Abstract
Summary
In this cross-sectional study, we found that areal bone mineral density (aBMD) at the knee and specific tibia bone geometry variables are associated with fragility fractures in men and women with chronic spinal cord injury (SCI).
Introduction
Low aBMD of the hip and knee regions have been associated with fractures among individuals with chronic motor complete SCI; however, it is unclear whether these variables can be used to identify those at risk of fracture. In this cross-sectional study, we examined whether BMD and geometry measures are associated with lower extremity fragility fractures in individuals with chronic SCI.
Methods
Adults with chronic [duration of injury≥2 years] traumatic SCI (C1-L1 American Spinal Cord Injury Association Impairment Scale A-D) reported post injury lower extremity fragility fractures. Dual-energy X-ray absorptiometry (DXA) was used to measure aBMD of the hip, distal femur, and proximal tibia regions, while bone geometry at the tibia was assessed using peripheral quantitative computed tomography (pQCT). Logistic regression and univariate analyses were used to identify whether clinical characteristics or bone geometry variables were associated with fractures.
Results
Seventy individuals with SCI [mean age (standard deviation [SD]), 48.8 (11.5); 20 females] reported 19 fragility fractures. Individuals without fractures had significantly greater aBMD of the hip and knee regions and indices of bone geometry. Every SD decrease in aBMD of the distal femur and proximal tibia, trabecular volumetric bone mineral density, and polar moment of inertia was associated with fracture prevalence after adjusting for motor complete injury (odds ratio ranged from 3.2 to 6.1).
Conclusion
Low knee aBMD and suboptimal bone geometry are significantly associated with fractures. Prospective studies are necessary to confirm the bone parameters reported to predict fracture risk in individuals with low bone mass and chronic SCI.
Keywords: Bone density, Bone geometry, Fracture, Osteoporosis, Peripheral quantitative computed tomography, Spinal cord injury
Introduction
Individuals with spinal cord injury (SCI) experience a substantial decline in bone mass in the lower extremity following their injury, predisposing them to an increased risk of low-energy, lower extremity fracture [1–3]. Low-energy fractures or fragility fractures are common after SCI, often occurring during activities of daily living such as transferring from wheelchair to bed, rolling in bed, or bumping into unseen objects [4–7]. The majority of fragility fractures among individuals with SCI occur at the distal femur or proximal tibia [1, 3]. An individual with SCI has approximately twice the risk of experiencing a lower extremity fracture for each 1 standard deviation (SD) decrement in hip and femoral neck T-score when compared to age-matched and gender-matched individuals without SCI [8]. Fragility fractures often result in increased healthcare costs, short-term hospitalization, increased disability, and mortality [4, 9]. Therefore, establishing strategies to improve the identification of individuals at high risk of fragility fracture would enable the implementation of fracture prevention strategies.
Dual-energy X-ray absorptiometry (DXA) is routinely used to assess areal bone mineral density (aBMD), diagnose osteoporosis, and quantify fracture risk among postmenopausal women and men over the age of 50 [10]. Clinical practice guidelines recommend that risk factors such as age, sex, prior fragility fractures, and glucocorticoid use be incorporated with aBMD to assess fracture risk [11, 12]. However, current fracture risk assessment paradigms are not designed for use in individuals <50 years of age [10], which may limit their applicability in the SCI population as the average age at injury in this population is 39.5 years [13]. Further, the risk factors for fracture after SCI that have emerged to date, such as low body mass index, completeness of injury, and duration of injury (DOI), are not accounted for in current risk assessment tools [14]. Finally, standard assessments of aBMD are performed at the spine and hip, but in SCI, studies often measure aBMD of the distal femur and proximal tibia [15–17] as it is the most common site of significant bone loss [18] and fractures in this population [19, 20]. Garland et al. have reported DXA-based aBMD fracture thresholds, values below which fractures occur, at the knee of 0.78 g/cm2 [19].
However, there are no available prospective data or large cross-sectional studies to verify correlates of fracture using validated knee region DXA protocols. Further, in the SCI population, it is often difficult to obtain accurate scans of the lumbar spine due to the presence of artifacts, including posterior element changes, laminectomy, hardware, and/or scoliosis, and the hip region due to subluxation, dislocation, flexion contractures, or heterotopic bone formation.
aBMD assessments using DXA cannot distinguish between cortical and trabecular bone compartments or evaluate bone quality, geometry, or microarchitecture. Peripheral quantitative computed tomography (pQCT) is a noninvasive technique that provides a measurement of volumetric bone mineral density (vBMD), and is able to differentiate between trabecular and cortical bone compartments, with no need for the participant to transfer from their wheelchair for scan acquisition. Assessment of trabecular and cortical structure and geometry may improve fracture risk prediction [21–23]. Currently, there have been a limited number of studies that have examined bone quality among individuals with SCI using pQCT. These studies report decreases in trabecular volumetric bone mineral density (TbvBMD), cortical thickness (CTh), and stress–strain index by 15–49 % [15, 24], 17–47 % [15, 25], and 17–19 % [25], respectively. It has been suggested that TbvBMD at the distal tibia epiphysis below 72 mg/cm3 is associated with prevalent fractures in males with complete SCI [20]; however, it is unknown if this finding extends to females or individuals with incomplete injuries (American Spinal Cord Injury Association Impairment Scale (AIS) C-D).
The objective of this study was to examine whether DXA-based measures of BMD or pQCT-based estimates of bone geometry at the tibia are associated with lower extremity fragility fractures in men and women with chronic SCI.
Methods
Study participants
We used the baseline data from a 2-year prospective longitudinal study being conducted at the University of Waterloo and the Lyndhurst Centre, Toronto Rehabilitation Institute, UHN for the current cross-sectional study. Baseline assessments were completed between April 2009 and June 2012. To establish a representative sample of the SCI population; men and women ≥18 years of age with spinal cord impairment [C1-L2 AIS A-D] of sudden onset (<24 h) were included in this study. To ensure that participants were neurologically and medically stable and had low bone mass typical of sublesional osteoporosis, participants included were at least 2 years post injury prior to enrolment. Participants were excluded if they had (a) a current or prior known condition, other than paralysis, known to influence bone metabolism including oral glucocorticoid use ≥3 months, malignancy, and known liver disease or malabsorption condition; (b) a body weight ≥270 lbs (maximum body weight for DXA); (c) planned to become pregnant or were pregnant at enrolment; or (d) contraindications to pQCT including bilateral lower extremity metal implants or severe hip and knee flexion contractures. Participants were recruited from the Lyndhurst Long-term Follow-up Database and the Outpatient Service Programs at Toronto Rehab’s Lyndhurst Centre and Hamilton Health Sciences, Chedoke site. All participants gave written informed consent for participation. The appropriate institutional review boards approved study conduct.
Medical history and demographics
Past and current medical history, current medication (including bisphosphonate use), demographic, lifestyle, and impairment data were obtained via participant interview and chart abstraction by a research coordinator or research associate. The participants’ neurological level of injury and AIS classification were determined by a physiatrist (BCC) using the International Standards for Neurologic Classification of SCI [26]. Height (in centimeters), weight (in kilograms), and waist circumference (in centimeters) were also recorded. Height was self-reported, while weight was measured using a scale (Model 6059, BMH Medical Inc., Addison, IL, USA) attached to the ceiling lift during transfer from wheelchair onto the DXA scanning bed. If the participant did not require a ceiling lift to transfer, they were weighed in their wheelchair to the nearest 0.1 kg on a floor scale (Model 513-417, Stathmos Scale Manufacturing Limited, Milliken, ON, USA). Once transferred to the DXA plinth, the participant’s wheelchair was weighed, and this weight was subtracted from the total weight (participant and wheelchair) to calculate the participant’s body weight. Waist circumference was measured after normal expiration below the lowest rib in the supine position with a Gulick II Tape Measure (Model 67019, Country Technology Inc., Gay Mills, WI, USA) [27].
Lower extremity fragility fractures
Participants were asked about the time, cause, and location of any fractures that occurred following their injury. Fragility fractures were verified through the participants’ medical records and X-rays, in which a written informed consent was obtained for health record abstraction. Protocols for verifying fractures and obtaining records were modeled after those used in the Canadian Multicentre Osteoporosis Study, a population-based cohort study of 10,000 individuals across Canada [28].
Individuals with a history of at least one lower extremity fragility fracture (cases), excluding toes, were compared to those without a fracture (controls). Fragility fractures were those that occurred due to minimal or no trauma. Fractures caused by high-energy trauma or fractures that occurred prior to injury, or at the time of the injury, were removed from the data set and excluded from the analysis.
aBMD by DXA
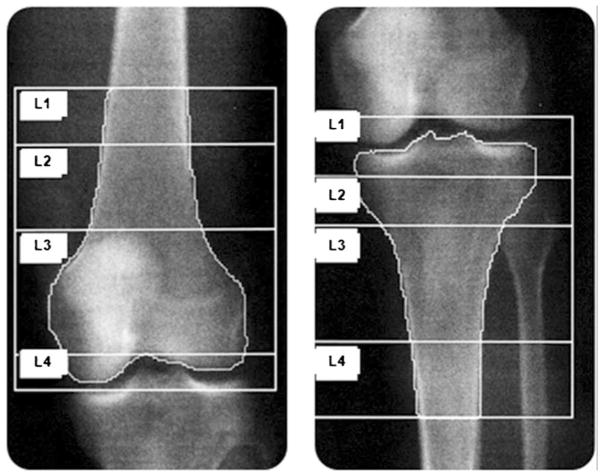
aBMD (in grams per square centimeter) was assessed by DXA (4500A, Hologic Inc., Waltham, MA, USA). The left hip (total hip and femoral neck), right distal femur, and right proximal tibia were scanned; in cases of severe spasticity, prior left hip pathology or regional surgery, or other contraindications, the alternate limb was scanned. The site was equipped with a ceiling lift to transfer participants on and off the scanning table. Participants were positioned supine on the scanning table. Each skeletal site took approximately 6 min to scan. The hip scans were analyzed using commercially available software from Hologic Inc. A lower extremity positioning device and knee aBMD protocol, whose reliability and accuracy have been previously determined [29], was used to acquire the scans for the distal femur and proximal tibia. A lower extremity polycarbonate positioning device, not recognized by the densitometer, is used to maintain optimal positioning during scan acquisition, ensuring the knee joint is perpendicular to the long axis of the femur, ensuring reliable overlap of the patella above the knee joint line, and limiting overlap of the fibular head over the proximal tibia. Scan acquisition places the laser crosshair 5 cm distal to the inferior border of the patella for the distal femur and 23 cm distal to the superior border of the patella for the proximal tibia. Total scan length is 24 cm for both the distal femur and proximal tibia. Scans are acquired and analyzed using the Hologic lumbar spine software (Fig. 1). The width of the distal femur and proximal tibia are measured in pixels and multiplied by 5.36 for the femur and 4.64 for the tibia, for a total region interest which is 25 % of femur estimated bone length and 30 % of proximal tibia estimated bone length. The relationships between the measured width and estimated length of the femur and tibia are derived from anatomic data published by Yoshioka et al. [30]. All scans were acquired and analyzed by a trained technologist in the Bone Density Lab at Lyndhurst Centre, Toronto Rehabilitation Institute. In a sample of 110 participants, the least significant change for DXA is 2 % for the distal femur and 3 % for the proximal tibia.
Fig 1.
DXA scans of the distal femur (left) and proximal tibia (right)
pQCT assessment
A pQCT scanner (XCT-2000, Stratec Mezintecknik, Pforzheim, Germany) was used to scan two sites on the tibia. The distal end and the tibia shaft were scanned to obtain information about trabecular bone and cortical bone, respectively. The right tibia was scanned, except in those participants with severe spasticity or other contraindications in which the left tibia was scanned instead (n=36). Reconstructing the 145 projection angles obtained by a narrow fan beam emitted from an X-ray tube creates an image. Bony landmarks at the medial condyle and medial malleolus were palpated, and a measuring tape was used to measure the distance between the two points. A line was marked on the leg at a spot corresponding to 66 % of the tibia length, measuring proximally from the distal landmark. The tibia distal endplate (anatomical reference point) was identified on a 30-mm coronal view of the joint line from the scout scan. The ultradistal tibia scan site was automatically located proximal to the tibia distal endplate at 4 % of the tibia length measuring proximally. The scanner was manually repositioned at the 66 % line to measure BMD and geometry at the tibia shaft. A single 2.5-mm slice was acquired at each site. A voxel size of 0.2 mm was used at the 4 % site, while a voxel size of 0.5 mm was used at the 66 % site. The 66 % site was not scanned for participants (n=3) whose calf circumference exceed the 15-cm gantry opening. To ensure consistency, the same technologist performed all the scans. We have established precision in our setting in individuals with and without SCI; the root mean square coefficient of variation was ≤2 % for all bone measures of interest [31].
Analyses of the pQCT scans were performed using the manufacturer’s software (Stratec XCT-2000 version 6.00). Contour mode 3 and peel mode 2 with an outer threshold of 130 mg/cm3 and an inner threshold of 400 mg/cm3 was used in CALCBD mode to assess TbvBMD (in milligrams per cubic centimeter). Contour mode 1 and a threshold of 710 mg/cm3 were used in CORTBD mode to determine CTh (in millimeters), cross-sectional moment of inertia (CSMI, in millimeters raised to the fourth power), polar moment of inertia (PMI, in millimeters raised to the fourth power), and buckling ratio (BR) as it is the default threshold [32]. CSMI and PMI represent the ability of bone to resist bending and torsion, respectively. BR is defined as the subperiosteal radius divided by the mean CTh [(PERI×PERI)/(CRT_A)(2π)]; it expresses the likelihood of bone to buckle due to excessive cortical thinning. Higher values of BR suggest greater instability of thin walls, contributing to fractures [33].
Statistical analysis
To ensure a stable model, 10–15 events are needed for every included correlate. There were 19 participants with fragility fractures, limiting our ability to include multiple correlates in a single statistical model. Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). We used descriptive statistics to describe all participant characteristics, anthropometric measurements, and indices of bone strength. Dichotomous variables were presented as counts (percent), while continuous variables were presented as mean (SD). Comparisons between the group with fragility fractures and the group without fractures were performed using unpaired two-sided t tests for continuous variable and chi-square tests for dichotomous variables. The criterion for statistical significance was set at α=0.05. We did not adjust the overall level of analyses for multiple testing, as the analyses are primarily exploratory. Univariate analyses were used to determine whether clinical characteristics, including age, gender, motor complete injury (AIS A-B), DOI, and past bisphosphonate use, were significant correlates of fragility fracture. We used logistic regression to examine the relationship between DXA-based and pQCT-based measurements of BMD or geometry and fracture history. Results are expressed as odds ratios (OR) per SD decrease in BMD and geometry, 95 % confidence intervals (CI), and associated p values. All p values are reported to three decimal places, with those <0.001 reported as p<0.001.
Results
Participants’ characteristics
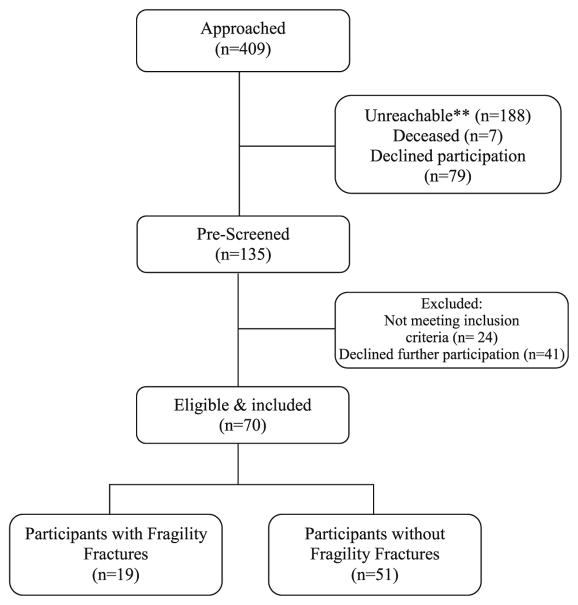
Of the 409 individuals approached, 274 were unreachable, deceased, or declined to participate. Following screening, we identified a total of 70 consenting men and women eligible for study participation. Figure 2 presents a study flowchart summarizing the recruitment and prescreening process prior to cohort assembly.
Fig 2.
Flow diagram for the study. Double asterisks denote that letter mail or telephone follow-up was unsuccessful
The anthropometric characteristics of the study participants are provided in Table 1. The sample consisted of 70 individuals with chronic SCI; 50 males (71.4 %) and 20 females (28.6 %; 8 postmenopausal). The mean (SD) age was 48.8 (11.5) years with a mean (SD) time post injury of 15.5 (10.0) years. There were 23 individuals with motor complete (AIS A-B) paraplegia, 11 with motor incomplete (AIS C-D) paraplegia, 22 with motor complete (AIS A-B) tetraplegia, and 14 with motor incomplete (AIS C-D) tetraplegia.
Table 1.
Sociodemographic and impairment characteristics
| All SCI participants (n=70) | Participants with fragility fractures (n=19) | Participants without fragility fractures (n=51) | p value | |
|---|---|---|---|---|
| Gender (female): n (%) | 20 (29) | 6 (32) | 14 (27) | 0.734 |
| Age (years): mean (SD) | 48.8 (11.5) | 48.9 (10.6) | 48.8 (11.9) | 0.976 |
| DOI (years): mean (SD) | 15.5 (10.0) | 19.4 (11.8) | 14.0 (8.9) | 0.082 |
| Height (cm): mean (SD) | 174.5 (10.3) | 174.1 (13.7) | 174.7 (8.9) | 0.874 |
| Weight (kg): mean (SD) | 80.1 (18.4) | 80.0 (20.8) | 80.6 (17.5) | 0.965 |
| Waist circumference (cm): mean (SD) | 97.4 (14.8) | 98.1 (16.7) | 97.2 (14.2) | 0.837 |
| Motor complete injury: n (%) | 45 (64) | 17 (89) | 28 (55) | 0.007 |
| LEMS: mean (SD) | 11.2 (15.8) | 2.3 (5.4) | 14.3 (17.1) | <0.001 |
| Sensory score: mean (SD) | 101.8 (53.6) | 102.1 (55.8) | 101.7 (53.4) | 0.984 |
| Past bisphosphonate user, n (%) | 38 (54) | 12 (63) | 26 (51) | 0.363 |
| Past calcium supplement, n (%) | 57 (81) | 18 (95) | 39 (76) | 0.081 |
| Past vitamin D supplement, n (%) | 61 (87) | 19 (100) | 42 (82) | 0.050 |
| Past multivitamin supplement, n (%) | 34 (49) | 11 (58) | 23 (45) | 0.341 |
| Smoking history: n (%) | 40 (57) | 11 (58) | 29 (57) | 0.938 |
| Alcohol history: n (%) | 43 (61) | 15 (79) | 28 (55) | 0.066 |
LEMS lower extremity motor scores
Twenty-seven percent of participants (n=19) had a history of fragility fracture following their SCI, where 13 (68.4 %) were men and 6 (31.6 %) were women. The number of individual fracture events per participant ranged from 1 to 7, with a total of 38 fragility fractures among 19 participants. Fragility fractures occurred as a result of torsion (n=5), low-velocity falls (n=20), transfers (n=13), low-velocity fall during a transfer (n=2), and other methods (n=5) such as intercourse, spasms, and being hit in the leg. Lower extremity fractures occurred more frequently at the femur (n=16), followed by the tibia (n=11), ankle (lateral/medial malleolus or anterior and posterior surfaces of the distal tibia; n=5), knee and fibula (n=3), and hip (femoral neck or intertrochanteric regions, n=3). One participant reported three fractures around the knee (femur, tibia, and fibula) for which X-rays were unavailable to confirm fracture location; however, this participant sustained two other fractures, one at the left femur and one at the right hip, which were verified.
Indices of bone strength in cases and controls
DXA scans of the distal femur could not be acquired on six participants, half due to prior bilateral fracture and half due to hardware presence. One proximal tibia scan could not be acquired due to the presence of hardware. During the pQCT scans, five ultradistal tibia scans and seven 66 % of the tibia length scans were not obtained because participants either missed their appointment, had severe spasticity, declined, had a health complication, or had a calf circumference which exceeded the gantry opening precluding accurate positioning for scan acquisition. Of the participants in whom a pQCT scan was not acquired, four had sustained a prior fragility fracture.
Table 2 summarizes the mean and SD for indices of BMD and geometry obtained by pQCT and DXA. Individuals with SCI who had sustained a lower extremity fracture had lower aBMD of the total hip and femoral neck (p<0.05) and distal femur and proximal tibia (p<0.0001). Cases also had a much lower TbvBMD, CTh, CSMI, and PMI in comparison to the control group (p<0.05).
Table 2.
Mean (SD) of indices of bone density and geometry between individuals with SCI with and without lower extremity fragility fractures
| Participants with fragility fractures | Participants without fragility fractures | p value | |
|---|---|---|---|
| DXA | |||
| Distal femur aBMD (g/cm2) | 0.454 (0.11) | 0.667 (0.20) | <0.001 |
| Proximal tibia aBMD (g/cm2) | 0.371 (0.10) | 0.541 (0.16) | <0.001 |
| Total hip aBMD (g/cm2) | 0.730 (0.19) | 0.769 (0.17) | 0.021 |
| Femoral neck aBMD (g/cm2) | 0.689 (0.13) | 0.595 (0.14) | 0.010 |
| pQCT | |||
| TbvBMD (mg/cm3) | 84.4 (33.3) | 145.7 (56.3) | <0.001 |
| CTh (mm) | 2.66 (0.79) | 3.47 (0.88) | 0.003 |
| BR | 6.1 (2.0) | 5.0 (1.4) | 0.057 |
| CSMI (mm4) | 4,603.1 (2,264.3) | 7,129.5 (4,782.7) | 0.009 |
| PMI (mm4) | 31,983.6 (10,278.1) | 46,971.8 (19,030.7) | <0.001 |
aBMD areal bone mineral density, TbvBMD trabecular volumetric bone mineral density, CTh cortical thickness, BR buckling ratio, CSMI cross-sectional moment of inertia, PMI polar moment of inertia
Relationship between bone mineral density and geometry and fracture risk
Table 3 shows the association between BMD and geometry and fragility fractures among individuals with SCI. Each SD decrease in DXA-based and pQCT-based measures of BMD and geometry were associated with increased risk of fractures, except for CSMI (OR=2.0, 95 % CI=1.0–4.8, p=0.07).
Table 3.
Associations between fragility fractures and indices of bone density and geometry obtained by pQCT and DXA
| Fractures (unadjusted), OR (95 % CI) | p value | Fractures (adjusted)a, OR (95 % CI) | p value | |
|---|---|---|---|---|
| DXA | ||||
| Distal femur aBMD (g/cm2) | 4.9 (2.0, 15.9) | 0.002 | 4.9 (1.7, 17.5) | 0.006 |
| Proximal tibia aBMD (g/cm2) | 6.5 (2.5, 23.0) | <0.001 | 6.1 (2.1, 23.6) | 0.003 |
| Total hip aBMD (g/cm2) | 2.4 (1.3, 5.1) | 0.009 | 1.9 (1.0, 4.1) | 0.083 |
| Femoral neck aBMD (g/cm2) | 2.1 (1.2, 4.0) | 0.019 | 1.7 (0.9, 3.4) | 0.093 |
| pQCT | ||||
| TbvBMD (mg/cm3) | 5.9 (2.2, 24.6) | 0.003 | 6.5 (1.9, 32.9) | 0.010 |
| CTh (mm) | 2.8 (1.4, 6.5) | 0.006 | 2.2 (1.1, 5.5) | 0.053 |
| BR | 0.5 (0.2, 0.9) | 0.033 | 0.7 (0.3, 1.2) | 0.176 |
| CSMI (mm4) | 2.0 (1.0, 4.8) | 0.073 | 1.8 (0.8, 4.3) | 0.158 |
| PMI (mm4) | 3.9 (1.5–13.3) | 0.013 | 3.2 (1.2–11.4) | 0.038 |
aBMD areal bone mineral density, TbvBMD trabecular volumetric bone mineral density, CTh cortical thickness, BR buckling ratio, CSMI cross-sectional moment of inertia, PMI polar moment of inertia
OR per SD decrease, adjusted for motor complete injury
Motor complete injury (p=0.01) was one clinical characteristic found to be associated with fragility fractures; age (p=0.98), gender (p=0.73), and prior bisphosphonate therapy (p=0.36) were not associated with lower extremity fragility fractures; however, longer DOI did trend towards fracture occurrence (p=0.08). After adjusting for motor complete injury, a 1 SD decrease in aBMD at the distal femur (where 1 SD refers to that obtained from a distribution obtained from the current sample of individuals with SCI) was associated with increased odds of fracture (OR=4.9; 95 % CI=1.7–17.5), as was a 1 SD decreases in aBMD at the proximal tibia (OR=6.1; 95 % CI=2.1–23.6). Decreased TbvBMD (OR=6.5; 95 % CI=1.9–32.9) and PMI (OR=3.2; 95 % CI=1.2–11.4) was associated with increased risk of fracture, while CTh (OR=2.2; 95 % CI=1.1–5.5) derived from pQCT at the 66 % site approached statistical significance in individuals with SCI. There were no associations found between fractures and total hip and femoral neck aBMD assessed by DXA or BR and CSMI assessed by pQCT.
Discussion
We found that men and women with chronic SCI who have sustained a fragility fracture post-SCI had significantly lower indices of BMD and bone geometry than those without fractures. In addition, we found that knee region aBMD was a strong correlate of fragility fractures, as were several other indices of bone geometry. However, after the addition of motor complete injury, only aBMD of the distal femur and proximal tibia, TbvBMD, and PMI were significantly associated with fragility fractures.
Among the clinical characteristics we examined, motor complete injury (AIS A-B) was the only variable associated with fragility fractures; individuals with motor complete injury were more likely to have experienced a fracture than those with incomplete injury, which is consistent with prior studies [6, 19]. Completeness of injury has been previously determined to be associated with aBMD at the knee. One study suggests that men with complete injuries are ≥6 times more likely to have aBMD of the knee low enough to place them into the osteoporotic category [34]. These findings may be explained by Frost’s mechanostat theory [35], which suggests that bone strength is adapted by strains created by physiological loads; muscle contractions provide the largest load on bone. Individuals with incomplete injuries have the ability to contract their muscles, and possibly bear weight, which may explain the higher aBMD among individuals with incomplete injuries. A recent cross-sectional study performed on men and women with chronic SCI found that surrogate measures of muscle force production are strongly correlated with vBMD and BMC at the distal tibia, with the strongest correlations being seen in individuals with complete injury [36]. Given that a muscle–bone relationship exists in individuals with SCI, muscle atrophy is a probable explanation for ensuing decreases in bone strength. Therefore, completeness of injury greatly influences the loss of bone in the lower extremity and should be considered as an important nonmodifiable fracture risk factor in individuals with SCI.
We found that there is an association between aBMD in the distal femur and proximal tibia and fragility fractures in individuals with SCI, and PMI was also associated with fragility fractures. Our findings are consistent with Garland et al. [19] who found significant differences between knee aBMD in individuals with SCI with fractures and those without fractures. However, our results are unique from the aforementioned study in that aBMD in the knee remains a strong correlate of fractures even after adjusting for completeness of injury. Furthermore, our study used a validated protocol for obtaining knee region aBMD [29], with established precision in individuals with SCI. It has also been previously determined that aBMD is a significant correlate of increased frequency of fractures in individuals with SCI after adjusting for age, DOI, and level of SCI [8]. Unfortunately, we were unable to assess the association between aBMD and the number of fractures per participant as the median number of fracture events was not large enough; 14 of our 19 individuals with fractures had only 1 or 2 fracture events, while the remaining individuals had either 3, 5, or 7 fractures events. Similar to our findings, De Bruin et al. [37] reported that men with complete SCI with a history of lower extremity fractures had a substantially lower area moment of inertia of the tibia, suggesting that the distribution of bone mineral around the bone’s bending axis is decreased in the SCI population with fragility fractures compared to those without. The association between CTh and PMI and fragility fractures may explain why individuals with SCI are susceptible to fractures as a result of torsional forces (e.g., during transfers).
Femoral neck aBMD was confirmed to be associated with fragility fractures in our sample [8]; however, it was no longer significant after adjusting for completeness of injury. The hip region is not the most common fracture site in the SCI population; however, we assessed it because it is the standard to assess the 10-year fracture risk in postmenopausal women and men over the age of 50 years [11, 12], and it is continuously used to assess fracture risk in individuals with neurological and muscular impairments. Our findings suggest that distal femur or proximal tibia aBMD in individuals with SCI is more strongly correlated with fracture than femoral neck aBMD. However, not all centers have standardized protocols for measuring knee aBMD. We suggest that the interpretation of femoral neck aBMD be made with an understanding that it may not accurately reflect fracture risk in SCI as aBMD of the distal femur and proximal tiba are fracture-prone sites and that other clinical correlates, such as completeness of injury, should be considered in decision-making. Further, it would be advisable to include a knee aBMD outcome or pQCT-based measures of bone geometry in any future trials evaluating interventions or fracture risk assessment protocols.
Individuals with SCI present with a unique pattern of regional low bone mass, the pathophysiology which is likely multifactorial. Muscle atrophy, lack of weight-bearing activity, autoimmune-mediated mechanisms, and loss of neural innervation to bone are among the proposed mechanisms [38]. Low bone mass and poor bone quality among individuals with SCI may be mediated in part by modifiable risk factors, such as secondary hyperparathyroidism, a common health concern among individuals with SCI [39–42]. We [43] and others [44] have observed that hyperparathyroidism can persist in chronic SCIs [43]. Indeed, parathyroid hormone (PTH) level was positively correlated with 25-hydroxyvitamin D status and the serum bone resorption marker, CTX-I, in individuals with chronic SCI [43]. We chose not to examine bone turnover or hormone levels as potential correlates of fracture a priori because of our cross-sectional design; any bone loss attributable to elevated bone turnover or hormone levels in the past should have manifested as altered BMD or structure. However, a future aim is to examine whether the presence of elevated bone turnover or PTH or suboptimal vitamin D status is a contributor to bone loss in the chronic stages of SCI through long-term follow-up of our cohort. We have observed that changes in aBMD and cortical bone geometry in individuals with SCI may contribute to fracture risk, and the identification of modifiable risk factors for fracture in SCI, such as elevated PTH, may facilitate the development of targeted fracture prevention strategies.
The most notable strength of our study is that we have a population consisting of both men and women with a diverse level of neurological impairment (C1-L1 AIS A-D). We acknowledge that there are limitations to the study. This study was a cross-sectional study; therefore, a causal relationship between fragility fractures and indices of BMD and geometry could not be established. An additional limitation was that we are unable to confirm the generalizability of the data from our sample; many of the individuals approached regarding study participation declined to participate, were unreachable, had deceased, or did not meet the study inclusion. However, given the difficulties recruiting individuals with SCI and the paucity of data on correlates of fracture after SCI, the current study adds important insight on fracture risk assessment in SCI. Due to the limited number of events, we were unable to perform multivariable analysis assessing whether or not the addition of pQCT parameters to the aBMD model explains any additional variance or perform logistic regression examining correlates of specific types of fractures. Our participants were also recruited from a tertiary osteoporosis clinic; for that reason, 54 % of our participants were on bisphosphonate treatments to improve their bone health. The average duration of bisphosphonate use in our sample was approximately 32 months. However, in our sample, bisphosphonate use was not correlated with fragility fracture. Finally, we did not exclude total hip DXA scans that contained heterotopic ossification and other clinical irregularities proximal to the lesser and/or greater trochanter. We also included pQCT scans with movement artifacts. Movement artifacts can occur with spasticity and could have contributed to overestimations and underestimations in bone measures. However, we found that removing the scans with movement artifacts did not result in changes to the mean values of the pQCT-based measurements of bone or the OR.
Conclusion
In summary, we found that distal femur and proximal tibia BMD and bone geometry parameters are associated with fragility fractures in individuals with SCI. The findings presented in this study provide the framework for future enquiry into the relationship between fragility fractures and indices of bone quality assessed noninvasively by pQCT and risk factors associated with sublesional osteoporosis. The results also provide preliminary evidence to support the current routine practice of assessing distal femur and proximal tibia aBMD as a means of predicting individual fracture risk. A larger prospective study is required to confirm the clinical characteristics, BMD, and bone strength measures ideal for fracture risk assessment in individuals with chronic SCI.
Acknowledgments
The authors acknowledge the support from the Ontario Neurotrauma Foundation (grant #2009-SC-MA-684), the Canadian Institutes of Health Research (grant #86521), the Spinal Cord Injury Solutions Network (RHI; grant #2010-43), and the Toronto Rehabilitation Institute who receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-Term Care. The views expressed do not necessarily reflect those of the ministry.
Footnotes
Conflicts of interest
A. Papiaoannou, JD. Adachi - Consultant/Speaker: Amgen, Eli Lillly, GSK, Merk, Novartis, Warner-Chilcott; Clinic Trials: Eli Lilly, Merck, Novartism, Pfizer.
M. Popovic - Share holder in MyndTec.
L. Giangregorio - Research Support: Merck.
Contributor Information
D. Lala, Department of Kinesiology, University of Waterloo, 200 University Avenue West, Waterloo, ON, Canada. Lyndhurst Centre, Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada
B. C. Craven, Department of Medicine, University of Toronto, Toronto, ON, Canada. Lyndhurst Centre, Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada
L. Thabane, Department of Clinical Epidemiology and Biostatistics, McMaster University, Hamilton, ON, Canada
A. Papaioannou, Department of Medicine, McMaster University, Hamilton, ON, Canada
J. D. Adachi, Department of Medicine, McMaster University, Hamilton, ON, Canada
M. R. Popovic, Institute of Biomaterials and Biomedical Engineering, University of Toronto, Toronto, ON, Canada. Lyndhurst Centre, Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada
L. M. Giangregorio, Department of Kinesiology, University of Waterloo, 200 University Avenue West, Waterloo, ON, Canada. Lyndhurst Centre, Toronto Rehabilitation Institute, University Health Network, Toronto, ON, Canada
References
- 1.Garland DE, Adkins RH. Bone loss at the knee in spinal cord injury. Top Spinal Cord Inj Rehabil. 2001;6:37–46. [Google Scholar]
- 2.Garland DE, Stewart CA, Adkins RH, et al. Osteoporosis after spinal cord injury. J Orthop Res. 1992;10:371–378. doi: 10.1002/jor.1100100309. [DOI] [PubMed] [Google Scholar]
- 3.Zehnder Y, Lüthi M, Michel D, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004;15:180–189. doi: 10.1007/s00198-003-1529-6. [DOI] [PubMed] [Google Scholar]
- 4.Vestergaard P, Krogh K, Rejnmark L, et al. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–796. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 5.Ragnarsson KT, Sell GH. Lower extremity fractures after spinal cord injury: a retrospective study. Arch Phys Med Rehabil. 1981;62:418–423. [PubMed] [Google Scholar]
- 6.Comarr AE, Hutchinson RH, Bors E. Extremity fractures of patients with spinal cord injuries. Top Spinal Cord Inj Rehabil. 2005;11:1–10. doi: 10.1016/0002-9610(62)90256-8. [DOI] [PubMed] [Google Scholar]
- 7.Freehafer AA, Hazel CM, Becker CL. Lower extremity fractures in patients with spinal cord injury. Spinal Cord. 1981;19:367–372. doi: 10.1038/sc.1981.69. [DOI] [PubMed] [Google Scholar]
- 8.Lazo MG, Shirazi P, Sam M, et al. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–214. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- 9.Carbone LD, Chin AS, Burns SP, et al. Morbidity following lower extremity fractures in men with spinal cord injury. Osteoporos Int. 2013 doi: 10.1007/s00198-013-2295-8. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 11.Papaioannou A, Morin S, Cheung AM, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. Can Med Assoc J. 2010;182:1864–1873. doi: 10.1503/cmaj.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanis JA, Johnell O, Odén A, et al. FRAX™ and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farry A, Baxter D. Overview and estimates based on current evidence. Rick Hansen Institute and Urban Futures; 2010. The incidence and prevalence of spinal cord injury in Canada. [Google Scholar]
- 14.Craven B, Giangregorio L, Robertson L, et al. Sublesional osteoporosis prevention, detection, and treatment: a decision guide for rehabilitation clinicians treating patients with spinal cord injury. Crit Rev Phys Rehabil Med. 2008;20:277–321. [Google Scholar]
- 15.Eser P, Frotzler A, Zehnder Y, et al. Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone. 2004;34:869–880. doi: 10.1016/j.bone.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Slade JM, Bickel CS, Modlesky CM, et al. Trabecular bone is more deteriorated in spinal cord injured versus estrogen-free post-menopausal women. Osteoporos Int. 2005;16:263–272. doi: 10.1007/s00198-004-1665-7. [DOI] [PubMed] [Google Scholar]
- 17.Modlesky CM, Majumdar S, Narasimhan A, Dudley GA. Trabecular bone microarchitecture is deteriorated in men with spinal cord injury. J Bone Miner Res. 2004;19:48–55. doi: 10.1359/JBMR.0301208. [DOI] [PubMed] [Google Scholar]
- 18.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Investig. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 19.Garland DE, Adkins RH, Stewart CA. Fracture threshold and risk for osteoporosis and pathologic fractures in individuals with spinal cord injury. Top Spinal Cord Inj Rehabil. 2005;11:61–69. [Google Scholar]
- 20.Eser P, Frotzler A, Zehnder Y, Denoth J. Fracture threshold in the femur and tibia of people with spinal cord injury as determined by peripheral quantitative computed tomography. Arch Phys Med Rehabil. 2005;86:498–504. doi: 10.1016/j.apmr.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 21.Sheu Y, Zmuda JM, Boudreau RM, et al. Bone strength measured by peripheral quantitative computed tomography and the risk of nonvertebral fractures: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2011;26:63–71. doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamal SA, Gilbert J, Gordon C, Bauer DC. Cortical pQCT measures are associated with fractures in dialysis patients. J Bone Miner Res. 2006;21:543–548. doi: 10.1359/jbmr.060105. [DOI] [PubMed] [Google Scholar]
- 23.Taes Y, Lapauw B, Griet V, et al. Prevalent fractures are related to cortical bone geometry in young healthy men at age of peak bone mass. J Bone Miner Res. 2010;25:1433–1440. doi: 10.1002/jbmr.17. [DOI] [PubMed] [Google Scholar]
- 24.Frey-Rindova P, De Bruin ED, Stüssi E, et al. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 25.Dionyssiotis Y, Trovas G, Galanos A, et al. Bone loss and mechanical properties of tibia in spinal cord injured men. J Musculoskelet Neuronal Interact. 2007;7:62–68. [PubMed] [Google Scholar]
- 26.Maynard FM, Jr, Bracken MB, Creasey G, et al. International standards for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Spinal Cord. 1997;35:266–274. doi: 10.1038/sj.sc.3100432. [DOI] [PubMed] [Google Scholar]
- 27.Edwards LA, Bugaresti JM, Buchholz AC. Visceral adipose tissue and the ratio of visceral to subcutaneous adipose tissue are greater in adults with than in those without spinal cord injury, despite matching waist circumferences. Am J Clin Nutr. 2008;87:600–607. doi: 10.1093/ajcn/87.3.600. [DOI] [PubMed] [Google Scholar]
- 28.Krieger N, Tenenhouse A, Joseph L, et al. The Canadian Multicentre Osteoporosis Study. Can J Aging. 1999;18:376–387. [Google Scholar]
- 29.Moreno JC. Master’s thesis. McMaster University; Hamilton: 2001. Protocol for using dual photon absorptiometry software to measure BMD of distal femur and proximal tibia. [Google Scholar]
- 30.Yoshioka Y, Siu DW, Scudamore RA, Cooke TD. Tibial anatomy and functional axes. J Orthop Res. 1989;7:132–137. doi: 10.1002/jor.1100070118. [DOI] [PubMed] [Google Scholar]
- 31.Giangregorio L, Lala D, Hummel K, et al. Measuring apparent trabecular density and bone structure using peripheral quantitative computed tomography at the tibia: precision in participants with and without spinal cord injury. J Clin Densitom. 2012 doi: 10.1016/j.jocd.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Ashe MC, Khan KM, Kontulainen SA, et al. Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int. 2006;17:1241–1251. doi: 10.1007/s00198-006-0110-5. [DOI] [PubMed] [Google Scholar]
- 33.Melton LJ, Beck TJ, Amin S, et al. Contributions of bone density and structure to fracture risk assessment in men and women. Osteoporos Int. 2005;16:460–467. doi: 10.1007/s00198-004-1820-1. [DOI] [PubMed] [Google Scholar]
- 34.Garland DE, Adkins RH, Kushwaha V, et al. Risk factors for osteoporosis at the knee in the spinal cord injury population. J Spinal Cord Med. 2004;27:202–206. doi: 10.1080/10790268.2004.11753748. [DOI] [PubMed] [Google Scholar]
- 35.Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 36.Totosy de Zepetnek JO, Craven BC, Giangregorio LM. An evaluation of the muscle–bone unit theory among individuals with chronic spinal cord injury. Spinal Cord. 2012;50:147–152. doi: 10.1038/sc.2011.99. [DOI] [PubMed] [Google Scholar]
- 37.De Bruin ED, Herzog R, Rozendal RH, et al. Estimation of geometric properties of cortical bone in spinal cord injury. Arch Phys Med Rehabil. 2000;81:150–156. [PubMed] [Google Scholar]
- 38.Jiang S-D, Dai L-Y, Jiang L-S. Osteoporosis after spinal cord injury. Osteoporos Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 39.Duan Y, De Luca V, Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab. 1999;84:718–722. doi: 10.1210/jcem.84.2.5498. [DOI] [PubMed] [Google Scholar]
- 40.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 41.Negri AL, Barone R, Lombas C, et al. Evaluation of cortical bone by peripheral quantitative computed tomography in continuous ambulatory peritoneal dialysis patients. Hemodial Int. 2006;10:351–355. doi: 10.1111/j.1542-4758.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 42.Bauman WA, Zhong YG, Schwartz E. Vitamin D deficiency in veterans with chronic spinal cord injury. Metabolism. 1995;44:1612–1616. doi: 10.1016/0026-0495(95)90083-7. [DOI] [PubMed] [Google Scholar]
- 43.Hummel K, Craven BC, Giangregorio L. Serum 25(OH)D, PTH and correlates of suboptimal 25(OH)D levels in persons with chronic spinal cord injury. Spinal Cord. 2012;50:812–816. doi: 10.1038/sc.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauman WA, Spungen AM. Metabolic changes in persons after spinal cord injury. Phys Med Rehabil Clin N Am. 2000;11:109–140. [PubMed] [Google Scholar]