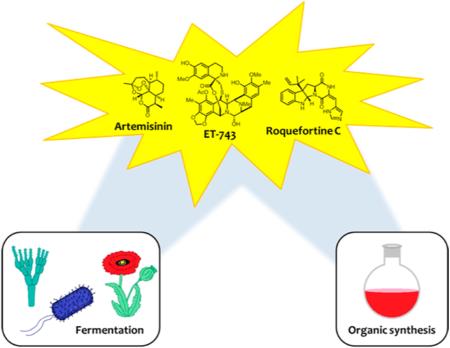
Abstract
Commercial application of many promising heterocyclic natural products is limited by their natural abundance. While organic synthesis provides access to many natural products, total synthesis of numerous complex molecules is not economically feasible. In recent years, the combination of fermentation and organic synthesis has provided a new route for the production of complex heterocycles that are inaccessible by typical synthetic methods. This JOCSynopsis will review examples of how this union of disciplines has overcome obstacles in both academia and industry.
Heterocyclic natural products play an essential role in numerous industries, particularly pharmaceuticals. A majority of today's small molecule drugs and drug candidates contain at least one heterocyclic functionality.1 Additionally, just over half of all small molecules approved as therapeutics between 1981 and 2014 are natural products or natural product based compounds.2 However, the implementation of many promising biologically active compounds as pharmaceuticals is limited by natural product isolation. Many of these compounds are produced in very limited amounts by their natural source, and isolation of these compounds from their host organisms depletes natural supply. Such methods of production are not sustainable for clinical trials, much less commercialization. Historically, chemists have attempted to circumvent this problem through organic synthesis. While organic synthesis has allowed affordable access of natural products, total synthesis of many natural products is still not economically feasible for mass production.
Recent trends in the synthesis and production of heterocyclic molecules have shown increased interest in green synthetic processes. In particular, there has been a movement toward implementation of fermentation to solve problems commonly encountered in organic synthesis. Though much of fermentation in industry is employed for the production of food and alcoholic beverages, this technology can be expanded to produce advanced intermediates in the synthetic process. Microorganisms such as bacteria, yeast, and filamentous fungi can convert simple starting materials into stereochemically complex natural products. Fermentation limits the use of organic solvents and organic reagents, and in turn reduces waste products generated during the synthetic process. In addition, it allows for rapid and stereospecific production of large quantities of advanced intermediates. Natural products obtained by fermentation can be efficiently converted into new structures that would be difficult to obtain synthetically. The combination of fermentation and organic chemistry offers an alternative approach for the production and derivatization of biological compounds and could produce new therapeutic agents. Biosynthetic pathways offer complex scaffolds that can be used to synthesize other compounds that may exhibit novel biological properties and provide a new approach to drug development.
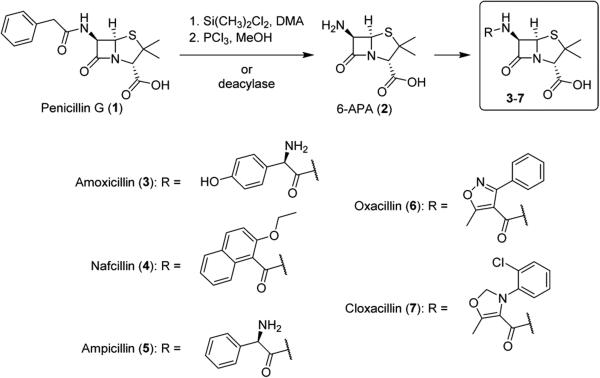
Natural products of bacterial or fungal origin are often amenable to production by fermentation. Besides sulfonamides and fluoroquinolones, the other major groups of antibiotics (penicillins, cephalosporins, tetracyclines, and macrolides) are all produced industrially by fermentation. The quintessential example of a fermented natural production is penicillin G (1), which is produced industrially by fermentation of Penicillium notatum. With the widespread availability of penicillin G following World War II, a number of bacterial strains developed resistance, and researchers sought to develop semisynthetic derivatives to combat bacterial resistance. (+)-6-Aminopenicillanic acid (6-APA, 2), also found in P. notatum cultures,3 was used as the base scaffold for new penicillin compounds (3–7), which are usually produced via acylation of 6-APA with the corresponding acid chloride or acid chloride hydrochloride (Scheme 1).4 6-APA can be prepared from the natural 1 via formation of the imino ether with PCl3 in methanol (using silyl esters as protecting groups) followed by hydrolysis4,5 or via deacylation enzymes.6
Scheme 1.
Synthesis of Semisynthetic Penicillins 3–7
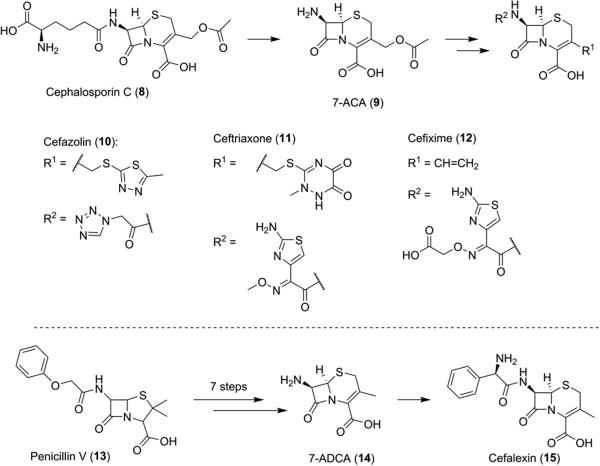
Cephalosporins, another class of beta-lactam antibiotics, are also produced industrially by combining fermentation and organic synthesis. The natural cephalosporins initially isolated from Acremonium fungi, cephalosporins P, N, and C, displayed antibiotic activity but not at the same potency of the penicillins. Analogous to 6-APA (2) for penicillin, hydrolysis of cephalosporin C (8) produced 7-aminocephalosporanic acid (7-ACA, 9), which became the platform upon which more potent cephalosporin compounds were built.7,8 Most commercial cephalosporins display varying functionalities on the C3 side arm of the β-lactam (10–12 and 15). These moieties are generated via synthetic manipulation of 7-aminodesacetoxycephalosporanic acid (7-ADCA, 14) from penicillin V (13, Scheme 2)9,10 or through transformation of the ester functionality of 9 (Schemes 2).11–14
Scheme 2.
Semisynthesis of Cephalosporins 10–12 and 15
Fermentation has also contributed to the efficient production of vancomycin. Vancomycin is a commercial antibiotic used to treat a number of bacterial infections, particularly those resistant to penicillins. Its structure consists of an arylglycine-rich heptapeptide aglycon to which is appended an array of sugar residues. Since its isolation in 1956 from Amycolatopsis orientalis, several total syntheses of vancomycin have been completed, each requiring extraordinary efforts but giving low overall yields. The total synthesis of vancomycin illustrates the formidable power of organic synthesis. The challenge of these syntheses was the development of stereoselective methods for controlling the three stereochemical elements of the atropisomerism present in the molecule.15–21
Vancomycin was first prepared by fermentation by Eli Lilly in 1962,22 and fermentation remains the most efficient, cost-effective method of production. In 1996, it was shown that vancomycin can be produced by A. orientalis in both batch and continuous culture.23 Additionally, purification of vancomycin can be achieved through simple precipitation at pH > 7.8.24
Microbial Synthesis of Plant-Based Natural Products
Plant-based natural products make up a large proportion of current pharmaceuticals. In a number of cases, plant-based harvest is feasible; however, in many cases, plants may not be suitable for fermentation due to factors such as low isolation titers, poor extraction, or difficult purification due to many metabolites produced by the plant. Additionally, plant-based fermentation may not be sustainable, as it requires large amounts of space and because it depletes natural resources. Efforts to engineer the production of heterocyclic natural products in native plant hosts can be accompanied by adverse morphological effects, as plant metabolism is highly regulated.
With the recent advances in genome mining and characterization of biosynthetic pathways, researchers have been able to genetically engineer microorganisms to produce plant-based natural products. There are several advantages to transgenic production of plant metabolites: (1) the techniques used to manipulate genes and expression of proteins are well established for microorganisms such as yeast and bacteria, (2) these microorganisms tend to grow at faster rates, and (3) techniques for large scale fermentation are well established for these organisms. Efficient biosynthesis of complex molecules is still challenging, however, because it involves optimization of many enzyme-catalyzed reactions.
Genetically engineered yeast has been used in the production of the antimalarial drug artemisinin (19, Scheme 3). This tetracyclic sesquiterpene bearing a peroxide bridge was first isolated from the plant Artemisia annua by Youyou Tu in 1971, and artemisinin (19) as well as its semisynthetic derivatives were found to exhibit potent antimalarial activity against Plasmodium falciparum malaria.25 The discovery of 19 has been one of the most important medical developments of the 20th century, earning Youyou Tu half of the 2015 Nobel Prize in Medicine.
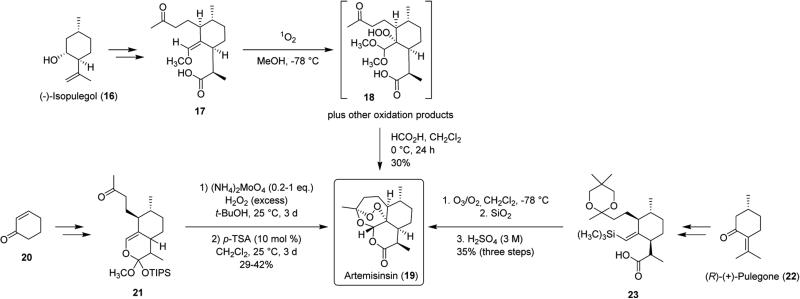
Scheme 3.
Synthetic Approaches to Artemisinin (19)
Artemisinin (19) has been synthesized a number of times (Scheme 3), notably from (−)-isopulegol (16) by Schmid and Hofheinz (13 steps, ~5% overall yield),26 from cyclohexenone (20) by Zhu and Cook (9 steps, 7.6% overall yield),27 and from (R)-(+)-pulegone (22) by Avery, Chong, and Jennings-White (9 steps, ~3% overall yield).28 The primary bottleneck of these total syntheses has been the construction of the peroxide bridge. In the synthesis by Schmid and Hofheinz, addition of singlet oxygen to compound 17 followed by addition of methanol is proposed to form hydroperoxide 18, which undergoes acid-promoted ring closure to give artemisinin (19) in 30% yield.26 The synthesis by Zhu and Cook features addition of singlet oxygen formed from ammonium molybdate-promoted decomposition of hydrogen peroxide to give a complex mixture of oxidation products, which upon addition of acid provided artemisinin (19) in 29–42% yield.27 Construction of the peroxide bridge in the Avery synthesis is carried out by a one-pot ozonolysis, deprotection, and cyclization reaction sequence to give artemisinin (19) in 35% yield.28
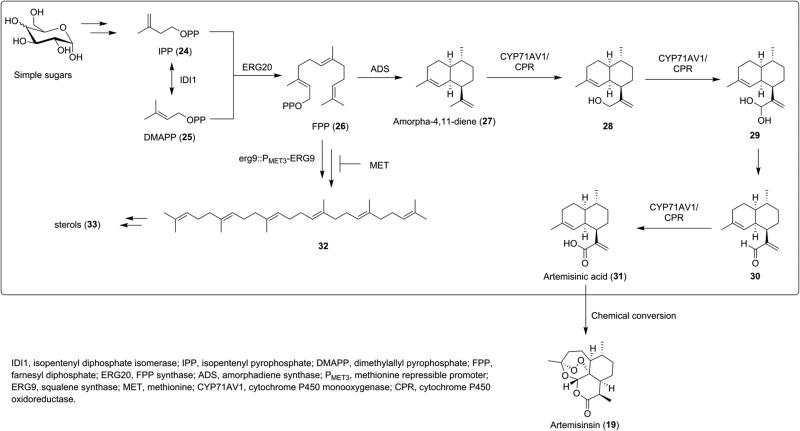
The semisynthesis of 19 from natural precursor artemisinic acid (31, Scheme 4) has been demonstrated by sequential oxidations29 and by multistep reaction cascades;30,31 however, like artemisinin (19), artemisinic acid (31) suffers from low natural abundance, thus limiting this method for commercial use. Recently, the production of 31 was reported in engineered yeast Saccharomyces cerevisiae (Scheme 4).32,33 An engineered mevalonate pathway was optimized for the production of artemisinic acid precursor amorpha-4,11-diene (27) through upregulation of farnesyl diphosphate (FPP, 26) production and inhibition of sterol (33) biosynthesis. Artemisinic acid was then synthesized from 27 by cytochrome P450 monooxygenase CYP71AV1. Employment of a high-copy plasmid system increased production of 31 from 100 to 250 mg/L in shake-flask cultures and 1 g/L in bioreactors.33
Scheme 4.
Engineering Pathway for the Biosynthesis of Artemisinic Acid (31) and Semisynthesis of Artemisinin (19) from Artemisinic Acid (31)
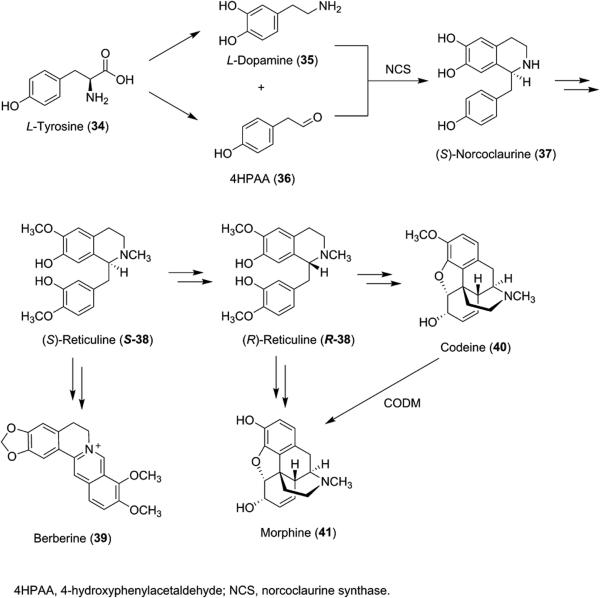
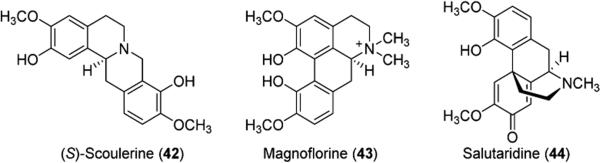
The benzylisoquinoline alkaloids (BIA) make up a family of heterocyclic compounds with a large presence in the pharmaceutical industry. These compounds include a number of common analgesic compounds such as morphine (41, Scheme 5) and codeine (40, Scheme 5), as well as the muscle relaxant (+)-tubocurarine and antibacterial agents such as berberine (39, Scheme 5), palmatine, and magnoflorine (43, Figure 1). These alkaloids are produced by a number of flowering plant families, including the Papaveraceae, or poppy, family and are known to be derived from tyrosine (34) and share (S)-reticuline (S-38, Scheme 5) as a precursor. Current production of opioids such as morphine (41) involves extraction of opium or poppy straw, which are the raw materials obtained from the poppy plant Papaver somniferum. While 41 is produced in high yields from the poppy plant, environmental factors such as weather and fertile soil can cause variations in the amount produced each year, making plant-based production an unsustainable practice. Additionally, many other alkaloids in this family are not produced in high levels.
Scheme 5.
Biosynthesis of Benzylisoquinoline Alkaloids
Figure 1.
Benzylisoquinoline alkaloids produced by genetic engineering.
Several publications describe the reconstitution of the BIA biosynthetic pathway in microorganisms. Minami et al. have developed a method for microbial production of BIAs (S)-scoulerine (42) and magnoflorine (43) (Figure 1). The BIA biosynthetic pathway was reconstituted in Escherichia coli for production of S-38 from 35. S. cerevisiae was used to reconstitute downstream enzymes as some enzymes needed to form BIAs from S-38 are not expressed in bacteria in their active form. The combination of the two transgenic microbes led to production of 42 and 43 in 8.3 and 7.3 mg/L, respectively.34
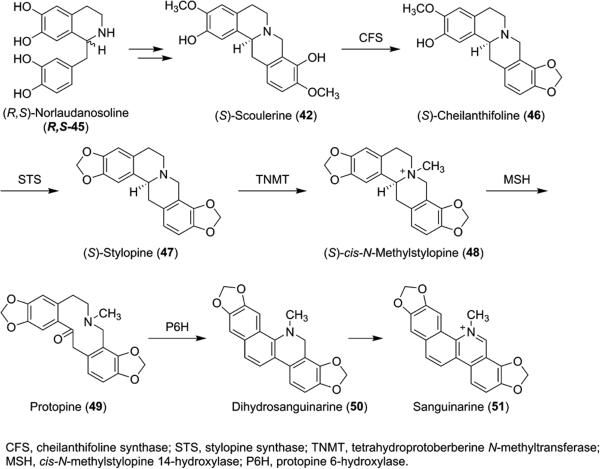
Additional work by the Smolke group involved the engineering of S. cerevisiae for the production of S-38 from precursor (S)-norcoclaurine (37) as well as the production of sanguinarine and berberine intermediates from S-38. Morphine precursor salutaridine (44, Figure 1) was also produced via a two-step isomerization of S-38 to R-38 followed by oxidation with human cytochrome P450 CYP2D6.35 Further work by the Smolke group demonstrated production of protoberberine alkaloids 42, 46, 47, and 48, protopine (49), and benzophenanthridine alkaloids 50 and 51 in the sanguinarine biosynthetic pathway, achieving high titers for each of the metabolites (Scheme 6).36 As a proof of concept, Smolke et al. have also engineered yeast to produce thebaine and hydrocodone starting from sugar.37
Scheme 6.
Production of Protoberberine Alkaloids 42, 46, 47, and 48, Protopine (49), and Benzophenanthridine Alkaloids 50 and 51 in S. cerevisiae
Microbial production of natural BIAs could provide more efficient routes to less abundant natural BIAs such as tubocurarine as well as semisynthetic opioids such as hydrocodone, hydromorphone, and oxycodone. While the titers of opioid alkaloids are not yet sufficient for commercial production, the research offers other possibilities such as synthetic production of superior drugs and drugs with more favorable pharmacokinetic properties. While major challenges remain, this approach has already proven feasible.
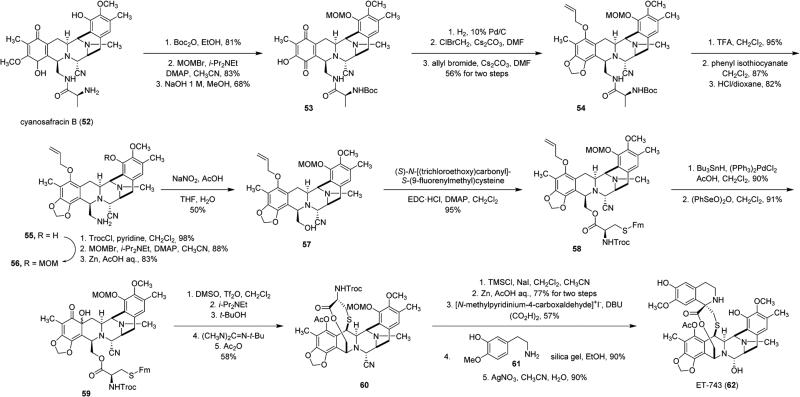
Knowledge of metabolites produced by organisms can be used for creative solutions to natural products that are not easily obtained. Reconstituting biosynthetic pathways may not be necessary if precursors or related compounds are naturally produced by microorganisms amenable to fermentation. Ecteinascidin 743 (ET-743, 62, Scheme 7) is a tetrahydroisoquinoline compound produced by Caribbean marine tunicate Ecteinascidia turbinata38 and is the first marine compound to be approved for the treatment of cancer. ET-743 was first reported in 1969, and following structure elucidation in 1986,39 it was found to be very similar to safracin compounds produced by Pseudomonas fluorescens.40,41 Preclinical and early clinical trials were completed using ET-743 obtained from aquaculture, but it was clear that this method of production would not be sustainable should the compound be approved for commercial use.42 Fermentation of cyanosafracin B (52) on kilogram scale has allowed for the efficient semisynthesis of 62 from 52 to be developed (Scheme 7).43 Though the total synthesis of 62 was completed in 2002,44 semisynthesis still remains the most efficient method of production. ET-743 has been approved for the treatment of sarcoma and ovarian cancer by the European Union's EMEA, for the treatment of ovarian cancer in the Philippines, and for malignant soft tissue tumors in Japan. It is currently in clinical trials in the United States for the treatment of a number of cancers.
Scheme 7.
Semisynthesis of ET-743 (62) from Cyanosafracin B (52)
Mutasynthesis as a Substitute for Late-Stage Modification
Despite the great potency of many heterocyclic natural products, unfavorable solubility, bioavailability, and pharmacokinetics often require drug candidates to be derivatized prior to commercialization. Recent breakthroughs in late stage modification have enabled derivatization of many complex molecules; however, a number of these methods are not general for heterocyclic compounds. Many total syntheses of heterocyclic natural product analogs require introduction of new functional groups at the beginning of the synthetic route and thus require many additional steps to generate synthetic derivatives.
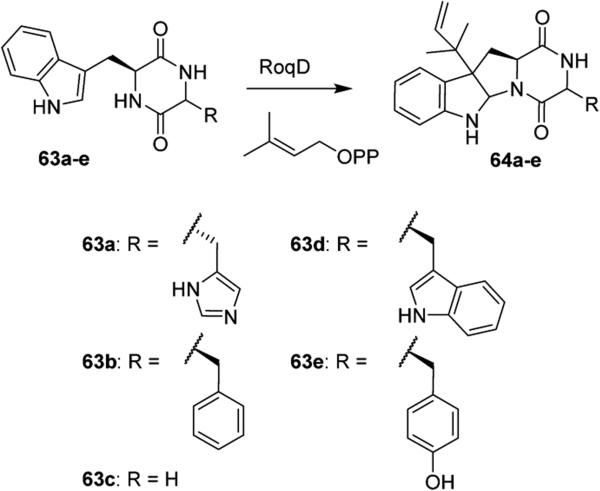
Mutasynthesis can be used to efficiently prepare derivatives of heterocyclic natural products. In this process, micro-organisms lack the machinery to generate a biosynthetic precursor of a natural product, and only when the precursor is supplied by the researcher can the natural product be generated. These mutant strains were initially developed through random mutagenesis; however, with the advent of genome mining and thorough characterization of biosynthetic pathways, targeted gene disruptions have allowed for the facile production of mutant strains. Natural product analogues can be easily synthesized through mutasynthesis by simple addition of unnatural precursors. Mutasynthesis can be extremely beneficial for the inclusion of moieties like halogens, which seldom occur in natural products but are highly prevalent in pharmaceuticals. For example, RNAi-mediated knockdown of tryptophan decarboxylase (TDC) in tryptamine biosynthesis has been implemented to generate halogenated monoterpene indole alkaloids.45 Stereoisomers of heterocyclic natural products can also be generated far more efficiently by mutasynthesis, as is evidenced in the production of roquefortine D analogues 64a–e by Driessen, Overkleeft et al. (Scheme 8).46
Scheme 8.
Synthesis of Roquefortine D Analogues 64a–e by Mutasynthesis
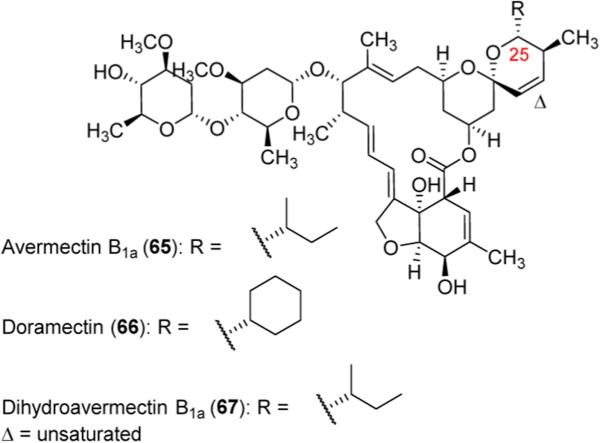
Commercial application of mutasynthesis is seen in the production of doramectin (66), an antiparasitic veterinary drug. Avermectin (65, Figure 2), whose discovery was awarded half of the Nobel Prize in Medicine in 2015, is a macrolide produced naturally by Streptomyces avermitilis. In a search for novel, more potent avermectin derivatives, researchers at Pfizer developed a mutant strain of S. avermitilis that blocked production of branched chain carboxylic acids. Supplementing the fermentation culture with various carboxylic acids led to a number of C25 analogues.47 Doramectin (66), an avermectin analogue synthesized via supplementation with cyclohexanoic acid, was found to have superior plasma concentrations and a longer half-life as compared to dihydroavermectin B1a (67), the main component of semisynthetic avermectin analogue ivermectin, while still possessing comparable, and in some cases superior, activity against a number of parasites.48
Figure 2.
Avermectin B1a (65), doramectin (66), and dihydroavermectin B1a (67).
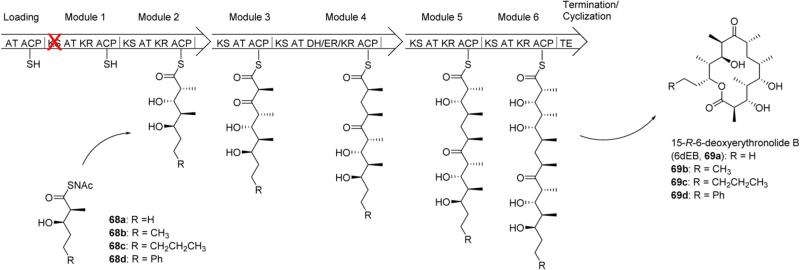
Chemobiosynthesis is a particular kind of mutasynthesis that involves disabling a particular enzymatic function in multi-enzyme polyketide synthases. The first instance of chemobiosynthesis was implemented in the synthesis of erythromycin analogues 69a–d (Scheme 9). 6-Deoxyerythronolide B (6-dEB, 69a) is a precursor to erythromycin, and it is synthesized by polyketide synthase 6-deoxyerythronolide B synthase (DEBS). These proteins are composed of several modules, each containing domains of varying enzymatic functions. The polyketide chain is constructed by passing it from one module to the next via transacylations of terminal thiols. The Khosla laboratory was able to produce 6-dEB analogues by introducing a point mutant to the ketosynthase of module 1 in DEBS, rendering this module ineffective. N-Acetylcysteamine thioesters 68a–d were shown to be transformed by module 2 and subsequently incorporated in 6-dEB analogues 69a–d.49 Point mutations have also been introduced into the DEBS loading module and have shown incorporation of nonchiral acyl thioesters into 6dEB analogues.50
Scheme 9.
Chemobiosynthesis of Erythromycin Derivatives 69a–d via Point Mutant to the Ketosynthase of Module 1
Saga of the Roquefortines
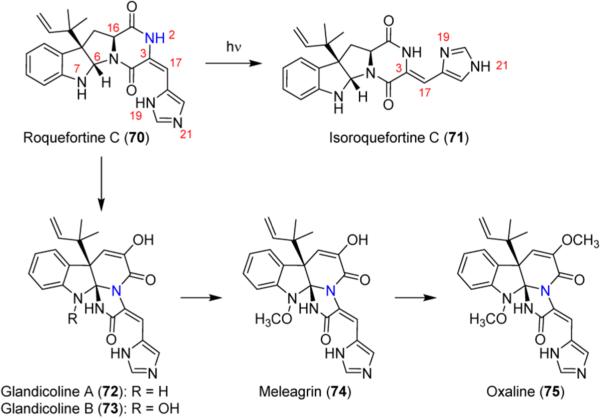
Penicillium roqueforti, a fungus found in Roquefort cheese and a number of other blue-veined cheeses, produces a variety of complex and structurally interesting compounds including the roquefortine family. Roquefortine C (70, Scheme 10) is the most investigated member of the family and exhibits a very rare and photo-chemically unstable E-configuration of its C3–C17 double bond. This configuration was established by comparison to its photoisomer isoroquefortine (71), which has the Z-configuration.51 Isoroquefortine has never been found in Nature and exhibits very different biological properties. As a consequence of the difference in stereochemistry, N19 of the imidazole is a hydrogen-bond donor in 70 and a hydrogen-bond acceptor in 71. As a result, 70 exhibits a strong Fe–N interaction with hepatic cytochrome P450 and microperoxidase8 due to the available lone pair on N21, thus inhibiting hemeproteins.52
Scheme 10.
Roquefortine C (70) and Related Compounds
In addition to its unique chemical structure, 70 has acquired interest in recent years due to its role as a biosynthetic precursor to biologically active triazaspiro indole alkaloids glandicoline B (73), meleagrin (74), and oxaline (75). These compounds have shown a range of interesting biological properties, including antimicrobial activity,53 antifouling activity,54 and inhibition of tubulin polymerization in T lymphocyte cells.55
The mechanism proposed for the conversion of 70 into the triazaspiro structures was of interest to our research group. Radiolabeling studies in the 1980s were the first to suggest a biosynthetic pathway containing 70 and 75.56 This proposed path, which remained the accepted biosynthetic pathway for these metabolites until 2013, began with enzymatic hydroxylation of 70 at C16, followed by rearrangement to give 72. The mechanism was thought to occur through the following steps: conversion of the hemiaminal to an open 9-membered dicarbonyl species, dehydrogenation at the C6 and N7 positions, nucleophilic attack of C6 by N2 to give 72, oxidation at N7, and methylation to give 75. A report that two diastereomers of 16-hydroxyroquefortine were characterized from a Penicillium crustosum species supported this mechanism.57 It was proposed that enzymatic hydroxylation occurred in either a nonspecific fashion or through specific oxidation followed by isomerization to form both stereoisomers.
To investigate this mechanism, however, a sufficient supply of 70 was needed. The total synthesis of 70, published by our research group in 2008,58 presented several challenges and could not produce sufficient material for detailed mechanistic investigation. As 70 is produced by several fungi, we looked into fermentation as a source of 70. As such, a procedure was developed by our group for the large scale fermentation of 70 from P. crustosum. This method allowed for the production of 70 at 125 mg/L for a fraction of the cost of total synthesis.59
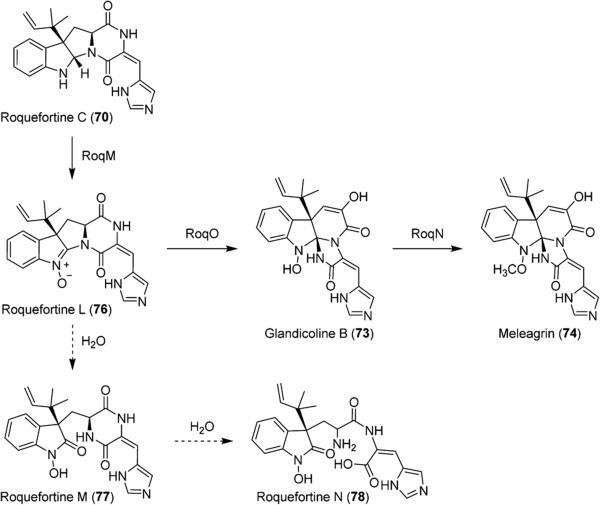
As we were concluding our work in the development of the roquefortine C fermentation procedure, a major breakthrough in the elucidation of the biosynthetic pathway of roquefortine C derived metabolites was made by the Vreeken laboratory (Scheme 11).60 Vreeken et al. observed several new metabolites in Penicillium chrysogenum, including novel indole nitrone roquefortine L (76). Due to their identical molecular weights, 76 had been previously misidentified as 72, which was not found by Vreeken et al. in any cultures of P. chrysogenum. Roquefortines M (77) and N (78) were observed to form from the degradation of 76 in acidic aqueous conditions. Through a series of gene silencing studies, 76 was found to play a vital role in the biosynthesis of related metabolites 73 and 74 in P. chrysogenum.
Scheme 11.
Biosynthesis of Glandicoline B (73) and Meleagrin (74) from Roquefortine L (76) and Roquefortine C (70)
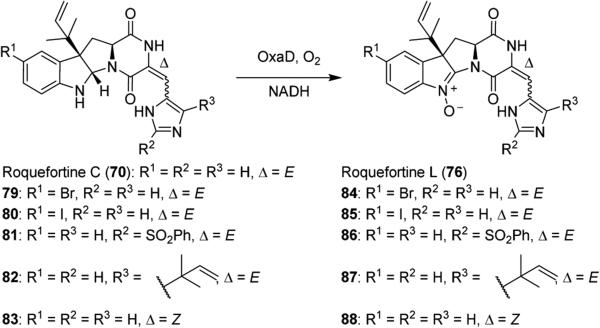
Based on this work, it is clear that 76 is an important intermediate in the conversion of 70 to the triazaspirocyclic indole alkaloids. Our research group is currently investigating the oxidation of 70 to 76 as indole nitrones are uncommon in natural products. Through a collaboration with the Sherman research group, we have shown that this oxidation can be accomplished by flavin monooxygenase OxaD from Penicillium oxalicum (Scheme 12). A number of semisynthetic derivatives of 70 were generated, and OxaD was shown to tolerate substitution on the indole benzene ring and the imidazole as well as isomerization about the C3–C17 double bond.61 Concurrently, we have been able to develop special conditions to perform the oxidation chemically using a variety of electrophilic oxidizing reagents.62
Scheme 12.
N-Oxidation of Roquefortine C (70), Roquefortine E (82), and Semisynthetic Derivatives 79–81 and 83 by OxaD
Our results have shown that the combination of fermentation and organic synthesis can not only produce new heterocycles for use in the pharmaceutical industry but also achieve a better understanding of biosynthetic pathways and the discovery of novel mechanistic transformations.
CONCLUSION
Although the production of heterocyclic natural products by fermentation is a well-investigated field, as is organic synthesis, there are few examples of combining both disciplines. The merging of both fields could result in new research directions and innovative ideas, advancing knowledge and fostering creativity in both areas. Despite its many benefits, the field of fermentation is not without limitations. The implementation of fermentation for industrial purposes is currently limited by microbial output and cost. Currently, many genetically engineered systems are limited to biosynthetic precursors and other closely related natural products as opposed to simple, inexpensive starting materials. Additionally, many biosynthetic pathways of natural products have yet to be identified or characterized, precluding their eligibility for genetic engineering. As evidenced by the work described in this review, the combination of fermentation and organic synthesis provides a new approach to heterocyclic compounds. Production of organic compounds has becoming more and more interdisciplinary, and we must begin to embrace emerging technology for efficient methods of producing complex heterocyclic compounds.
ACKNOWLEDGMENTS
We thank Dr. David Sherman and his research group for their contributions toward the development of flavin monooxygenase OxaD. We also acknowledge the NSF (Grant No. CHE-0951394) for support of our research and the NIH (Grant Nos. 5T32GM071339-09, 5T32GM071339-10, 2T32GM071339-11) for predoctoral training grant fellowships awarded to C.G.
Biography
 Claire Gober received an A.B. in Chemistry and Chemical Biology from Cornell University in 2012, where she carried out research in the laboratory of Professor Bruce Ganem. In 2012, she began her graduate studies at the University of Pennsylvania and joined the research group of Professor Madeleine Joullié. Her current research is focused on the transformation of indole alkaloid roquefortine C to triazaspirocyclic biosynthetic derivatives glandicoline B, meleagrin, and oxaline.
Claire Gober received an A.B. in Chemistry and Chemical Biology from Cornell University in 2012, where she carried out research in the laboratory of Professor Bruce Ganem. In 2012, she began her graduate studies at the University of Pennsylvania and joined the research group of Professor Madeleine Joullié. Her current research is focused on the transformation of indole alkaloid roquefortine C to triazaspirocyclic biosynthetic derivatives glandicoline B, meleagrin, and oxaline.
 Madeleine M. Joullié obtained a B.S. in Chemistry from Simmons College in 1949, after which she earned a Ph.D. from the University of Pennsylvania in 1953 under the guidance of Professor Allan R. Day. She then joined the faculty at the University of Pennsylvania, where she was one of the first female professors to earn tenure in Chemistry at a major university in the U.S. Her laboratory has focused on the chemistry of cyclopeptide alkaloids and the roquefortine and didemnin families of natural products as well as the development of compounds for the visualization of latent fingerprints as a forensic tool for law enforcement.
Madeleine M. Joullié obtained a B.S. in Chemistry from Simmons College in 1949, after which she earned a Ph.D. from the University of Pennsylvania in 1953 under the guidance of Professor Allan R. Day. She then joined the faculty at the University of Pennsylvania, where she was one of the first female professors to earn tenure in Chemistry at a major university in the U.S. Her laboratory has focused on the chemistry of cyclopeptide alkaloids and the roquefortine and didemnin families of natural products as well as the development of compounds for the visualization of latent fingerprints as a forensic tool for law enforcement.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.Pharmaceutical Sales US. [May 12, 2016];2013 http://www.drugs.com/stats/top100/2013/sales.
- 2.Newman DJ, Cragg GM. J. Nat. Prod. 2016;79:629. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor FR, Doyle FP, Nayler JHC, Rolinson GN. Nature. 1959;183:257. doi: 10.1038/183257b0. [DOI] [PubMed] [Google Scholar]
- 4.Chester Sapino J, Vulcano AL, Brundidge SP, Mahan JD. Production of semisynthetic penicillins. 1975 U.S. Patent 3912719.
- 5.Sellstedt JH. Process for preparation of 6-aminopenicillanic acid. 1975 U.S. Patent 3896110.
- 6.Kaufmann W, Bauer K. Process for the production of 6-aminopenicillanic acid. 1966 U.S. Patent 3260653.
- 7.Johnson DA, Richardson EJ, Roubie JM, Silvestri HH, Smith RR. Process for the preparation of 7-aminocephalosporanic acid. 1971 U.S. Patent 3573296. [Google Scholar]
- 8.Macher I, Widschwenter G. Production of cefotaxime and new sodium salts. 1998 U.S. Patent 5831086.
- 9.Burton B, Graham W. Process for preparing cephalosporin compounds from 7-ADCA. 1976 U.S. Patent 3957773.
- 10.Morin RB, Jackson BG. Certain 3-methyl-cephalosporin compounds. 1970 U.S. Patent 3507861.
- 11.Takano T, Kurita M, Nikaido H, Mera M, Konishi N, Nakagawa R. 3,7-Disubstituted cephalosporin compounds and preparation thereof. 1970 U.S. Patent 3516997.
- 12.Takaya T, Takasugi H, Masugi T, Yamanaka H, Kawabata K. 7-Acylamino-3-vinylcephalosporanic acid derivatives and processes for the preparation thereof. 1983 U.S. Patent 4409214.
- 13.Yamanaka H, Chiba T, Kawabata K, Takasugi H, Masugi T, Takaya T. J. Antibiot. 1985;38:1738. doi: 10.7164/antibiotics.38.1738. [DOI] [PubMed] [Google Scholar]
- 14.O'Callaghan CH, Livermore DGH, Newall CE. (6R,7R)-7-[(Z)-2-(2-Aminothiazol-4-yl)-2-(2-carboxyprop-2-oxyimino)acetamido]-3-(1-pyridiniummethyl)ceph-3-em-4-carboxy-late and salts thereof. 1981 U.S. Patent 4258041.
- 15.Nicolaou KC, Mitchell HJ, Jain NF, Bando T, Hughes R, Winssinger N, Natarajan S, Koumbis AE. Chem. - Eur. J. 1999;5:2648. [Google Scholar]
- 16.Nicolaou KC, Mitchell HJ, Jain NF, Winssinger N, Hughes R, Bando T. Angew. Chem., Int. Ed. 1999;38:240. [Google Scholar]
- 17.Evans DA, Wood MR, Trotter BW, Richardson TI, Barrow JC, Katz JL. Angew. Chem., Int. Ed. 1998;37:2700. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2700::AID-ANIE2700>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaou KC, Li H, Boddy CNC, Ramanjulu JM, Yue T-Y, Natarajan S, Chu X-J, Brase S, Rubsam F. Chem. - Eur. J. 1999;5:2584. [Google Scholar]
- 19.Nicolaou KC, Boddy CNC, Li H, Koumbis AE, Hughes R, Natarajan S, Jain NF, Ramanjulu JM, Brase S, Solomon ME. Chem. - Eur. J. 1999;5:2602. [Google Scholar]
- 20.Nicolaou KC, Koumbis AE, Takayanagi M, Natarajan S, Jain NF, Bando T, Li H, Hughes R. Chem. - Eur. J. 1999;5:2622. [Google Scholar]
- 21.Boger DL, Miyazaki S, Kim SH, Wu JH, Castle SL, Loiseleur O, Jin Q. J. Am. Chem. Soc. 1999;121:10004. [Google Scholar]
- 22.McCormick MH, McGuire JM. Vancomycin and method for its preparation. 1962 U.S. Patent 3067099.
- 23.McIntyre JJ, Bull AT, Bunch AW. Biotechnol. Bioeng. 1996;49:412. doi: 10.1002/(SICI)1097-0290(19960220)49:4<412::AID-BIT8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Catt HR, Hayes HB. Vancomycin precipitation process. 1991 U.S. Patent 5037652.
- 25.Tu Y. Nat. Med. 2011;17:1217. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 26.Schmid G, Hofheinz W. J. Am. Chem. Soc. 1983;105:624. [Google Scholar]
- 27.Zhu C, Cook SP. J. Am. Chem. Soc. 2012;134:13577. doi: 10.1021/ja3061479. [DOI] [PubMed] [Google Scholar]
- 28.Avery MA, Chong WKM, Jennings-White C. J. Am. Chem. Soc. 1992;114:974. [Google Scholar]
- 29.Acton N, Roth RJ. J. Org. Chem. 1992;57:3610. [Google Scholar]
- 30.Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR, Teoh KH, Reed DW, Treynor T, Lenihan J, Fleck M, Bajad S, Dang G, Dengrove D, Diola D, Dorin G, Ellens KW, Fickes S, Galazzo J, Gaucher SP, Geistlinger T, Henry R, Hepp M, Horning T, Iqbal T, Jiang H, Kizer L, Lieu B, Melis D, Moss N, Regentin R, Secrest S, Tsuruta H, Vazquez R, Westblade LF, Xu L, Yu M, Zhang Y, Zhao L, Lievense J, Covello PS, Keasling JD, Reiling KK, Renninger NS, Newman JD. Nature. 2013;496:528. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 31.Haynes RK, Vonwiller SC. J. Chem. Soc., Chem. Commun. 1990:451. [Google Scholar]
- 32.Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD. Nature. 2006;440:940. doi: 10.1038/nature04640. [DOI] [PubMed] [Google Scholar]
- 33.Ro DK, Ouellet M, Paradise EM, Burd H, Eng D, Paddon CJ, Newman JD, Keasling JD. BMC Biotechnol. 2008;8:83. doi: 10.1186/1472-6750-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minami H, Kim JS, Ikezawa N, Takemura T, Katayama T, Kumagai H, Sato F. Proc. Natl. Acad. Sci. U. S. A. 2008;105:7393. doi: 10.1073/pnas.0802981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkins KM, Smolke CD. Nat. Chem. Biol. 2008;4:564. doi: 10.1038/nchembio.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thodey K, Galanie S, Smolke CD. Nat. Chem. Biol. 2014;10:837. doi: 10.1038/nchembio.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galanie S, Thodey K, Trenchard IJ, Interrante MF, Smolke CD. Science. 2015;349:1095. doi: 10.1126/science.aac9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sigel MM, Wellham LL, Lichter W, Dudeck LE, Gargus JL, Lucas LH. Food−Drugs from the Sea: Proceedings 1969. Marine Technology Society; 1970. [Google Scholar]
- 39.Holt TG. Ph.D. Thesis. University of Illinois at Urbana− Champaign; 1986. [Google Scholar]
- 40.Rinehart KL, Holt TG, Fregeau NL, Stroh JG, Keifer PA, Sun F, Li LH, Martin DG. J. Org. Chem. 1990;55:4512. [Google Scholar]
- 41.Wright AE, Forleo DA, Gunawardana GP, Gunasekera SP, Koehn FE, McConnell OJ. J. Org. Chem. 1990;55:4508. [Google Scholar]
- 42.Newman DJ. Pharmacol. Ther. 2016;162:1. doi: 10.1016/j.pharmthera.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 43.Cuevas C, Pérez M, Martín MJ, Chicharro JL, Fernández-Rivas C, Flores M, Francesch A, Gallego P, Zarzuelo M, de la Calle F, García J, Polanco C, Rodríguez I, Manzanares I. Org. Lett. 2000;2:2545. doi: 10.1021/ol0062502. [DOI] [PubMed] [Google Scholar]
- 44.Endo A, Yanagisawa A, Abe M, Tohma S, Kan T, Fukuyama T. J. Am. Chem. Soc. 2002;124:6552. doi: 10.1021/ja026216d. [DOI] [PubMed] [Google Scholar]
- 45.Runguphan W, Maresh JJ, O'Connor SE. Proc. Natl. Acad. Sci. U. S. A. 2009;106:13673. doi: 10.1073/pnas.0903393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ouchaou K, Maire F, Salo O, Ali H, Hankemeier T, van der Marel GA, Filippov DV, Bovenberg RA, Vreeken RJ, Driessen AJ, Overkleeft HS. ChemBioChem. 2015;16:915. doi: 10.1002/cbic.201402686. [DOI] [PubMed] [Google Scholar]
- 47.Dutton CJ, Gibson SP, Goudie AC, Holdom KS, Pacey MS, Ruddock JC, Bu'Lock JD, Richards MK. J. Antibiot. 1991;44:357. doi: 10.7164/antibiotics.44.357. [DOI] [PubMed] [Google Scholar]
- 48.Goudie AC, Evans NA, Gration KAF, Bishop BF, Gibson SP, Holdom KS, Kaye B, Wicks SR, Lewis D, Weatherley AJ, Bruce CI, Herbert A, Seymour DJ. Vet. Parasitol. 1993;49:5. doi: 10.1016/0304-4017(93)90218-c. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsen JR, Hutchinson CR, Cane DE, Khosla C. Science. 1997;277:367. doi: 10.1126/science.277.5324.367. [DOI] [PubMed] [Google Scholar]
- 50.Murli S, MacMillan KS, Hu Z, Ashley GW, Dong SD, Kealey JT, Reeves CD, Kennedy J. Appl. Environ. Microb. 2005;71:4503. doi: 10.1128/AEM.71.8.4503-4509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scott PM, Polonsky J, Merrien MA. J. Agric. Food Chem. 1979;27:201. [Google Scholar]
- 52.Aninat C, Andre F, Delaforge M. Food Addit. Contam. 2005;22:361. doi: 10.1080/02652030500073287. [DOI] [PubMed] [Google Scholar]
- 53.Zheng CJ, Sohn M-J, Lee S, Kim W-G. PLoS One. 2013;8:e78922. doi: 10.1371/journal.pone.0078922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han Z, Sun J, Zhang Y, He F, Xu Y, Matsumura K, He LS, Qiu JW, Qi SH, Qian PY. J. Proteome Res. 2013;12:2090. doi: 10.1021/pr301083e. [DOI] [PubMed] [Google Scholar]
- 55.Koizumi Y, Arai M, Tomoda H, Ōmura S. Biochim. Biophys. Acta, Mol. Cell Res. 2004;1693:47. doi: 10.1016/j.bbamcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 56.Steyn PS, Vleggaar R. J. Chem. Soc., Chem. Commun. 1983;10:560. [Google Scholar]
- 57.Trimble LA, Sumarah MW, Blackwell BA, Wrona MD, Miller JD. Tetrahedron Lett. 2012;53:956. [Google Scholar]
- 58.Shangguan N, Hehre WJ, Ohlinger WS, Beavers MP, Joullié MM. J. Am. Chem. Soc. 2008;130:6281. doi: 10.1021/ja800067q. [DOI] [PubMed] [Google Scholar]
- 59.Gober C, Joullie MM. Athens Journal of Science. 2015;3:257. [PMC free article] [PubMed] [Google Scholar]
- 60.Ries MI, Ali H, Lankhorst PP, Hankemeier T, Bovenberg RA, Driessen AJ, Vreeken RJ. J. Biol. Chem. 2013;288:37289. doi: 10.1074/jbc.M113.512665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newmister SA, Gober CM, Romminger S, Yu F, Tripathi A, Parra LLL, Williams RM, Berlinck RGS, Joullié MM, Sherman DH. OxaD, a versatile indole nitrone synthase from the marine-derived fungus Penicillium oxalicum F30. J. Am. Chem. Soc. 2016 doi: 10.1021/jacs.6b04915. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gober C, Joullié MM. Isr. J. Chem. 2016 submitted for publication. [Google Scholar]