Abstract
Injured patients with lung contusion (LC) are at risk of developing bacterial pneumonia (PNA) followed by sepsis and death. A recent Genome-wide Association Study, showed FER gene expression positively correlating with survival rates among individuals with above conditions. We sought to determine if electroporation-mediated (EP) delivery of FER gene could indeed improve survival, in a lethal model of combined LC and PNA. C57BL/6 mice sustained unilateral LC, which preceded a 500 Klebsiella CFU inoculation by 6 hrs. In-between these insults, human FER plasmid (pFER) was introduced into the lungs followed by eight EP pulses applied externally (10ms at 200V/cm). Control groups included EP of empty vector (pcDNA3) or Na+/K+-ATPase genes (pPump) and no treatment (LC + PNA). We recorded survival, histology, lung mechanics, bronchial alveolar fluid (BAL), FER and inflammatory gene expression and bacteriology. The data shows that 7-day survival was significantly improved by pFER compared to control groups. pFER increased BAL monocytes and activated antibacterial response genes (NOS, Fizz). pFER treatment showed decreased lung and blood Klebsiella counts reaching, in some cases, complete sterilization. In conclusion, FER gene delivery promoted survival in LC+PNA mice via recruitment of activated immune cells, improving efficiency of bacterial clearance within contused lung
Keywords: Acute Lung Injury, Secondary Bacterial Pneumonia, Lung Electroporation Gene Therapy, FER tyrosine kinase
INTRODUCTION
Trauma to the chest produces lung contusion injury (LC), impairs lung function, and accounts for a significant number of admissions to adult intensive care units. Findings from our laboratory have shown several immunological and physiological derangements in the lung following LC that increase the susceptibility of these patients to develop severe bacterial pneumonia (PNA)(1–5). The combination of LC and PNA can lead to severe respiratory failure with need for endotracheal intubation and mechanical ventilation support. LC patients are at high risk for pneumonia, sequential sepsis and Adult Respiratory Distress Syndrome (ARDS). Despite improvements in critical care, severe ARDS continues to be associated with significant (~40%) mortality (6, 7). Elderly patients are increasing in prevalence and are at an elevated risk for falls and subsequent complications from traumatic injury. In the injured elderly (> 65 years of age), the presence of rib fractures from traumatic closed chest injury is associated with an odds ratio of dying of 1.98 (1.86–2.11, 95% CI). The odds ratio of mortality in the elderly increases dramatically to 5.24 (3.51–87.82, 95% Cl) when combined with the presence of pneumonia(8).
Klebsiella is an important pathogen of the so-called ESKAPE organisms (Enterococcus faecium, Staphylococcus aureus/MRSA, Klebsiella pneumoniae, Acintenobacter baumannii, Pseudomonas aeruginosa and various Enterobacter species), all constitute the bulk of nosocomial infections worldwide and are particular associated with high mortality thus studying their pathogenicity and virulence in clinical relevant models of lung injury simulating susceptible populations is extremely important. (2, 9, 10).
A recent Genome-wide Association Study (GWAS) from four independent cohorts showed that common variants of the FER gene were strongly associated with a reduced risk of death from sepsis due to pneumonia(11). The true functional significance of FER is highly controversial and the subject of great debate. FER is an evolutionary conserved, ubiquitously expressed, non-transmembrane receptor tyrosine kinase within the fes/fps family(12). It has been described in normal tissues to be a downstream integrator of membrane and cytosolic signaling pathways(13–15) boasting a beneficial participation in cell survival, cytoskeleton re-arrangement, epithelial maintenance and leukocyte differentiation and chemotaxis. However in several cancer models, FER has been implied to be involved in mechanisms of malignant cell transformation, tumoral invasion and metastatic potential(16–19). FER overexpression is a negative predictor of survival in Non-Small Cell Lung Cancer (NSCLC)(20). This ambivalent characteristic suggests that FER is tightly regulated. Interestingly, its role in lung acute inflammatory states has never been extensively studied.
It has been proposed that transient genomic editing and manipulation of protein expression by gene therapy can potentially restore known altered aspects of lung biology that favor resolution of lung injury (21–23). However, in the injured lung, traditional viral techniques may worsen inflammation and could be neutralized by natural lung physical and immunological barriers that prevent such transfection(24). To circumvent these challenges, our laboratory has adopted in vivo electroporation (EP) as a simple, reproducible and inexpensive method of gene transfer. The possible events of in vivo electroporation have been elegantly described elsewhere (25, 26). In the lung, however it does confer advantages over other gene therapy techniques, among these the lack of inflammation, as well as the lack of integration into the chromosome making transgene expression transient and short-lived, ideal for the resolution of acute lung injury without concern for long term effects or mutagenesis(21, 27). We have been successful in achieving high levels of expression both in naïve and injured lungs by using in vivo electroporation(22, 27–29). Furthermore, we have shown that EP-mediated delivery of genes of the Na+/K+-ATPase pump, an important regulator of water and salt balance in the lung, was successful in reducing lung edema and inflammation after lipopolysaccharide (LPS) or LC insults.
In this study, we examined the role of EP-mediated delivery of the FER gene in an acutely injured lung. We hypothesized that electroporation-mediated delivery of the FER gene would improve survival in our lethal murine model of trauma complicated by PNA. The data presented here indicates a favorable phenotypic induction with survival advantage given by EP-mediated delivery of FER gene, characterized by recruitment of inflammatory monocytes and up-regulation of bactericidal response gene activity. This outcome yielded an improved capacity to clear the lung of bacteria and decrease subsequent inflammation. We describe a promising alternative strategy towards the resolution of acute lung injury and a complementary treatment to antibiotics for severe trauma-related secondary bacterial pneumonia.
RESULTS
The following describes a transient 1–2 day increase of expression of human FER tyrosine-kinase gene introduced by electroporation-mediated gene transfer of a naked DNA plasmid encoding human FER (pSG5-FER) into injured mouse lungs.
1. FER Gene Delivery by Electroporation increases FER transcription and FER protein expression in areas of contusion-damaged lungs
Initially, we sought to delineate if electroporation-mediated delivery of FER gene would be successful at clearing bacteria in severely damaged lungs after unilateral LC injury followed by bacterial pneumonia. At 24 hr., harvested right contused lung lobes (Figure 1, Panel D) were subjected to immunohistochemistry to reveal the expression of FER post electroporation of pSG5-FER and control plasmids encoding Na+/K+ ATPase or pcDNA-3. In Figure 1, the immunofluorescent stained specimens depict overexpression of FER in bronchial airway epithelial cells, underlying bronchial stromal tissue (A) and in lung parenchyma cells (B) only in the pFER electroporated treatment group. Very minimal background expression could be seen in the electroporated Na+/K+-ATPase gene group whereas all other control groups had no evident signal (data not shown). In BAL and total lung lysates we performed real-time PCR assays showing increased transcription of human transgene FER at 24 hr (Figure 1, Panel E). This expression correlated with Western Blot data showing increased total protein FER at 24 hr within the FER electroporated group and increased phosphorylated isoform of FER at 72 hr, confirming gene transfer (Figure 1, Panel F).
Figure 1. Expression of FER after electroporation-mediated gene transfer of PSG5-FER plasmid in murine model of lung contusion injury and pneumonia.

FER expression localization by immunohistochemistry on frozen sections of right lung lobe: A. Airway; B. Alveoli; C. FER expression in the Na+/K+-ATPase gene electroporated group in similar injury conditions; D. Aspect of lungs at 24 hr time point after unilateral (right-sided) contusion followed by administration at (LC+PNA group); E. Western Blot; F. Quantitative RT-PCR (N=10 mice per group). Statistical analysis performed one-way ANOVA followed by Tukey’s multiple comparison test; * p < 0.01
2. Electroporation-mediated Delivery of FER Gene Decreases Injury and Inflammation Following Combined Lung Contusion and Pneumonia
a. Histological Analysis
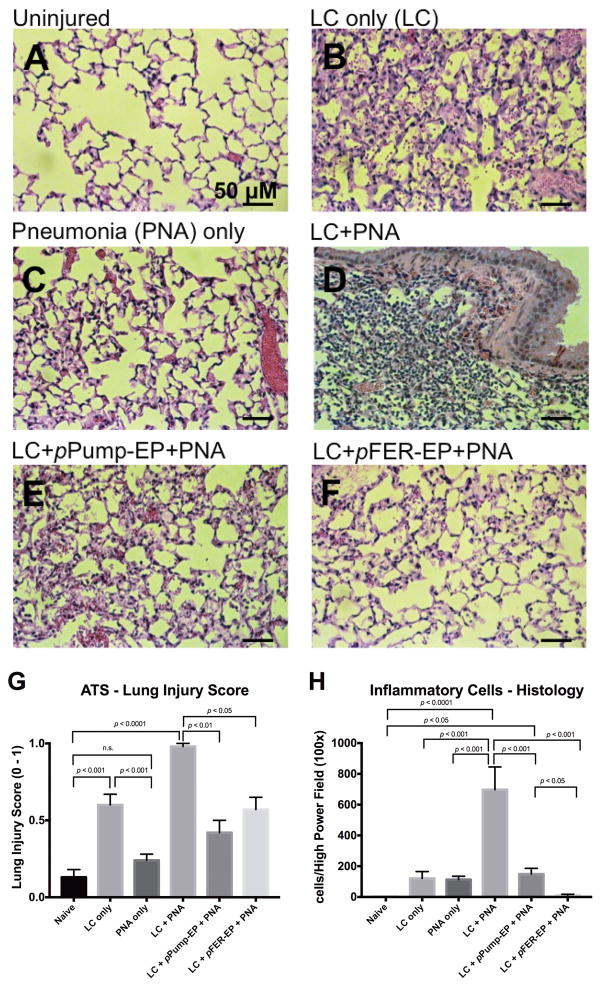
We performed assessment of the distinct area of injury distributed among the right contused lung lobes in animals at 24 hr (Figure 2, panels B, D, E, and F). Histological evaluation of these contused areas showed extensive hemorrhage, infiltration of inflammatory cells with pulmonary consolidation, proteinaceous exudate, decreased airspaces and edema. After lung contusion, infiltrative cells are predominantly mononuclear in nature and neutrophils are very low in number (Panel B). In contrast, when bacteria are innoculated into the lungs, neutrophils are rapidly recruited (Panel C). The inflammatory response is vastly amplified in the combined LC + PNA model of injury (Panel D). Thus the deleterious effects of LC injury and bacterial pneumonia appear to be summative as measured with the American Thoracic Society (ATS) Lung Injury Scoring System (30). After electroporation of either Na+/K+ ATPase or FER gene, we observed a relative reduction of tissue inflammation characteristics present in the parenchyma as compared to LC+PNA animals (Figure 2, panel E or F).
Figure 2. Lung Histology. Hematoxylin and Eosin-stained lung sections at 24 hr. after 500 CFU Klebsiella pneumoniae administration and/or 30 hr. post lung contusion injury.
A. Uninjured-Naive; B. Lung Contusion only; C. Pneumonia only; D. Lung Contusion plus Pneumonia; E. Lung Contusion plus electroporation of the Na+/K+-ATPase gene followed by Pneumonia; F. Lung Contusion plus electroporation of FER gene followed by Pneumonia; G. American Thoracic Society - Lung Injury Score; H. Inflammatory Cell Infiltrate per High Power Field (100x). Microphotographs were obtained from contusion portion of right mouse lung. Right lung lobes in Uninjured-Naive and Pneumonia only groups were also used for comparison. Significant inflammation characterized by inflammatory cell infiltrate, intra-alveolar hyaline membrane (proteinaceous deposit), cellular debris, interstitial septal edema was found and separate components were used to score severity of lung injury blindly. Inflammatory response was mitigated in the FER treated group, less so in the Na+/K+-ATPase group. Bar represents 50 μM (20x). N=3 mice per group. Statistical analysis performed one-way ANOVA followed by Tukey’s multiple comparison test; 5 fields per lobe.
b. Alveolar-Capillary Integrity
At the 24 hr. time point, ELISA assays measuring the levels of albumin in BAL were elevated in all groups compared with the naïve controls (Figure 3), indicating different degrees of severity from lung permeability injury and alveolar-capillary disruption. LC injury with ensuing pneumonia had the highest level of permeability injury as compared to pneumonia exposure alone. In animals with these combined injury insults, treatment with EP-mediate delivery of Na+/K+-ATPase gene was able to show a significant reduction of BAL albumin levels at 24 hours after injury, consistent with our previously reported data in LPS-only and LC-only injury models(28, 29). Importantly, FER gene delivery was also able to show this positive effect in reducing the degree of permeability injury as compared to controls.
Figure 3. Levels of albumin present in Bronchial Alveolar Lavage (BAL) fluid as a measure of lung permeability injury 24 hr. after administration of 500 CFU Klebsiella pneumoniae and 30 hr. after lung contusion.
N= 6–8 mice per group. Statistical analysis performed one-way ANOVA against naive (bracket *** p < 0.0001) and post-hoc Tukey’s multiple comparison test * p < 0.05.
c. Pressure – Volume (P–V) Curves
Electroporation-mediated delivery of both FER and Na+/K+-ATPase genes showed favorable responses in quasi-static P–V curves (Figure 4, panel A–B) generated over 4,000 data points with the SCIREQ Flexivent ventilator. This indicates better compliance and higher inflatable volume at Pressure-30 (TLC-30) than the LC + PNA control (Figure 4, panel C–D) in the assessed time points. For the LC + PNA only control group, the large separation found between the inspiratory and expiratory limbs of the curve signified a larger driving pressure needed to open areas of atelectatic lung, making these lungs, in theory, more susceptible to atelectrauma (31). Both gene therapy treatments, either Na+/K+-ATPase (to reduce edema) or FER (to reduce inflammation), showed smoother and parallel curves indicating much more harmonic ventilation.
Figure 4. Pressure Volume Curves after established combined injury of Lung Contusion and Pneumonia.
A. Pressure Volume Curves at 24 hr B. Pressure Volume Curves at 72 hr. C. Total Lung Capacity (TLC) at 30 cm H2O pressure at 24 hr after PNA D. Total Lung Capacity (TLC) at 30 cm H2O pressure at 72 hr. Using a SCIREQ FlexVent ® (Montreal, Canada) quasi-static pressure volume curves with a maximal inflation to 30 cm H2O were generated from over 4000 data points. Bottom half of each loop represents the inspiratory portion of a P–V curve perturbation maneuver. Top half represents the expiratory portion of each graph. LC+PNA reduces overall compliance requiring higher pressures to open the same amount of lung units than other groups. Representative curve from uninjured group for same age cohort animals. Both the Na+/K+-ATPase and FER gene delivery were effective in improving lung mechanics at 24 and 72 hrs as compared to control. N= 6 – 8 mice per group. Analysis of results for the Naïve, LC-only, LC+PNA, LC + pPump + PNA and LC + pFER + PNA groups was performed using one-way analysis of variance (p <0.001)
d. Bronchial Lavage Fluid (BAL) Cytology
LC, PNA and combo LC + PNA conditions resulted in the production of a significant inflammatory response discernable by changes in cellular subpopulations in BAL. At 24 hrs, in LC only conditions, analysis of cytospin slides stained with Diff-Quik revealed significant recruitment of macrophages and sparse recruitment of neutrophils. 72 hrs after the same condition (LC-only), the majority of cells appear as apoptotic macrophages, indicating the resolution of trauma-related sterile inflammation (Figure 5, panels B and G). In contrast, mice challenged both with LC + PNA demonstrated equal recruitment of macrophages and neutrophils in BAL within 24 hours post pneumonia challenge (Figure 5, panel C). The response was continued at 72 hours (Figure 5, panel H) illustrating the non-resolution of inflammation. For animals receiving electroporation-mediated delivery of Na+/K+ ATPase genes under similar combined injury conditions, we found an elevated number of macrophages in BAL and almost a complete absence of neutrophils (Figure 5, panel D). This neutropenic response to LC + PNA was persistent in the Na+/K+ ATPase group at 72 hours with significant ghost cells (dead necrotic cells) present in BAL (Figure 5, panel I). The electroporation-mediated delivery of FER gene was distinguishable from all other groups in showing a greatly boosted immune response, with equally recruited and relatively larger numbers of macrophages and neutrophils at 24 hours post pneumonia challenge (Figure 5, panel E). This response however is not lasting and by 72 hours post pneumonia, the FER electroporated group shows a return of the BAL cellular populations to almost baseline pre-injury characteristics with much lower number of macrophages and absence of neutrophils, suggesting resolved infection and inflammation (Figure 5, panel J).
Figure 5. Bronchial Alveolar Lavage Cells.
A. Naïve; B and G. Lung Contusion only; C and H. Lung Contusion plus Pneumonia; D. and I. Lung Contusion plus electroporation of the pNa+/K+-ATPase ATPase/EP plus Pneumonia; E. and J. Lung Contusion plus pFER/EP plus Pneumonia. B–E. 24 hr. time point(s); G–J. 72 hr. time point(s); F. Experimental model.
e. Flow Cytometry
Flow Cytometry experiments (Figure 6) confirmed the observations from our cytospin slides. Our data suggests that electroporation of FER gene delivery stimulated a multi-lineage cellular response in BAL, with a predominance in the number of Inflammatory Monocytes (GR-1+; F4/80+) at all time points. As for assessing for neutrophils (GR-1+; F4/80−), the electroporation of Na+/K+-ATPase treated group showed a noticeable reduction in their numbers both at 24 and 72 hrs, whereas the FER treated group had an initial peak at 24 hrs which rapidly dropped by 72 hrs. In regards to macrophages (GR-1−; F4/80+), no differences were seen in the first 24 hrs among groups, however these were significantly increased in LC + PNA group at 72 hrs.
Figure 6. Flow Cytometric analysis of Bronchial Alveolar Lavage Cells shows that FER gene therapy works by enhancing the lung immune system.
Cell counts and types of cells present in equal volumes of BAL fluid as identified using flow cytometry. Samples were collected 24 and 72 hr. after administration of Klebsiella. N= 5 mice per group, 100000 cells per sample. Analysis of results for the LC+PNA, LC+pFER+PNA and LC+pPump+PNA groups was performed using one-way ANOVA followed by Tukey’s multiple comparison test. *p < 0.05 post hoc test.
3. EP-mediated Gene Delivery of FER was associated with Increased Survival following Combined Lung Contusion and Pneumonia
a. Survival Curve data
Pneumonia induced by Klebsiella pneumoniae (500 CFU) post Lung Contusion dramatically increased mortality. None of the animals died from LC alone (data not shown). The electroporation of FER plasmid resulted in 90% 7-day survival, which was significantly different (p = .004, long-rank test) than all other groups: Lung Contusion + Pneumonia group (20%-survival; p < 0.003); Lung Contusion + Na+/K+-ATPase pump genes electroporation treated group + Pneumonia (0%-survival; p <0.001) (Figure 7 Panel A). To assess that the survival benefit was not related to the electroporation of a non-specific plasmid DNA, two additional groups were electroporation-treated. One group was delivered with an empty plasmid DNA and another one was delivered with a reporter gene (luciferase) plasmid DNA under same injury conditions. However neither of these groups had increased survival outcomes after lung contusion and pneumonia injury (Figure 7 Panel B).
Figure 7. Survival Curve Data.
A, Electroporation of FER significantly enhanced (p = .004, long-rank test) survival in the settings of lung contusion and pneumonia. Survival after lung contusion followed by 500 CFU Klebsiella pneumonia created 6 hours after chest injury. LC+PNA: lung contusion and pneumonia; LC + pPump + PNA: lung contusion followed by EP-mediated delivery of genes for the Na+/K+-ATPase pump followed by PNA; LC + pFER + PNA: lung contusion followed by EP-mediated delivery of human FER gene followed by PNA. B, Electroporation of empty plasmid or luciferase plasmid were out of survival benefit. N = 10 mice per group. Significance placed in graph. Log- rank (Mantel-Cox) test.
4. FER Gene Therapy Significantly Reduced Klebsiella pneumoniae Counts in Blood Circulation and Lungs
Whole blood and lung tissue were assayed for the formation colony forming units (CFU) of Klebsiella pneumoniae normalized to 1 mL of blood and total lung tissue, respectively (Figure 8). Not surprisingly, in the LC + PNA group bacterial counts had a 1-log increase between 24 and 72 hrs, especially in blood, confirming continued uncontrolled multiplication of organisms and suggesting probable death due to invasion and sepsis at later time points. Electroporation of FER was very effective in suppressing bacteria from reaching blood circulation at all time points. Only 3 out of 18 animals revealed a positive culture in any of time points assessed. While the electroporation of Na+/K+ ATPase was able to contain bacterial blood invasion at 24 hr, this effect was lost at 72 hr. At 24 hr post pneumonia delivery, EP-mediated transfer of both Na+/K+ ATPase and FER genes showed capacity to inhibit bacterial growth in the lung; however, only FER was capable of a statistically significant decrease in CFUs. Importantly, FER was able to completely clear infection of several animals at 72 hrs.
Figure 8. Whole blood and lung tissue culture to quantify bacterial colony forming units assay.
Electroporation of FER significantly suppressed bacteria in the lung and in circulation. Electroporation of Na+/K+-ATPase was also efficient in suppressing bacteremia but was time-limited for only for 24 hrs. Meanwhile FER treated animals continue to avoid major bacteremia at 72 hrs and cleared bacteria from 6 out of 11 animals. N= 10 – 11 mice per group. (circle) Lung Contusion + pneumonia; (triangle) LC + EP-pPump+ Pneumonia; (square) LC + EP-pFER + pneumonia; Bar indicates median for each group. Statistical analysis performed one-way ANOVA and post-hoc Tukey’s multiple comparison test.
5. EP-mediated delivery of FER induces Expression of Nos-2 in BAL and Lung Cells
Noting the increase in recruitment of inflammatory monocytes upon FER electroporation, we set out to perform real-time PCR. We found that EP-mediated delivery of FER showed a significantly enhanced expression of Nos-2, Fizz1, and IL-1β in BAL cells (Figure 9A) known genes to be critical in the fight against bacterial infection in the lung. Additionally, we looked at the expression of these genes within washout lung parenchymal tissue and found that Nos-2, IL-1β and TNF-α expression was significantly higher in the FER group (Figure 9B), suggesting that these activation effects were also present in interstitial macrophages and other inflammatory cells distributed within lung tissue and thus primed to create a second barrier of protection against bacterial invasion.
Figure 9. Expression of key genes, involved in anti-bacterial immune response, are enhanced after EP-mediated delivery of FER gene in BAL cells and lung parenchyma tissue.
Real-time PCR TaqMan indicate that FER gene therapy produces a boost in Nitric Oxide Synthase (NOS-2) and similar genes (Fizz1, IL-1β and IL-6) at 24 hours indicating a potentiated immune system to fight infection. N= 3–10 mice per group. Statistical analysis performed one-way ANOVA
6. Over-expression of FER did not Induce Fibrosis or Epithelial Mesenchymal Transformation in the Lung
Mice with LC and PNA and who had EP-mediated delivery of FER gene surviving over 7-days were euthanized and their lungs were subject to Masson’s Trichrome staining (Figure 10). All areas of the lung were assessed. Based on our model, the right lung (subject to contusion) still had mild evidence of inflammation; however, there was no evidence of excess fibrosis or collagen deposition as compared to bleomycin control. EP-delivery of human FER gene and its expression did not linger nor did it induce Mesenchymal Transformation or a fibrosis-type of recovery in these treated lungs.
Figure 10. Masson’s Trichrome Staining does not show excess fibrosis or Mesenchymal Epithelial Transformation in lungs of among FER treated survivors.

Areas representative in the graphs are right lung contusion with pneumonia (compare to Figure 1-Panel D) and surviving after treatment with EP-mediated delivery of FER gene. Bleomycin a known induction agent for pulmonary fibrosis was used as positive control after harvesting animal at 8 days post insult. N= 3 mice per group.
DISCUSSION
The use of FER electroporation therapy was effective in containing bacterial infection within the lung and resulted in prevention of bacteremia following LC injury. Moreover, a single application of FER was able to boost host immune response counteracting known deleterious effect of LC injury and pneumonia(2, 5). Unfortunately, the alternative Na+/K+ ATPase gene delivery therapy, previously reported to be beneficial in reducing lung edema and inflammation in the settings of sterile models of LC and LPS injury(28, 29), was not protective against secondary Klebsiella pneumonia. Based on BAL cytospin and Flow Cytometry experiments, our findings suggest that the overexpression of Na+/K+ ATPase induces an excessive inhibition of neutrophilic recruitment, impeding bacterial clearance. In consequence, as bacteria multiply they will overwhelm lung barriers allowing bloodstream invasion and animals will die from sepsis as seen in the survival experiments.
Following LC, there are several pathological features that increase the susceptibility of the lung to pneumonia and affect severity. LC injury produces an area of epithelial disruption causing alveolar hemorrhage, which constitutes an excellent culture media for bacteria (2, 32). The presence of a protein-rich exudate interferes with surfactant lipids, changing surface-tension properties within alveoli and thus inhibiting immune protective effects. The loss of integrity of the epithelial barrier permits the invasion of microorganisms into the bloodstream causing bacteremia and sepsis. Our laboratory has previously reported that lung contusion injury also forges a depletion of alveolar macrophages and is an important feature in potentiating the vulnerability of lungs to fulminant infection (2, 5). Therefore, the development of short-lasting gene therapy to counter this phenomena and boost the host’s a balanced response against bacterial pneumonia is of therapeutic interest (33–35).
Electroporation is an effective technological platform for introducing genes into both intact and diseased lung
Electroporation-technology has been around for almost 40 years with the first report of single cell electroporation in 1982 (36). However, it is only recently that is has been considered for clinical application. As mentioned in comprehensive review by Mir (2014)(26), there are not only technological hurdles present to be solved with smart biomedical engineering but it also requires a significant change in heart and acceptance in medical society. In regards to lung electroporation, we have recently demonstrated that external chest electroporation achieves high expression levels of newly introduced genes in the lung, comparable to other leading viral vector techniques (27). Using reporter gene assays, we have demonstrated extensive expression throughout many pulmonary cells (alveolar epithelia, bronchial epithelia, bronchial/vascular smooth muscle)(22). One of the greatest advantages of electroporation over viruses is that it does not increase inflammation levels in the surrounding tissues. A second advantage is that its activity is only limited to the area of electrical field, thus avoiding potential systemic effects derived from vector dissemination. Studies from the laboratory of David Dean have demonstrated the applicability of this method of gene delivery in large animal models such as swine (37). The amount of energy used has been calculated in 0.2 joules/kg, much less than defibrillators in hospital practice (38). Our preliminary data has also shown that electroporation can effectively introduce therapeutic gene(s) not only into the intact, but also the injured lung (27–29). This opens the possibility of electroporation mediated gene transfer to be translated into clinical applications.
FER gene expression improved survival from death related to lung contusion complicated by pneumonia
Our results support the recent genome-wide association study (GWAS) which points to enhanced expression of common variants FER gene and reduced the risk of death by 44% from sepsis due to pneumonia (11). Although no particular mechanism was described in this population study, it was suggested that FER could been implied in the regulation of host defense mechanisms along with preservation of epithelial and endothelial barrier function, which could explain many of its protective effects (12, 39–45).
In our experimental model, we found that innate defense mechanisms were indeed enhanced with electroporation-mediated delivery of FER gene. This was not found in the similarly EP-delivered Na+/K+-ATPase gene group, implying gene specificity and that it is not a side effect of EP pulses and/or the presence of foreign DNA (a known stimulator of TLR-8). The overexpression of FER showed a rapid recruitment of inflammatory monocytes at 24 hrs. Additionally, Nos-2 and FIZZ-1, genes important for white blood cells’ bactericidal activity, were increased after single electroporation treatment of FER.
The role of FER gene in normal lung biology and its function during acute inflammatory lung states has not been studied in detail. Fps/Fes and FER are the only known members of a distinct subfamily of the non-receptor protein-tyrosine kinases. These proteins have autocatalytic properties that induce phosphorylation of their own SH-2 domains, as well as similar domains belonging to other membrane bound tyrosine kinases(13, 15, 18, 46, 47). Many studies indicate that these kinases have roles in regulating inside-out signaling that accompany receptor-ligand, cell-matrix and epithelial-immune cell interactions in the gut(42), which are probably of equal importance in lung epithelia. Using a kinase deficient transgenic model, significant but nonlethal intestinal epithelial disruption and exaggerated neutrophilia were found after insult (42, 45, 48, 49). Other authors have observed that members of this family are important in myeloid cell recruitment and differentiation (14, 41, 49).
We recognize several limitations to our study that need to be pointed out. First, we have yet to identify the actual downstream effectors of FER expression in this particular model lung injury and gene transfer. Future experiments involving transgenic kinase deficient mice and adoptive cell transfer are planned that will allow to further ascertain the mechanisms of FER-induced host response in this model. Second, we have yet to know if these protective effects can be expanded to other bacterial species, especially of the ESKAPE spectrum. This therapy will need to be empirically tested against these organisms in this murine model as well as other larger animal models of lung injury.
Knowing the reported association as a negative predictive marker for cancer-survival in non-small cell lung cancer (NSCLC) and addressing any other safety concerns we performed histological analysis of 10-day survivors after FER treatment. We did not find any major dysplastic, fibrotic or mesenchymal transformation features in electroporated lungs. Our RT-PCR and Western Blot data also showed a very limited, short lived expression of FER, which is ideal in our disease model of critical illness. These observations, in combination with findings from other investigators showing that gene expression driven by viral promoters (like SV40 such as in this case) is deactivated rapidly(21, 50, 51), should not prohibit the use of FER gene delivery for fear of malignant transformation. As plasmid DNA rarely integrates unassisted into the host chromosome, concerns for mutagenesis and activation of other oncogenes should be minimal.
In summary, the transient increase of FER expression was advantageous in successfully clearing bacterial pneumonia complicating lung contusion. The FER-treated mice displayed 90% survival compared < 20% survival among control groups, thus supporting the findings from the GWAS study of the beneficial role of FER expression in sepsis and pneumonia.
MATERIALS AND METHODS
Animals
Male C57BL/6 wild-type mice (Harlan, Indianapolis, Ind.) were used in all experiments. Mice were housed under specific pathogen-free conditions and were allowed a 1-week acclimation period to their new surroundings before being used in experiments. All experiments were performed in accordance with National Institutes of Health guidelines for care and use of animals. Approval for of all experimental protocols was obtained from the University of Michigan Committee on Use and Care of Animals (UCUCA) and the Institutional Biotechnology Committee (IBC).
Lung Contusion Injury
We followed modified methods as described by Hoth et al (2007)(52). At time minus 6 hours, a unilateral blunt lung contusion injury was induced using an electric cortical contusion impactor apparatus (23, 35). Procedural details for the closed chest lung contusion injury model has been described extensively in previous publications from our laboratory(2, 3, 29, 35, 53). All animals received buprenorphine immediately after contusion to avoid post-treatment discomfort. Mice were then allowed to recover spontaneously.
Recombinant Plasmid DNA
pSG5-FER (human FER tyrosine kinase) plasmid was purchased from Addgene (Cambridge, MA) and propagated in E. coli. Plasmids were then harvested using QIAGEN Giga-prep kits (Valencia, CA)(54) per manufacturer’s instructions. Original pSG5-FER plasmid was a gift from Nora Heisterkamp deposited in Addgene DNA repository (Addgene plasmid # 30191)(54). The plasmid contains an insert that encodes human FER (hFER) which is a non-receptor tyrosine kinase located on human chromosome 5q21 (Gene ID 2241; HPRD 01491; NM_005246). Two separate plasmids containing the α and β subunits of the Na+/K+-ATPase were a gift from David A. Dean (University of Rochester, NY) and were harvested in similar fashion as described above. A luciferase reporter gene plasmid (Promega, Madison, WI) was used as a control for effects of electroporation process over results, containing similar promoter sequence of the FER plasmid (SV40). Similarly an empty plasmid (pcDNA3) was used for same purposes in survival curve experiments. All plasmids of DNA were stored in 10 mM Tris- 1 mM EDTA buffer (pH 8.0) at −20°C until the day of gene delivery. Protocols for the use of recombinant DNA technology required prior approval from the University of Michigan Institutional Biosafety Committee.
Electroporation-mediated delivery of genes into mouse lungs
Electroporation mediated gene transfer was performed on the same day of injury, in-between lung contusion and deep oral delivery of bacteria. We modified methodology as described by Dean et al (2003) and Machado-Aranda et al (2012). Briefly, 150 μg of DNA suspended in 100 mM NaCl were delivered to the lungs via hypopharyngeal drop in anesthetized animals. After several regular breaths, metal plated electrodes were placed under each forelimb assuring contact with an electroconductive gel. Using a BTX Harvard Apparatus ECM 830 generator (Holliston, MA) eight square wave electroporation 200 V/cm pulses of 10 ms duration and one second apart were given. Animals were allowed to recover while kept under BSL2 containment.
Induction of Secondary Bacterial Pneumonia
Six hours after lung contusion injury and lung electroporation, we administered Klebsiella pneumoniae, strain 43816, serotype 2 (American Type Culture Collection, Manassas, VA). We followed our previously reported methodology of combined LC and pneumonia, which produces consistent and significant (> 80% at 7 days)(2, 55, 56). Appropriate serial dilutions were subsequently performed to achieve a concentration of 500 CFU’s of bacteria per 30 μL of inoculum, which corresponds to LD50 for C57/Bl6 mice using this particular strain of Klebsiella. Administration of 30 μL of bacterial suspension was performed via deep oral injection into the trachea under isoflurane anesthesia. Animals were allowed to recover spontaneously and transported back to BSL2 housing where animal health and survival data were recorded every 8 hr.
Lung Mechanics Measurements and Bronchoalveolar Lavage Collection
Based on luciferase reporter gene assays (data not shown) and in parallel to survival curve generation experiments, we selected two time points, one before (24 hr) and one after (72 hr) after the peak of maximal expression (48 hr) of luciferase under similar injury conditions. At these times points, mice were euthanized and a tracheal cut down was performed. A blunt metal tube was inserted gently into the trachea and connected to the circuit of a FlexiVENT® ventilator (Montreal, Quebec, Canada) and ex-vivo ventilation was initiated as described in Mutlu et al (2007). A quasi-static pressure volume curve (P–V curve) was obtained after an initial recruitment maneuver to a pressure of 30 cm H2O and recorded three times during a 15 min. ventilation course. Immediately afterwards, the lungs were lavaged with 1.5 mL of sterile PBS using several 1 mL syringes. Bronchoalveolar lavage (BAL) fluid was centrifuged at 400 g for 8 minutes. Following centrifugation, supernatant was separated from precipitated cells, and stored frozen at −80°C until use. Spun down cells were resuspended in appropriate buffer and used either in flow cytometry assay or for quick staining.
Quantitative Bacterial Culture Assessments
Blood Culture
In separate experiments, at similar time points as above, animals were euthanized according to UCUCA Approved Protocol. Blood was obtained via cardiac puncture using a sterile 18-gauge needle. To measure bacteria titers in blood, 100 μL of undiluted blood was plated onto 5% sheep blood agar plates (Thermo Fisher Scientific, Remel Products, Lenexa, KS). Plates were incubated overnight at 37°C and colony forming units (CFU) per mL of blood were counted after 16 hours.
Total Lung Tissue Culture
Additionally, the thoracic cavity was opened and right ventricle and trachea were cannulated, and lungs were flushed of blood via right heart with 2 mL of ice cold PBS. Both lungs were removed and homogenized in 1 mL of ice cold PBS. Serial 10-fold dilutions were made then made in the same buffer and 100 μL volumes of homogenate were plated on 5% blood agar plates. Plates were incubated overnight at 37°C and CFU per total lung tissue were counted after 16 hours. If no colonies were present, culture plates were kept for additional 48 h in the incubator to confirm no growth of delayed organisms.
Cytospins BAL Cell Staining
Cytospins were performed by loading 100 μL from spun down BAL into each cuvette and spinning against glass slides at 350g for 5 minutes. Slides were fixed with Diff-Quik (Baxter, Detroit, MI) based on Wright-Giemsa stain for microscopic examination.
Flow Cytometry
Flow-cytometric analysis of 100000 cells per sample was performed using a BD LSR II Flow Cytometer (BD Biosciences) following protocol as described in Dolgachev et al (2014)(56) and using the following antibodies Gr-1-PE, CD11c-APC-Cy7, F4/80-AF488, CD11b-PE-Cy7, and CD206-APC (BioLegend and BD Biosciences, San Jose, CA). Obtained data was plotted and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Western Blot Analysis
For FER expression determination, whole lung extracts were run on 12.5% SDS-polyacrylamide gels, with detailed protocol for sample processing as described in our previous publications (4, 53, 57, 58). Polyvinylidene difluoride membranes were probed sequentially with rabbit anti-FER or anti-phospho-FER (1:2000); followed by horseradish peroxidase-conjugated anti-rabbit IgG antibodies (1:3000) (AbCAM, Cambridge, MA). We used actin as a loading control using mouse anti-actin (1:100) (AbCAM, Cambridge, MA) followed by secondary as described above. Proteins were visualized by chemiluminiscense (Super Signal West Pico chemiluminescent substrate, Pierce, Rockford, IL, USA) using a Kodak photo imager.
Real time-Polymerase Chain Reaction (RT-PCR)
In parallel experiments, from control and experimental group animals under same injury conditions, treatments and time points as described above, after euthanasia the chest cavity was opened. Cannulating the heart, lung circulation was perfused blood free with ice-cold PBS prior to their removal from chest cavity. RNA was isolated from the upper right lobes of lung using TRIzol (Ambion, Invitrogen, Carlsbad, CA) and from BAL cells using Qiashredder columns (QIAGEN, Valencia, CA) per manufacturer instructions. Levels of mRNA for FIZZ1, Arginase 1, nitric oxide synthase 2 (inducible) (NOS2), interferon gamma (IFN-γ), interleukin 1 beta (IL-1β), and interleukin 10 (IL-10) were assessed using quantitative PCR analysis (TaqMan®) with predeveloped primers and probe sets (Applied Biosystems, Carlsbad, CA). Quantification of the genes of interest were normalized to GAPDH and expressed as fold increases over the negative control for each treatment at each time point.
Histopathological examination and Masson’s Trichrome staining
At 24-hr post insult, lungs of individual mice were fixed in 4% formaldehyde solution, and then 4-μm thick sections were cut, stained with hematoxylin and eosin and examined by light microscopy. Slides (4 for each group) were sent to be analyzed blindly by our resident pathologist measuring parameters to achieve a composite Lung Injury Score, as stated in the consensus document from the American Thoracic Society (ATS)(30). Representative pictures were taken at 600x magnification using Tx2 Nikon microscope. Long term survivors (10 days) were euthanized and lung were extracted and processed as above and following methods described elsewhere(59) for Masson’s Trichrome staining. Pulmonary fibrosis was induced by single introduction of 2.5 U/kg of bleomycin intratracheally at animals and animals sacrificed at comparable time point of long-term survivors.
In parallel experiments, right lungs of mice from electroporated and control groups were obtained at 24-hr post PNA and 4-μm frozen sections were stained using α-FER (Abcam, Cambrige, MA, cat#AB191060) and secondary α-rabbit-Alexa-594 (Invitrogen-Molecular Probes) following manufacturer instructions to demonstrate localization of expression after electroporation.
Statistical Methods
All statistical analysis and graphs were performed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA). Results are presented as mean values ± the SEM unless otherwise noted. Continuous variables were analyzed using an unpaired two-tailed Student’s t-test. Multiple groups were compared by one-way ANOVA with Tukey’s multiple comparison test used for post-hoc analysis or two-way ANOVA with Bonferroni posttests. Survival curves were generated using the Kaplan-Meier method. The Log-rank (Mantel-Cox) Test was used to compare the survival data between experimental groups. Statistical significance was defined as a p-value < 0.05.
Acknowledgments
The authors gratefully acknowledge the support of National Institutes of Health-R01HL102013 (KR). We thank Dr. J.E. Wilkinson (Department of Pathology/ULAM, University of Michigan Medical School) for the help with lung tissue histology scorings. We also thank Ms. Lane Kelley McCandless for her contribution in revising this manuscript.
Footnotes
Author contributions: VAD, RHG, MVS, BT, NT and DMA – conducting experiments, writing manuscript. DMA, MRH, KR – conceptual design, interpretation of data and critical review.
Authors Conflicts of Interest: None
CONFLICTS OF INTEREST
The authors do not report any competing financial interest with the work described.
References
- 1.Raghavendran K, Davidson BA, Woytash JA, Helinski JD, Marschke CJ, Manderscheid PA, et al. The evolution of isolated bilateral lung contusion from blunt chest trauma in rats: cellular and cytokine responses. Shock. 2005;24(2):132–8. doi: 10.1097/01.shk.0000169725.80068.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolgachev VA, Yu B, Reinke JM, Raghavendran K, Hemmila MR. Host susceptibility to gram-negative pneumonia after lung contusion. J Trauma Acute Care Surg. 2012;72(3):614–22. doi: 10.1097/TA.0b013e318243d9b1. discussion 22–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado-Aranda D, Wang Z, Yu B, Suresh MV, Notter RH, Raghavendran K. Increased phospholipase A2 and lyso-phosphatidylcholine levels are associated with surfactant dysfunction in lung contusion injury in mice. Surgery. 2013;153(1):25–35. doi: 10.1016/j.surg.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suresh MV, Ramakrishnan SK, Thomas B, Machado-Aranda D, Bi Y, Talarico N, et al. Activation of hypoxia-inducible factor-1alpha in type 2 alveolar epithelial cell is a major driver of acute inflammation following lung contusion*. Crit Care Med. 2014;42(10):e642–53. doi: 10.1097/CCM.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado-Aranda D, MVS, Yu B, Dolgachev V, Hemmila MR, Raghavendran K. Alveolar macrophage depletion increases the severity of acute inflammation following nonlethal unilateral lung contusion in mice. J Trauma Acute Care Surg. 2014;76(4):982–90. doi: 10.1097/TA.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–93. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 7.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Current opinion in critical care. 2014;20(1):3–9. doi: 10.1097/MCC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 8.Battle CE, Hutchings H, Evans PA. Risk factors that predict mortality in patients with blunt chest wall trauma: a systematic review and meta-analysis. Injury. 2012;43(1):8–17. doi: 10.1016/j.injury.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Taylor AP. Serious Putty. The Scientist. 2016 May;2016:15–6. [Google Scholar]
- 10.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- 11.Rautanen A, Mills TC, Gordon AC, Hutton P, Steffens M, Nuamah R, et al. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. The Lancet Respiratory medicine. 2015;3(1):53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao QL, Ferris DK, White G, Heisterkamp N, Groffen J. Nuclear and cytoplasmic location of the FER tyrosine kinase. Molecular and cellular biology. 1991;11(2):1180–3. doi: 10.1128/mcb.11.2.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senis YA, Sangrar W, Zirngibl RA, Craig AW, Lee DH, Greer PA. Fps/Fes and Fer non-receptor protein-tyrosine kinases regulate collagen- and ADP-induced platelet aggregation. Journal of thrombosis and haemostasis: JTH. 2003;1(5):1062–70. doi: 10.1046/j.1538-7836.2003.t01-1-00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Sangrar W, Gao Y, Bates B, Zirngibl R, Greer PA. Activated Fps/Fes tyrosine kinase regulates erythroid differentiation and survival. Exp Hematol. 2004;32(10):935–45. doi: 10.1016/j.exphem.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Sangrar W, Gao Y, Scott M, Truesdell P, Greer PA. Fer-mediated cortactin phosphorylation is associated with efficient fibroblast migration and is dependent on reactive oxygen species generation during integrin-mediated cell adhesion. Molecular and cellular biology. 2007;27(17):6140–52. doi: 10.1128/MCB.01744-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok E, Everingham S, Zhang S, Greer PA, Allingham JS, Craig AW. FES kinase promotes mast cell recruitment to mammary tumors via the stem cell factor/KIT receptor signaling axis. Molecular cancer research: MCR. 2012;10(7):881–91. doi: 10.1158/1541-7786.MCR-12-0115. [DOI] [PubMed] [Google Scholar]
- 17.Ahn J, Truesdell P, Meens J, Kadish C, Yang X, Boag AH, et al. Fer protein-tyrosine kinase promotes lung adenocarcinoma cell invasion and tumor metastasis. Molecular cancer research: MCR. 2013;11(8):952–63. doi: 10.1158/1541-7786.MCR-13-0003-T. [DOI] [PubMed] [Google Scholar]
- 18.Sangrar W, Shi C, Mullins G, LeBrun D, Ingalls B, Greer PA. Amplified Ras-MAPK signal states correlate with accelerated EGFR internalization, cytostasis and delayed HER2 tumor onset in Fer-deficient model systems. Oncogene. 2015;34(31):4109–17. doi: 10.1038/onc.2014.340. [DOI] [PubMed] [Google Scholar]
- 19.Rocha J, Zouanat FZ, Zoubeidi A, Hamel L, Benidir T, Scarlata E, et al. The Fer tyrosine kinase acts as a downstream interleukin-6 effector of androgen receptor activation in prostate cancer. Molecular and cellular endocrinology. 2013;381(1–2):140–9. doi: 10.1016/j.mce.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Kawakami M, Morita S, Sunohara M, Amano Y, Ishikawa R, Watanabe K, et al. FER overexpression is associated with poor postoperative prognosis and cancer-cell survival in non-small cell lung cancer. International journal of clinical and experimental pathology. 2013;6(4):598–612. [PMC free article] [PubMed] [Google Scholar]
- 21.Lin X, Dean DA. Gene therapy for ALI/ARDS. Crit Care Clin. 2011;27(3):705–18. doi: 10.1016/j.ccc.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GR, Yeldandi AV, et al. Gene transfer of the Na+,K+-ATPase beta1 subunit using electroporation increases lung liquid clearance. Am J Respir Crit Care Med. 2005;171(3):204–11. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado-Aranda DA, Suresh MV, Yu B, Raghavendran K. Electroporation-mediated in vivo gene delivery of the Na(+)/K(+)-ATPase pump reduced lung injury in a mouse model of lung contusion. J Trauma Acute Care Surg. 2012;72(1):32–9. doi: 10.1097/TA.0b013e31823f0606. discussion 9–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiss DJ. Delivery of gene transfer vectors to lung: obstacles and the role of adjunct techniques for airway administration. Mol Ther. 2002;6(2):148–52. doi: 10.1006/mthe.2002.0662. [DOI] [PubMed] [Google Scholar]
- 25.Somiari S, Glasspool-Malone J, Drabick JJ, Gilbert RA, Heller R, Jaroszeski MJ, et al. Theory and in vivo application of electroporative gene delivery. Mol Ther. 2000;2(3):178–87. doi: 10.1006/mthe.2000.0124. [DOI] [PubMed] [Google Scholar]
- 26.Mir LM. Electroporation-based gene therapy: recent evolution in the mechanism description and technology developments. Methods in molecular biology. 2014;1121:3–23. doi: 10.1007/978-1-4614-9632-8_1. [DOI] [PubMed] [Google Scholar]
- 27.Dean DA, Machado-Aranda D, Blair-Parks K, Yeldandi AV, Young JL. Electroporation as a method for high-level nonviral gene transfer to the lung. Gene Ther. 2003;10(18):1608–15. doi: 10.1038/sj.gt.3302053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mutlu GM, Machado-Aranda D, Norton JE, Bellmeyer A, Urich D, Zhou R, et al. Electroporation-mediated gene transfer of the Na+,K+-ATPase rescues endotoxin-induced lung injury. Am J Respir Crit Care Med. 2007;176(6):582–90. doi: 10.1164/rccm.200608-1246OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machado-Aranda DA, Suresh MV, Yu B, Raghavendran K. Electroporation-mediated in vivo gene delivery of the Na+/K+-ATPase pump reduced lung injury in a mouse model of lung contusion. J Trauma Acute Care Surg. 2012;72(1):32–9. doi: 10.1097/TA.0b013e31823f0606. discussion 9–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. The New England journal of medicine. 2015;372(8):747–55. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 32.Raghavendran K, Notter RH, Davidson BA, Helinski JD, Kunkel SL, Knight PR. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32(2):122–30. doi: 10.1097/SHK.0b013e31819c385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zisman DA, Kunkel SL, Strieter RM, Tsai WC, Bucknell K, Wilkowski J, et al. MCP-1 protects mice in lethal endotoxemia. J Clin Invest. 1997;99(12):2832–6. doi: 10.1172/JCI119475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raghavendran K, Davidson BA, Mullan BA, Hutson AD, Russo TA, Manderscheid PA, et al. Acid and particulate-induced aspiration lung injury in mice: importance of MCP-1. Am J Physiol Lung Cell Mol Physiol. 2005;289(1):L134–43. doi: 10.1152/ajplung.00390.2004. [DOI] [PubMed] [Google Scholar]
- 35.Suresh MV, Yu B, Machado-Aranda D, Bender MD, Ochoa-Frongia L, Helinski JD, et al. Role of Macrophage Chemoattractant Protein 1 in Acute Inflammation Following Lung Contusion. Am J Respir Cell Mol Biol. 2012 doi: 10.1165/rcmb.2011-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann E, Schaefer-Ridder M, Wang Y, Hofschneider PH. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–5. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emr BM, Roy S, Kollisch-Singule M, Gatto LA, Barravecchia M, Lin X, et al. Electroporation-mediated gene delivery of Na+,K+-ATPase, and ENaC subunits to the lung attenuates acute respiratory distress syndrome in a two-hit porcine model. Shock. 2015;43(1):16–23. doi: 10.1097/SHK.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean DA, Ramanathan T, Machado D, Sundararajan R. Electrical Impedance Spectroscopy Study of Biological Tissues. Journal of electrostatics. 2008;66(3–4):165–77. doi: 10.1016/j.elstat.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calautti E, Cabodi S, Stein PL, Hatzfeld M, Kedersha N, Paolo Dotto G. Tyrosine phosphorylation and src family kinases control keratinocyte cell-cell adhesion. The Journal of cell biology. 1998;141(6):1449–65. doi: 10.1083/jcb.141.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim L, Wong TW. Growth factor-dependent phosphorylation of the actin-binding protein cortactin is mediated by the cytoplasmic tyrosine kinase FER. The Journal of biological chemistry. 1998;273(36):23542–8. doi: 10.1074/jbc.273.36.23542. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Ogata Y, Feldman RA. Fes tyrosine kinase promotes survival and terminal granulocyte differentiation of factor-dependent myeloid progenitors (32D) and activates lineage-specific transcription factors. The Journal of biological chemistry. 2003;278(17):14978–84. doi: 10.1074/jbc.M212118200. [DOI] [PubMed] [Google Scholar]
- 42.Qi W, Ebbert KV, Craig AW, Greer PA, McCafferty DM. Absence of Fer protein tyrosine kinase exacerbates endotoxin induced intestinal epithelial barrier dysfunction in vivo. Gut. 2005;54(8):1091–7. doi: 10.1136/gut.2004.061887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh MA, Choi S, Lee MJ, Choi MC, Lee SA, Ko W, et al. Specific tyrosine phosphorylation of focal adhesion kinase mediated by Fer tyrosine kinase in suspended hepatocytes. Biochimica et biophysica acta. 2009;1793(5):781–91. doi: 10.1016/j.bbamcr.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Putzke AP, Rothman JH. Repression of Wnt signaling by a Fer-type nonreceptor tyrosine kinase. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(37):16154–9. doi: 10.1073/pnas.1006600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khajah M, Andonegui G, Chan R, Craig AW, Greer PA, McCafferty DM. Fer kinase limits neutrophil chemotaxis toward end target chemoattractants. Journal of immunology. 2013;190(5):2208–16. doi: 10.4049/jimmunol.1200322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Craig AW, Greer PA. Fer kinase is required for sustained p38 kinase activation and maximal chemotaxis of activated mast cells. Molecular and cellular biology. 2002;22(18):6363–74. doi: 10.1128/MCB.22.18.6363-6374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu G, Craig AW, Greer P, Miller M, Anastasiadis PZ, Lilien J, et al. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. Journal of cell science. 2004;117(Pt 15):3207–19. doi: 10.1242/jcs.01174. [DOI] [PubMed] [Google Scholar]
- 48.McCafferty DM, Craig AW, Senis YA, Greer PA. Absence of Fer protein-tyrosine kinase exacerbates leukocyte recruitment in response to endotoxin. Journal of immunology. 2002;168(10):4930–5. doi: 10.4049/jimmunol.168.10.4930. [DOI] [PubMed] [Google Scholar]
- 49.Zirngibl RA, Senis Y, Greer PA. Enhanced endotoxin sensitivity in fps/fes-null mice with minimal defects in hematopoietic homeostasis. Molecular and cellular biology. 2002;22(8):2472–86. doi: 10.1128/MCB.22.8.2472-2486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vacik J, Dean BS, Zimmer WE, Dean DA. Cell-specific nuclear import of plasmid DNA. Gene Ther. 1999;6(6):1006–14. doi: 10.1038/sj.gt.3300924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young JL, Dean DA. Electroporation-mediated gene delivery. Advances in genetics. 2015;89:49–88. doi: 10.1016/bs.adgen.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoth JJ, Hudson WP, Brownlee NA, Yoza BK, Hiltbold EM, Meredith JW, et al. Toll-like receptor 2 participates in the response to lung injury in a murine model of pulmonary contusion. Shock. 2007;28(4):447–52. doi: 10.1097/shk.0b013e318048801a. [DOI] [PubMed] [Google Scholar]
- 53.Suresh MV, Yu B, Machado-Aranda D, Bender MD, Ochoa-Frongia L, Helinski JD, et al. Role of macrophage chemoattractant protein-1 in acute inflammation after lung contusion. Am J Respir Cell Mol Biol. 2012;46(6):797–806. doi: 10.1165/rcmb.2011-0358OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao QL, Heisterkamp N, Groffen J. Isolation and sequence analysis of a novel human tyrosine kinase gene. Molecular and cellular biology. 1989;9(4):1587–93. doi: 10.1128/mcb.9.4.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Taddonio MA, Dolgachev V, Bosmann M, Ward PA, Su G, Wang SC, et al. Influence of lipopolysaccharide-binding protein on pulmonary inflammation in gram-negative pneumonia. Shock. 2015;43(6):612–9. doi: 10.1097/SHK.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dolgachev VA, Yu B, Sun L, Shanley TP, Raghavendran K, Hemmila MR. Interleukin 10 overexpression alters survival in the setting of gram-negative pneumonia following lung contusion. Shock. 2014;41(4):301–10. doi: 10.1097/SHK.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dolgachev V, Thomas M, Berlin A, Lukacs NW. Stem cell factor-mediated activation pathways promote murine eosinophil CCL6 production and survival. J Leukoc Biol. 2007;81(4):1111–9. doi: 10.1189/jlb.0906595. [DOI] [PubMed] [Google Scholar]
- 58.Dolgachev V, Farooqui MS, Kulaeva OI, Tainsky MA, Nagy B, Hanada K, et al. De novo ceramide accumulation due to inhibition of its conversion to complex sphingolipids in apoptotic photosensitized cells. J Biol Chem. 2004;279(22):23238–49. doi: 10.1074/jbc.M311974200. [DOI] [PubMed] [Google Scholar]
- 59.Ding L, Dolgachev V, Wu Z, Liu T, Nakashima T, Wu Z, et al. Essential role of stem cell factor-c-Kit signalling pathway in bleomycin-induced pulmonary fibrosis. The Journal of pathology. 2013;230(2):205–14. doi: 10.1002/path.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]