4 | +2.21 | 0.65 |
Fig. 2.
Observed and predicted effect of the rounded offset adjustment on fractures adjusted for FRAX major osteoporotic fracture probability (reference category is offset between −0.5 and +0.5). Error bars are 95% confidence intervals.
The contribution of using the T-score offset rounded to the nearest integer to adjust major osteoporotic fracture probability on an individual’s categorization was assessed. Area under the receiver operating characteristic curve for the conventional FRAX major osteoporotic fracture probability was 0.689 (95% CI: 0.667–0.711) and was 0.693 (95% CI: 0.671–0.714) after the rounded offset adjustment. Calibration plots without and with the offset adjustment showed Y-intercept terms not significantly different from zero. Calibration slopes were close to unity (0.94 without the offset adjustment and 1.01 with the offset adjustment).
Table 4 shows that 5.3% of subjects had their risk category reclassified after using the rounded offset adjustment. Of the 245 individuals who moved to a lower risk category (31 [12.7%] with a subsequent fracture) and 109 who moved to a higher risk category (18 [16.5%] with a subsequent fracture), 66.5% were appropriately reclassified. Most of the re-classification (3.3%) was among those at moderate risk using the conventional FRAX major osteoporotic fracture probability measurement. In this subgroup, 2.7% were reclassified from moderate to low risk, whereas 0.6% were reclassified from moderate to high risk. For those at moderate risk using the conventional FRAX probability measurement, there was a statistically significant relationship between observed fractures and the reclassified risk category (Cochran-Armitage p trend =0.027). Observed 10-yr fracture outcomes were 16.1% among those classified as moderate risk with the conventional FRAX major osteoporotic fracture probability measurement. After reclassification using the rounded offset adjustment, the observed 10-yr fracture outcomes were 9.2% in those allocated to the low-risk category, 17.0% in those allocated to moderate risk and 19.1% in those allocated to high risk. Among the 180 individuals who changed from moderate to low risk, 16 (8.9%) subsequently experienced a major osteoporotic fracture compared with 7 of 42 (19.4%) who changed from moderate to high risk (p = 0.142 by log rank). Overall fracture discrimination as measured by the IDI was not significantly improved (p =0.684).
Table 4.
Observed Fracture Probability at 10 Yr (Kaplan-Meier Estimate) According to FRAX Major Osteoporotic Fracture Category Before and After Risk Reclassification Using the Rounded Offset Adjustment
| FRAX (No offset adjustment) | FRAX (With offset adjustment)
|
||||
|---|---|---|---|---|---|
| Overall | Low | Moderate | High | p Trend* | |
| Low (<10%) | |||||
| N total | 4443 | 4376 | 67 | 0 | |
| N fracture | 277 | 266 | 11 | 0 | <0.001 |
| Estimated fracture probability at 10 yr (%) | 6.8 | 6.7 | 18.2 | — | |
| Total reclassified | 1.0% | — | 1.0% | 0.0% | |
| Moderate (10–20%) | |||||
| N total | 1476 | 180 | 1254 | 42 | |
| N fracture | 217 | 16 | 194 | 7 | 0.027 |
| Estimated fracture probability at 10 yr (%) | 16.1 | 9.2 | 17.0 | 19.1 | |
| Total reclassified | 3.5% | 2.8% | — | 0.7% | |
| High (>20%) | |||||
| N total | 469 | 0 | 65 | 404 | |
| N fracture | 107 | 0 | 15 | 92 | 0.957 |
| Estimated fracture probability at 10 yr (%) | 25.4 | — | 25.0 | 25.4 | |
| Total reclassified | 1.0% | 0.0% | 1.0% | — | |
| Total | |||||
| N total | 6388 | 4556 | 1386 | 446 | |
| N fracture | 601 | 282 | 220 | 99 | <0.001 |
| Estimated fracture probability at 10 yr (%) | 10.3 | 6.8 | 17.4 | 24.8 | |
| Total reclassified | 5.5% | 2.8% | 2.1% | 0.7% | |
p Trend is from the Cochran-Armitage test.
Table 5 summarizes a secondary analysis when offsets were adjusted for sex- and age-dependent differences (equivalent to an offset based on Z-scores). Results were generally similar to those seen in the primary analysis with a low overall rate of reclassification (5.4%) and without an overall improvement in fracture discrimination as measured by the IDI (p =0.663). After reclassification of those at moderate risk under the conventional fracture probability measurement, the observed 10-yr fracture outcomes were 7.5% in those reallocated to the low-risk category, 16.6% in those still designated at moderate risk and 22.1% in those reallocated to high risk. Among the 125 individuals who changed from moderate to low risk, 9 (7.2%) subsequently experienced a major osteoporotic fracture compared with 13 of 65 (20.0%) who changed from moderate to high risk (p =0.010 by log rank).
Table 5.
Observed Fracture Probability at 10 Yr (Kaplan-Meier Estimate) According to FRAX Major Osteoporotic Fracture Category Before and After Risk Reclassification Using the Sex- and Age-Adjusted Rounded Offset Adjustment
| FRAX (No offset adjustment) | FRAX (With offset adjustment)
|
||||
|---|---|---|---|---|---|
| Overall | Low | Moderate | High | p Trend* | |
| Low (<10%) | |||||
| N total | 4443 | 4327 | 116 | 0 | |
| N fracture | 277 | 258 | 19 | 0 | <0.001 |
| Estimated fracture probability at 10 yr (%) | 6.8 | 6.5 | 18.1 | — | |
| Total reclassified | 1.8% | — | 1.8% | 0.0% | |
| Moderate (10–20%) | |||||
| N total | 1476 | 125 | 1286 | 65 | |
| N fracture | 217 | 9 | 195 | 13 | 0.008 |
| Estimated fracture probability at 10 yr (%) | 16.1 | 7.5 | 16.6 | 22.1 | |
| Total reclassified | 3.0% | 2.0% | — | 1.0% | |
| High (>20%) | |||||
| N total | 469 | 0 | 38 | 431 | |
| N fracture | 107 | 0 | 10 | 97 | 0.592 |
| Estimated fracture probability at 10 yr (%) | 25.4 | — | 28.7 | 25.1 | |
| Total reclassified | 0.6% | 0.0% | 0.6% | — | |
| Total | |||||
| N total | 6388 | 4452 | 1440 | 496 | |
| N fracture | 601 | 267 | 224 | 110 | <0.001 |
| Estimated fracture probability at 10 yr (%) | 10.3 | 6.6 | 17.0 | 24.7 | |
| Total reclassified | 5.4% | 2.0% | 2.4% | 1.0% | |
p Trend is from the Cochran-Armitage test.
Discussion
We found that the lumbar spine T-score, through the offset difference from the femoral neck T-score, predicted fractures independent of FRAX major osteoporotic fracture probability derived with femoral neck BMD. This was most clearly seen in women but did not achieve statistical significance in men. An age-related increase in the offset measurement was seen in both women and men, but was more prominent among the latter, possibly reflecting age-related degenerative sclerosis in the lumbar spine. Sex- and age-dependent offsets (equivalent to an offset based on Z-scores) showed improved risk classification among individuals designated to be at moderate risk with the conventional FRAX probability measurement.
The small size of the change in risk stratification is consistent with a previous report (11), and simulation studies showing very little expected benefit from combining BMD measurement sites as results tend to be moderately correlated (23). The observed adjusted HR for each offset SD seen in this study was similar to a previous report using the Manitoba Bone Density cohort, with overlapping 95% CIs, despite the fact that the latter excluded vertebral artifacts. This would suggest that the rounded offset adjustment that was initially developed and internally validated in the Manitoba Cohort is applicable to other groups even when exclusion of vertebral artifact is not performed.
Femoral neck BMD or T-score measured by DXA is the only skeletal measure recommended as an input variable to FRAX, therefore 2 individuals with different lumbar spine T-scores but identical in all other respects would generate the same fracture probabilities under FRAX. Available data suggest that in this scenario the individual with the lower lumbar spine T-score would have higher fracture risk. A report from the Study of Osteoporotic Fractures found that discordant spine and hip BMD values predicted different fracture patterns, and that women who were osteoporotic only at the spine had elevated fracture risk compared with women that were not osteoporotic at the spine or hip (24). However, the latter used categorical criteria to define between-site BMD discordance and did not analyze data according to the magnitude of the T-score difference. A large clinical cohort study found that there was an incremental information in fracture risk prediction in women when lumbar spine BMD was included in a model that already included femoral neck BMD (25).
The strength of this study is that it is a population-based cohort with documented incident fractures up to 10 yr. Limitations of this report are acknowledged. The number of men was relatively small, and this may have contributed the fact that the offset did not achieve statistical significance in men. Although we excluded obviously abnormal vertebrae and also applied an automated algorithm for vertebral exclusions (17), this may not adequately account for site-specific differences in T-score declines with aging (26). Our analysis suggests that an approach based on Z-scores may be preferable for groups with a high prevalence of vertebral artifact. Finally, we were underpowered for analyses to identify specific subgroups where the offset contributes more or less information, and whether there are significant interactions with sex, age, or ethnicity. Detailed subgroup analyses would be better assessed in a meta-analysis using the multiple FRAX cohorts.
In summary, the T-score offset between the lumbar spine and femoral neck is an independent risk factor for major osteoporotic fractures that is independent of the FRAX probability calculated using the femoral neck BMD.
Acknowledgments
We would like to thank Ms. Helena Johansson and Dr. John Kanis for generating the FRAX results for the Manitoba cohort. We thank all those participants in CaMos whose careful responses and attendance made this analysis possible.
Disclosures: None relevant to the content of this paper. Sources of support: The Canadian Multicentre Osteoporosis Study was funded by the Canadian Institutes of Health Research; Merck Frosst Canada Ltd.; Eli Lilly Canada Inc.; Novartis Pharmaceuticals Inc.; The Alliance: sanofiaventis & Procter and Gamble Pharmaceuticals Canada Inc.; Servier Canada Inc.; Amgen Canada Inc.; The Dairy Farmers of Canada; and The Arthritis Society.
CaMos Research Group: David Goltzman (co-principal investigator, McGill University), Nancy Kreiger (co-principal investigator, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto); CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Claudie Berger (study statistician); Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator); Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator); Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator); Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator); University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie A Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator); McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator); University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator); University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator); University British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Milan Patel (co-director), Yvette Vigna (coordinator); Brian C. Lentle (radiologist).
References
- 1.Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. 2007;18:1033–1046. doi: 10.1007/s00198-007-0343-y. [DOI] [PubMed] [Google Scholar]
- 2.Kanis JA, Borgstrom F, De Laet C, et al. Assessment of fracture risk. Osteoporos Int. 2005;16:581–589. doi: 10.1007/s00198-004-1780-5. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Johnell O, Oden A, et al. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA, McCloskey EV, Johansson H, et al. A reference standard for the description of osteoporosis. Bone. 2008;42:467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Looker AC, Wahner HW, Dunn WL, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–489. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 7.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blake GM, Knapp KM, Spector TD, Fogelman I. Predicting the risk of fracture at any site in the skeleton: are all bone mineral density measurement sites equally effective? Calcif Tissue Int. 2006;78:9–17. doi: 10.1007/s00223-005-0127-3. [DOI] [PubMed] [Google Scholar]
- 9.Leslie WD, Tsang JF, Caetano PA, Lix LM. Number of osteoporotic sites and fracture risk assessment: a cohort study from the Manitoba Bone Density Program. J Bone Miner Res. 2007;22:476–483. doi: 10.1359/jbmr.061112. [DOI] [PubMed] [Google Scholar]
- 10.Leslie WD, Lix LM. Absolute fracture risk assessment using lumbar spine and femoral neck bone density measurements: derivation and validation of a hybrid system. J Bone Miner Res. 2011;26:460–467. doi: 10.1002/jbmr.248. [DOI] [PubMed] [Google Scholar]
- 11.Leslie WD, Lix LM, Johansson H, et al. Spine-hip discordance and fracture risk assessment: a physician-friendly FRAX enhancement. Osteoporos Int. 2011;22:839–847. doi: 10.1007/s00198-010-1461-5. [DOI] [PubMed] [Google Scholar]
- 12.Kreiger N, Tenenhouse A, Joseph L, et al. Research notes: the Canadian Multicentre Osteoporosis Study (CaMos)—background, rationale, methods. Can J Aging. 1999;18:376–387. [Google Scholar]
- 13.Lu Y, Fuerst T, Hui S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int. 2001;12:438–444. doi: 10.1007/s001980170087. [DOI] [PubMed] [Google Scholar]
- 14.Genant HK. Universal standardization for dual X-ray absorptiometry: patient and phantom cross-calibration results. J Bone Miner Res. 1995;10:997–998. doi: 10.1002/jbmr.5650100624. [DOI] [PubMed] [Google Scholar]
- 15.Berger C, Langsetmo L, Joseph L, et al. Change in bone mineral density as a function of age in women and men and association with the use of antiresorptive agents. Can Med Assoc J. 2008;178:1660–1668. doi: 10.1503/cmaj.071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenenhouse A, Joseph L, Kreiger N, et al. Estimation of the prevalence of low bone density in Canadian women and men using a population-specific DXA reference standard: the Canadian Multicentre Osteoporosis Study (CaMos) Osteoporos Int. 2000;11:897–904. doi: 10.1007/s001980070050. [DOI] [PubMed] [Google Scholar]
- 17.Barden HS, Markwardt P, Payne R, et al. Automated assessment of exclusion criteria for DXA lumbar spine scans. J Clin Densitom. 2003;6:401–410. doi: 10.1385/jcd:6:4:401. [DOI] [PubMed] [Google Scholar]
- 18.Fraser LA, Langsetmo L, Berger C, et al. Fracture prediction and calibration of a Canadian FRAX(R) tool: a population-based report from CaMos. Osteoporos Int. 2011;22:829–837. doi: 10.1007/s00198-010-1465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie WD, Lix LM, Johansson H, et al. Independent clinical validation of a Canadian FRAX tool: fracture prediction and model calibration. J Bone Miner Res. 2010;25:2350–2358. doi: 10.1002/jbmr.123. [DOI] [PubMed] [Google Scholar]
- 20.Siminoski K, Leslie WD, Frame H, et al. Recommendations for bone mineral density reporting in Canada. Can Assoc Radiol J. 2005;56:178–188. [PubMed] [Google Scholar]
- 21.Janes H, Pepe MS, Gu W. Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med. 2008;149:751–760. doi: 10.7326/0003-4819-149-10-200811180-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 23.Blake GM, Patel R, Knapp KM, Fogelman I. Does the combination of two BMD measurements improve fracture discrimination? J Bone Miner Res. 2003;18:1955–1963. doi: 10.1359/jbmr.2003.18.11.1955. [DOI] [PubMed] [Google Scholar]
- 24.Fink HA, Harrison SL, Taylor BC, et al. Differences in site-specific fracture risk among older women with discordant results for osteoporosis at hip and spine: study of osteoporotic fractures. J Clin Densitom. 2008;11:250–259. doi: 10.1016/j.jocd.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leslie WD, Lix LM, Tsang JF, Caetano PA. Single-site vs multisite bone density measurement for fracture prediction. Arch Intern Med. 2007;167:1641–1647. doi: 10.1001/archinte.167.15.1641. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner KG, von SE, Miller P. Discordance in patient classification using T-scores. J Clin Densitom. 1999;2:343–350. doi: 10.1385/jcd:2:3:343. [DOI] [PubMed] [Google Scholar]