Abstract
Thrombospondin-4 (TSP4) is a synaptogenic molecule that is upregulated in the spinal cord after painful facet joint injury and may contribute to spinal hyperexcitability. However, the mechanisms leading to increased spinal TSP4 are unclear. Because primary afferent activity is critical in the development of spinal hyperexcitability after facet joint injury, this study evaluated the role of afferent firing in the increase of spinal TSP4 and excitatory synapses. Intra-articular bupivacaine was administered immediately or 4 days after painful facet joint injury in male Holtzman rats, and TSP4 and excitatory synapses were quantified in the spinal cord at day 7. Immediate, but not delayed bupivacaine treatment, prevents the injury-induced increase in TSP4 and excitatory synapses in the dorsal horn (p<0.0001). Preliminary in vitro experiments suggest that the excitatory signaling molecules ATP and glutamate may stimulate astrocytic TSP4 expression (p≤0.04). Collectively, these results suggest that afferent activity early after facet joint injury is critical for the induction of spinal TSP4. This study advances the understanding of the timing and role of afferent activity in TSP4 expression after injury, which is critical for the therapeutic targeting of TSP4 to treat persistent pain conditions.
Key Terms: thrombospondin-4, astrocyte, bupivacaine, facet joint, pain
Introduction
Chronic pain affects at least one in three adults in the United States, and has an estimated annual cost of over $635 billion.33 Over 40% of the general population reports pain symptoms lasting longer than 3 years.4 Pain has a growing economic burden, in part, because of the inability of current therapeutic strategies to effectively treat the symptoms that develop in the long term, especially after injuries that produce sensitization of nociceptive processing in the central nervous system (CNS). In order to engineer more effective treatments for chronic pain, the neurophysiological processes that lead to dysfunction in pain processing in the CNS after injury must be defined and understood.
Structural plasticity, including neurite outgrowth and synaptogenesis, is one mechanism by which pain can be maintained through the rewiring of both nociceptive and non-nociceptive pathways in the spinal cord.17,24,40 Synapse growth can be stimulated by synaptogenic factors, including thrombospondins, which are a family of extracellular matrix proteins that are secreted by astrocytes in the CNS and are necessary for synapse development.6,11,13,32 One member of the thrombospondin family, thrombospondin-4 (TSP4), is increased in astrocytes in the spinal cord after painful mechanical joint injury that induces persistent spinal sensitization,7 and after nerve injuries that are associated with neuropathic pain.21,27,43 However, it is unknown which signals induce spinal TSP4 expression after injury. Thrombospondin-1 (TSP1) is the most-studied member of the thrombospondin family,1 and several factors have been identified that can modulate expression of TSP1. For example, activation of purinergic P2Y receptors on astrocytes by ATP, which functions as an excitatory signaling molecule in the nociceptive pathways in the spinal cord,5 increases astrocytic TSP1 expression.37 However, the potential role of excitatory signaling in modulating astrocytic TSP4 expression in the spinal cord is unknown.
In this study we used complementary in vivo and in vitro approaches to elucidate the neural signals inducing TSP4 expression after painful injury. First, we used an established rat model of painful cervical facet joint injury to evaluate the role of excitatory signaling in the induction of spinal astrocytic TSP4 after injury. The facet joints are injured during neck trauma, and their excessive loading can result in persistent pain.3,25,28 Painful facet joint loading in the rat increases activity in the primary afferents that innervate the joint28 in association with widespread changes in the spinal cord that enhance neuronal excitability,8 including increases in astrocytic TSP4 expression and excitatory synapse density.7 Transiently blocking afferent activity immediately after joint injury prevents the development of pain, but blocking afferent firing 4 days after injury does not attenuate pain.8 Based on those results, we hypothesized that excitatory signaling in the first hours after facet injury induces spinal astrocytic TSP4 expression and subsequent increases in excitatory synapse density. To test that hypothesis, TSP4 expression and excitatory synapses were quantified in the spinal cord after painful facet joint injury in rats receiving intra-articular bupivacaine to block excitatory afferent activity either immediately or 4 days after injury.
The results of our first study suggested that immediate injury-induced afferent activity increases spinal TSP4 expression. Glutamate and ATP, two key spinal excitatory signaling molecules, are elevated in dorsal root ganglia, spinal cord, and pain-processing regions of the brain in response to nociceptive stimulation and chronic neuropathic pain.9,29,31,36,39 Because of those pain-related increases in excitatory signaling molecules and the ability of ATP to induce TSP1 expression in astrocytes in vitro,37 we conducted a set of preliminary experiments by stimulating cultured astrocytes with glutamate or ATP to determine whether astrocytic TSP4 increases in direct response to either or both of those excitatory signals.
Materials and Methods
This study evaluated the potential roles of injury-induced afferent activity and excitatory signaling in the upregulation of spinal astrocytic TSP4. In the in vivo experiment, bupivacaine was used to transiently block joint afferent activity after painful facet joint injury. Rats received bilateral intra-articular injections of bupivacaine either immediately after painful facet joint loading (Inj-BP0h, n=6) or delayed until day 4 after painful joint loading (Inj-BPd4, n=6). Separate groups of rats received control injections of the saline vehicle at the same times after sham surgery (Sham-VEH0h, n=6; Sham-VEHd4, n=6) or after painful joint loading (Inj-VEH0h, n=6; Inj-VEHd4, n=5). Behavioral sensitivity was evaluated through day 7, at which time TSP4 expression and excitatory synapse density were quantified in the spinal dorsal horn. In a second in vitro experiment, purified astrocyte cultures were stimulated with ATP or glutamate, two signaling molecules that contribute to excitation of spinal neurons by primary sensory afferents.14,35 Expression of TSP4 and a marker of astrocytic activation, glial fibrillary acidic protein (GFAP), were quantified by immunolabeling after astrocytes were stimulated with ATP or glutamate to determine the effects of excitatory signaling on TSP4 expression and astrocytic activation.
Facet joint distraction and intra-articular bupivacaine treatment
All experimental procedures were approved by the University of Pennsylvania Institutional Animal Care and Use Committee and carried out under the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. All surgical procedures were performed using adult male Holtzman rats (362–464g) under inhalation isoflurane anesthesia (4% induction, 2–3% maintenance). Facet joint loading was performed by distracting the bilateral C6/C7 facet joints, which has been described previously.25,26 The C6 and C7 vertebrae were attached to microforceps on a custom loading device and the C6 vertebra was distracted 0.7mm rostrally to stretch the bilateral facet capsules across the C6/C7 joints. Bead markers were placed on the C6 and C7 vertebrae and on the surface of the right C6/C7 facet capsule to quantify the vertebral and capsule displacements, as well as the maximum principal strain (MPS) on the facet capsule surface. Sham surgeries included all of the same procedures with no joint distraction applied in order to control for the effect of the surgery.
Intra-articular injections of 10μL 0.5% bupivacaine or 0.9% saline solution were injected into the left and right facet joints using a microsyringe with a 33G beveled needle (Hamilton; Reno, NV).8 After injection, the microsyringe was held in the joint for at least 30 seconds in order to prevent fluid leakage from the joint space. Treatments given immediately after injury were administered following facet joint loading, prior to closing of the surgical incisions. For the intra-articular injections made on day 4, rats were anesthetized with isoflurane (4% induction, 2–3% maintenance), the paraspinal musculature was separated to re-expose the bilateral C6/C7 facet joints and injections were performed as described above. All incisions were closed using 3-0 polyester suture and surgical staples, and rats were monitored during recovery in room air.
Assessment of mechanical hyperalgesia
Mechanical hyperalgesia was assessed pre-operatively (at baseline) and on days 1 and 7 after facet joint distraction by quantifying the paw withdrawal threshold (PWT) to mechanical stimulation of the forepaws of each rat. A series of von Frey filaments (1.4, 2, 4, 6, 8, 10, 15, and 26g) was applied to the plantar surface of the forepaws.25 If a rat responded to two consecutive filament weights by withdrawing, licking, or shaking the forepaw, the lower of those filament weights was recorded as the PWT, with a maximum threshold of 26g. Testing was repeated in three rounds and the average of all rounds was calculated for each rat, by averaging the left and right paw withdrawal thresholds (mean±SD).22 PWT was compared over time and between groups by repeated-measures ANOVA with a post-hoc Tukey’s HSD test.
Immunolabeling of TSP4 and GFAP in spinal cord tissue
To quantify the expression of astrocytic TSP4 on day 7 after facet joint injury, spinal cord sections were immunolabeled for TSP4 and GFAP. Rats were anesthetized with sodium pentobarbital (65mg/kg, i.p.) and transcardially perfused with 250mL of chilled PBS followed by 250mL of 4% paraformaldehyde (PFA). The C6/C7 spinal cord was removed and post-fixed in 4% PFA overnight, then cryopreserved in 30% sucrose in PBS for seven days at 4°C. Samples were freeze-mounted in OCT medium (Fisher Scientific; Waltham, MA) and axial cryosections (14μm each, 5–6 sections per rat) were mounted on Superfrost Plus slides (Fisher Scientific; Waltham, MA).
Sections were blocked in 10% goat serum in PBS for 1h at room temperature. Sections were then incubated overnight at 4°C with chicken anti-TSP4 (1:1000; from F. Zaucke; Cologne, Germany) and rabbit anti-GFAP (1:500; Dako, Denmark) in 10% goat serum with 0.3% Triton-X PBS. Sections were incubated for 2h at room temperature with goat anti-chicken Alexa 488 and goat anti-rabbit Alexa 568 fluorescent secondary antibodies (1:1000, Invitrogen; Carlsbad, CA), and then incubated with DAPI (1:10,000, Invitrogen; Carlsbad, CA) for 10min and cover-slipped with Fluorogel mounting medium (EMS; Hatfield, PA). The superficial dorsal horn of each spinal tissue section was imaged using a Zeiss LSM510 confocal microscope. For each pixel in an image, the intensity of GFAP labeling was assessed using a custom MATLAB code.10 If the pixel was determined to be positively labeled for GFAP (relative to a threshold that was set using control tissue samples), then the intensity of TSP4 labeling was quantified. TSP4 intensity was averaged across all GFAP-positive pixels in each image.
Synapse quantification in the superficial dorsal horn
Spinal cord sections were immunolabeled with mouse anti-synapsin (1:100; Synaptic Systems; Goettingen, Germany) and rabbit anti-homer (1:200; Synaptic Systems; Goettingen, Germany) to quantify synapse densities in the dorsal horn at day 7 after facet joint injury. Excitatory synapses were quantified by counting puncta with co-localized synapsin and homer labeling, using previously published methods.7,16 Briefly, image stacks were acquired from the dorsal horn at 0.33μm increments up to 3μm of depth using a Zeiss LSM510 confocal microscope. The maximum intensity projection of each set of three sequential images was used to generate a single image (0.02mm2 tissue area) corresponding to 1μm of tissue depth. The Puncta Analyzer plugin for ImageJ (National Institutes of Health; Bethesda, MD) was used to identify puncta exhibiting co-localization of synapsin and homer in each maximum intensity projection. The area of the tissue parenchyma, excluding any holes and/or gaps in the tissue sections, was quantified using a custom MATLAB code.7 The number of co-localized puncta in the dorsal horn was normalized to the corresponding tissue area for each image. The excitatory synapse number per area was then averaged across all rats in each group and compared between groups using Student’s t-test.
Quantification of astrocytic TSP4 in vitro
Purified rat astrocyte cultures were used to evaluate changes in TSP4 expression induced by the excitatory signaling molecules ATP and glutamate. Cortical cultures were isolated from E18 rat pup brains by dissecting the cortices at 37°C in Neurobasal media (Invitrogen Corp., Carlsbad, CA) with trypsin (0.3 mg/ml; Sigma-Aldrich, St. Louis, MO) and DNase I (0.2mg/ml; Amersham Biosciences, Piscataway, NJ).30 Soybean trypsin inhibitor (0.5mg/ml; Gibco, Grand Island, NY) was added after 20min and the tissue was mechanically dissociated. Cell solutions were centrifuged at 1000rpm for 5min and the remaining pellet was resuspended in DMEM with Glutamax and FBS (Gibco, Grand Island, NY). Cell solutions were filtered sequentially through 60μm and 28μm Nitex meshes and plated on T75 tissue culture flasks precoated with poly-L-lysine (Sigma Aldrich, St. Louis, MO). Cultures were maintained at 37°C and 5% CO2 and the media was replaced every 3–4 days. To acquire pure astrocyte cultures, the flasks were placed on an orbital shaker overnight at 250rpm to dislodge the neuronal layer. Flasks were rinsed and the remaining adherent cells were collected by adding 4ml of trypsin/EDTA (0.25%, Invitrogen) for 2–3min at 37°C and mechanically dislodging the cells. The cells were centrifuged for 5min at 1000g and were resuspended in DMEM with 5% FBS before re-plating on poly-L-lysine-treated T75 flasks. Media was changed at 24h and then every 3–4 days.
Two weeks after purification, astrocytes were split onto 35mm glass-bottom wells at a density of 1×105 cells/mL. Two days before stimulating the cells, the media was changed to DMEM with 0.5% FBS. Cells were then stimulated for 12h with DMEM media containing 0.5% FBS and either ATP (0, 100, or 500μM; 3 wells each dose) or glutamate (0, 10, 100, 1000μM; 3 wells each dose). After the stimulation period, each well was rinsed with PBS and fixed for 15min in 4% paraformaldehyde. Cells were blocked with 10% goat serum in PBS, then incubated overnight at 4°C with chicken anti-TSP4 (1:1000; from F. Zaucke; Cologne, Germany) and rabbit anti-GFAP (1:500; Dako, Denmark) in 10% goat serum with 0.3% Triton-X PBS. Wells were incubated for 2h at room temperature with goat anti-chicken Alexa 488 and goat anti-rabbit Alexa 568 fluorescent secondary antibodies (1:1000, Invitrogen; Carlsbad, CA), and then incubated with DAPI (1:10,000, Invitrogen; Carlsbad, CA) for 10min. Glass coverslips were removed from the culture dishes and mounted on slides using Fluorogel mounting medium (EMS; Hatfield, PA). Five images were collected from each slide using a Zeiss LSM510 confocal microscope with a 40X water-immersion lens. GFAP-positive cells were traced using ImageJ, and the cell area and mean green (TSP4) and red (GFAP) pixel intensities were recorded for each cell profile (2–5 cells profiles per image). Cell areas and mean TSP4 and GFAP intensities were compared between treatment groups using a one-way ANOVA with post-hoc Tukey’s HSD testing.
Results
Early intra-articular bupivacaine prevents spinal astrocytic TSP4 expression & excitatory synaptogenesis
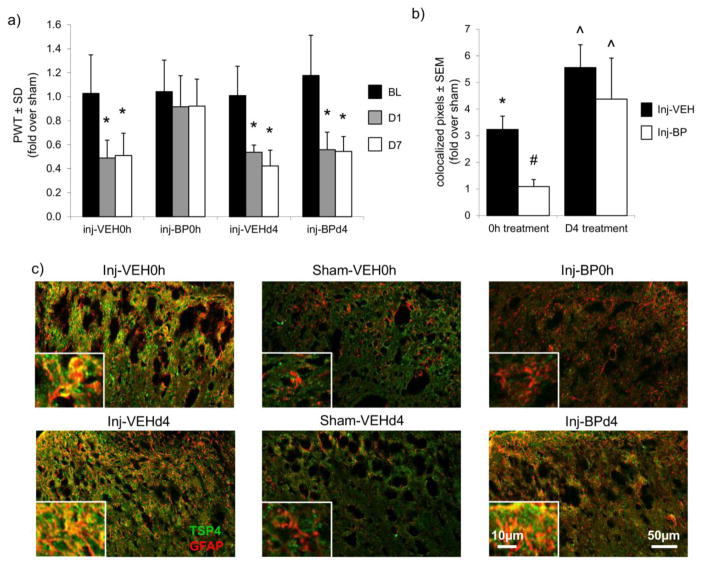
The imposed injury severities are the same across all groups. In fact, the vertebral distraction magnitude, average capsule distraction magnitude, and average MPS on the facet capsule are not different in any of the groups receiving facet joint distractions (Table 1). Facet capsule stretch induces a significant decrease from the baseline PWT at both day 1 and day 7 after injury with an immediate intra-articular injection of the saline control (Inj-VEH0h, p<0.004) (Figure 1A). Immediate treatment with bupivacaine attenuates that decrease in PWT, and maintains PWT in the Inj-BP0h group at baseline and sham levels at days 1 and 7 after injury (Figure 1A). The PWT remains decreased from baseline and sham levels at days 1 and 7 after injury when saline vehicle (Inj-VEHd4, p<0.021) or bupivacaine (Inj-BPd4, p<0.0001) injections are given at day 4 after injury (Figure 1A).
Table 1.
Facet joint distraction mechanics.
| vertebral distraction (mm) | capsule distraction (mm) | maximum principal strain (%) | |
|---|---|---|---|
| inj-VEH0h | 0.75±0.19 | 0.35±0.08 | 23.9±9.6 |
| inj-BP0h | 0.79±0.19 | 0.30±0.06 | 27.5±8.0 |
| inj-VEHd4 | 0.70±0.07 | 0.36±0.07 | 31.2±8.8 |
| inj-BP4h | 0.71±0.17 | 0.35±0.23 | 31±20 |
All data are shown as mean ± SD.
Figure 1.
Immediate intra-articular bupivacaine prevents behavioral sensitivity and spinal astrocytic TSP4 upregulation. (a) Paw withdrawal thresholds (PWT) are reduced on days 1 and 7 after facet joint injury (*p≤0.021), but immediate intra-articular bupivacaine treatment prevents the decreases in PWT. PWTs for the 0h and day 4 (d4) treatment groups are normalized to their respective sham groups. (b) Quantification of co-localized TSP4 (green) and GFAP (red) in the superficial dorsal horn, as shown in (c) representative images with enlarged insets depicting the co-localization of GFAP and TSP4. Increases in astrocytic TSP4 after painful joint loading (*p<0.0001 vs. Sham-VEH0h) are prevented by immediate treatment with bupivacaine (#p<0.0001 vs. Inj-VEH0h). However, increases in astrocytic TSP4 after painful joint loading (^p≤0.048 vs. Sham-VEHd4) are not blocked when bupivacaine is given at day 4. Data for the 0h and d4 treatment groups are normalized to their respective sham groups.
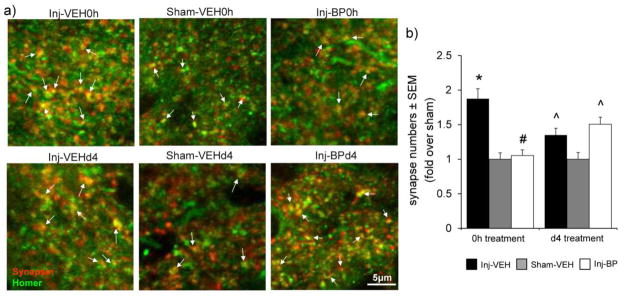
Astrocytic TSP4 expression increases in the superficial dorsal horn at day 7 after facet capsule stretch with immediate saline vehicle treatment (Inj-VEH0h) over sham levels (Sham-VEH0h) (p<0.0001) (Figure 1). However, immediate bupivacaine treatment (Inj-BP0h) attenuates that increase in astrocytic TSP4 in the superficial dorsal horn (p<0.0001) (Figure 1). In contrast, although astrocytic TSP4 increases in the dorsal horn after painful joint loading with vehicle treatment given at day 4 (Inj-VEHd4) (p≤0.0061); bupivacaine administered at day 4 (Inj-BPd4) does not attenuate the increase in spinal TSP4 expression (p≤0.048) (Figure 1). Immediate bupivacaine treatment also similarly prevents the injury-induced increase in excitatory synapses in the superficial dorsal horn at day 7 after facet capsule stretch (p<0.0001), but synapse numbers increase despite the administration of bupivacaine at day 4 (p≤0.043) (Figure 2).
Figure 2.
Immediate intra-articular bupivacaine prevents excitatory synaptogenesis. (a) Representative immunolabeling of co-localization of synapsin (red) and homer (green) identifies synaptic puncta (yellow, arrows). (b) Counting synapses in the superficial dorsal horn after sham or painful facet joint injury followed by immediate (Inj-VEH0h, Sham-VEH0h) or day 4 vehicle (Inj-VEHd4, Sham-VEHd4) or bupivacaine (Inj-BP0h, Inj-BPd4) injections shows that increases in excitatory synapses after painful joint loading (*p<0.0001 vs. Sham-VEH0h) are prevented by bupivacaine given immediately (#p<0.0001 vs. Inj-VEH0h) but not at day 4 (^p≤0.043 vs. Sham-VEHd4).
ATP and glutamate induce astrocytic TSP4 expression
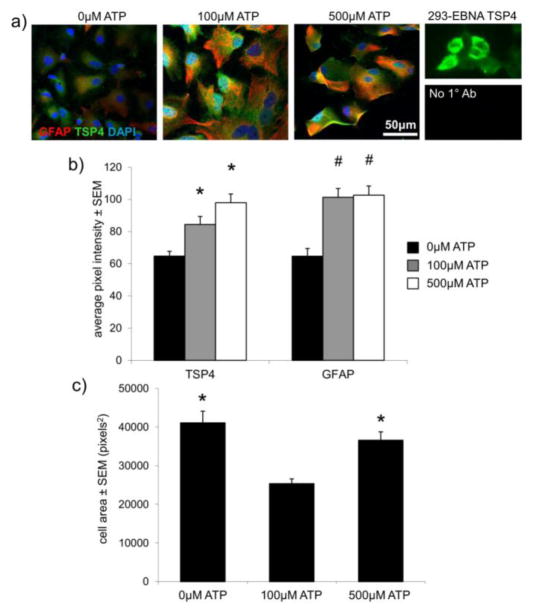
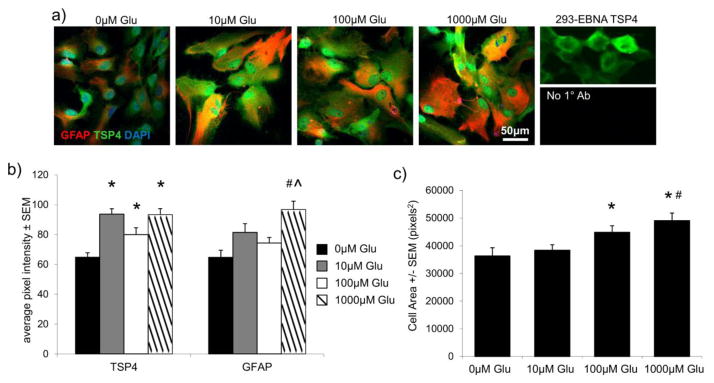
TSP4 and GFAP immunolabeling were quantified in 56±1 astrocytes in each group after ATP treatment, and 55±8 astrocytes per group after glutamate treatment. GFAP-positive astrocytes exhibit greater average TSP4 intensity (p≤0.009) and average GFAP intensity (p<0.001) following treatment with either 100μM or 500μM ATP, compared to astrocytes receiving no ATP stimulation (Figure 3). The mean area of the cells is unchanged after 500μM ATP treatment from the area of cells in the control group, but the area of the cells after 100μM ATP treatment is lower than both the control group and the 500μM-treated group (p≤0.0006) (Figure 3). Similarly, the average intensity of TSP4-labeled pixels increases after all three of the glutamate doses – 10μM, 100μM, or 1000μM glutamate – relative to untreated astrocytes (p≤0.04) (Figure 4). However, the intensity of GFAP-labeled pixels increases over the intensity in untreated cells only after 1000μM glutamate treatment (p<0.0001) (Figure 4). The mean area of the astrocytes is also greater after 1000μM glutamate treatment, relative to the control group (p<0.0001) and the 10μM-treated cells (p=0.001) (Figure 4) and the mean area after 100μM glutamate treatment is greater than the area of the cells in the control group (p=0.033) (Figure 4).
Figure 3.
ATP stimulates expression of TSP and GFAP in astrocytes. (a) Purified cultured astrocytes co-labeled for TSP4 (green), GFAP (red) and DAPI (blue) in the presence of 0μM, 100μM, or 500μM ATP for 12 hours. TSP4 labeling of TSP4-transfected 293-EBNA cells and astrocytes labeled with secondary, but no primary antibodies (No 1° Ab), are shown as positive and negative labeling controls, respectively. (b) Both 100μM and 500μM ATP induce greater TSP4 (*p≤0.009) and GFAP (#p<0.0001) expression than 0μM ATP, as measured by average pixel intensity in GFAP-positive astrocytes. (c) Mean cell profile areas are greater after both 0μM and 500μM ATP treatment relative to 100μM ATP (*p≤0.0006).
Figure 4.
Glutamate stimulates astrocytic expression of TSP4 and GFAP. (a) Purified cultured astrocytes co-labeled for TSP4 (green), GFAP (red) and DAPI (blue) after exposure to glutamate in 0μM, 10μM, 100μM, or 1000μM doses for 12 hours. TSP4 labeling of TSP4-transfected 293-EBNA cells and astrocytes labeled with secondary, but no primary antibodies (No 1° Ab), are shown as positive and negative labeling controls, respectively. (b) All glutamate concentrations increase the average TSP4 pixel intensity (*p≤0.04), but only 1000μM glutamate stimulation increases the average GFAP pixel intensity (#p<0.0001 vs. 0μM, ^p=0.023 vs. 100μM). (c) Mean cell profile areas are greater after 100μM or 1000μM glutamate treatment relative to 0μM glutamate (*p≤0.033), and greater after 1000μM glutamate relative to 10μM glutamate (#p=0.001).
Discussion
Blocking afferent activity from the facet joint immediately after painful joint loading prevents the injury-induced upregulation of astrocytic TSP4 and increase in excitatory synapses in the spinal dorsal horn (Figures 1 & 2). However, blocking activity from the facet joint after the development of behavioral sensitivity does not affect the injury-induced increases in TSP4 or excitatory synapses (Figures 1 & 2). These findings strongly support that excitatory afferent activity early after painful facet joint loading induces the upregulation of spinal TSP4 and excitatory synaptogenesis.
Painful facet joint injury induces a host of changes in the spinal cord that contribute collectively to spinal hyperexcitability and behavioral sensitivity. TSP4 expression and excitatory synaptogenesis can be induced by excessive mechanical loading of the joint, but both are blocked by the transient pharmacological inhibition of joint afferent activity (Figures 1 & 2). The absence of behavioral sensitivity when TSP4 expression and synapse numbers are not increased (i.e., after immediate bupivacaine treatment) is also consistent with the previous observation that increased TSP4 levels are sufficient to induce mechanical allodynia, but specifically blocking TSP4 expression after facet joint injury can prevent allodynia.7 These results also suggest that synapse number may correlate with allodynia, a potential relationship that bears further investigation. In addition to TSP4, painful facet joint injury modulates levels of many other spinal proteins, including the metabotropic glutamate receptor mGluR5 and phosphorylation of the NMDA-NR1 subunit (pNR1) and ERK1/2 (pERK),7 all of which are indicative of the central sensitization that develops in the first 7 days after injury.20,38 However much like TSP4, the increases in mGluR5, pNR1, and pERK are attenuated by immediate bupivacaine treatment after facet joint loading,8 suggesting that TSP4 and excitatory synaptogenesis are part of a more complicated cascade that is initiated by increased afferent activity after excessive loading of the facet joint.
This study supports the notion that early intervention is critical to prevent persistent pain and the spinal modifications that promote neuronal hyperexcitability following nerve injury34,41 or indirect nerve injury such as that experienced during joint loading.8,19 Bupivacaine administered 4 days after joint injury fails to attenuate the increases in TSP4 and excitatory synapses at day 7 (Figures 1 & 2), supporting that continuous afferent input from the injured facet is not required to maintain the hyperexcitable state in the spinal cord. These findings are consistent with the prior observation that delayed bupivacaine treatment also does not attenuate injury-induced increases in other excitatory signaling proteins in the spinal cord, including mGluR5 and pNR1.8
TSP4 represents a potential therapeutic target for persistent neuropathic pain,21 but the current study suggests that the timing of such treatments may be a key factor in their success. For example, intrathecal oligonucleotides that block translation of TSP4 protein transiently reduce behavioral sensitivity for only 3–4 days when they are delivered after sensitivity has already developed following spinal nerve ligation.21 When the same oligonucleotides are delivered intrathecally beginning 3 days before painful facet joint injury, no behavioral sensitivity develops for at least 7 days,7 which was the duration of the post-injury observation period. Because of the pre-treatment protocol in that study, anti-TSP4 oligonucleotides were likely present in the spinal cord at the time of injury, possibly preventing the increase in expression of TSP4 that would otherwise be induced by the injury event. Although the current study focuses on bupivacaine treatment to block afferent activity rather than the temporal aspects of specific anti-TSP4 therapeutics for neuropathic pain, its findings and those of both Kim et al.,21 and Crosby et al.7,8 together suggest that, much like intra-articular bupivacaine, specifically targeting spinal TSP4 is likely to be more effective before sustained pain develops.
Stimulation with either ATP or glutamate, two excitatory neurotransmitters that are involved in excitatory CNS signaling originating from primary afferent fibers that innervate the facet joint, induces an increase in TSP4 in cultured astrocytes (Figures 3 & 4), suggesting that both excitatory signaling molecules can separately stimulate TSP4 expression. However, the observations in our study are complicated by the fact that ATP can lead to glutamate release from astrocytes,18 so TSP4 upregulation could be an indirect effect of ATP treatment through self-induced glutamate release. Similarly, glutamate signaling can cause astrocytes to release ATP,2 so the increase in TSP4 evident after glutamate treatment may be due to indirect ATP signaling. Clearly, although excitatory signaling molecules seem to be important in modulating TSP4 after joint injury, the complicated relationships between ATP and glutamate signaling require determination of the exact mechanisms of astrocytic activation and TSP4 upregulation.
These in vitro experiments suggest a role for ATP and/or glutamate in TSP4 upregulation after painful facet joint injury, but several limitations restrict the scope of the findings. Of note, although each of the five TSP isoforms have synaptogenic properties,11 only TSP4 was investigated in the pilot in vitro study here. TSP4 has been reported to increase after peripheral nerve injury while TSP1 and TSP2 levels are unchanged,21 but the potential overlap of the mechanisms that induce astrocytic expression of each TSP isoform remains unclear, especially since TSP1 is also induced in astrocytes via a purinergic pathway.37 The in vitro experiments in this study were also limited in using embryonic rat cortical astrocytes. Primary astrocyte cultures derived from embryonic rat or mouse cortices are common, including in studies evaluating TSP expression.15,37,42 However, astrocytes from fully-developed, adult spinal cords would better control for the temporal changes in TSP production from development to adulthood32 and regional heterogeneity in astrocyte populations.12 Moreover, additional studies using human cell lines would provide comparative data regarding any species-dependent findings.
Neither of the markers of astrocyte activation used in this study (GFAP immunolabeling and cell area), did not increase consistently with larger concentrations of ATP or glutamate (Figures 3 & 4). Astrocyte cultures are subjected to a number of other conditions that can activate the cells even before stimulation, in spite of attempts to mitigate those factors. Serum components in cell culture medium can stress astrocytes,23 and despite reducing serum levels to 0.5% for 48 hours in our study to return astrocytes to the quiescent phase prior to stimulation,37 some baseline level of astrocyte activation is expected. The lack of a consistent response of astrocyte activation to stimulation is indicative of the limitations associated with studying astrocytes in vitro. Furthermore, TSP4 is a secreted extracellular matrix protein,1 so evaluating intracellular astrocytic TSP4 quantifies only a portion of the potential total TSP4. Total TSP4 levels in vivo or in vitro found using Western blot or ELISA would provide more information about TSP4 regulation after injury. Ultimately, although TSP4 and GFAP expression are correlated in vivo,21 the relationships between TSP4 expression and astrocyte activation remain unclear; further studies are needed to resolve these issues by evaluating the induction of TSP4 expression, both overall and specifically in astrocytes either in vivo or in more regulated in vitro conditions.
By demonstrating the importance of facet joint afferent activity in the induction of TSP4 and synaptogenesis, including the potential roles of specific excitatory neurotransmitters in TSP4 upregulation, this study supports a putative mechanism for the development of spinal hyperexcitability after painful facet joint injury. Excessive loading of the facet is known to increase firing in the afferents that innervate that joint.28 Astrocytes participate in synaptic transmission at tripartite synapses,2 so as primary afferent firing reaches secondary neurons in the spinal dorsal horn, astrocytes are exposed to synaptically-released neurotransmitters. Increases in glutamate and ATP in primary afferents29 and the spinal dorsal horn9,39 in response to painful stimuli or nerve injury support that dorsal horn astrocytes are exposed to greater levels of those signaling molecules. Although further studies are required to solidify the link between excitatory signaling molecules and TSP4 expression, such stimulation by ATP and/or glutamate may increase levels of astrocytic TSP4, which is a synaptogenic molecule that increases synapse numbers in the CNS.6,13 An increase in the overall number of excitatory synapses in the dorsal horn could partially contribute to the previously observed increases in spinal proteins like mGluR5, pNR1, and pERK,8 with an overall result of increased excitability of spinal neurons. Once spinal sensitization has developed, neuronal excitation is less directly related to the intensity or frequency of peripheral inputs,24 which also is supported by the ineffectiveness of intra-articular bupivacaine administered 4 days after injury (Figures 1 & 2).
In summary, this study shows that afferent activity early after painful joint injury is critical for the induction of spinal TSP4, and the subsequent increase in excitatory synapses in the dorsal horn that may result from increased TSP4 levels. Current findings also suggest that excitatory signaling molecules could directly induce TSP4 expression in astrocytes; after injury, astrocytes may be stimulated to release TSP4 as a by-product of primary afferent signaling to the dorsal horn following excessive joint loading. These data suggest a potential mechanism for the induction of TSP4 and the timing of that mechanism, both of which are important for the continued development of TSP4 as a potential therapeutic target for persistent pain conditions.
Acknowledgments
This work was supported by grants from the National Institutes of Health (#AR056288), the Catharine Sharpe Foundation, and a fellowship from the Ashton Foundation. The authors would also like to thank Frank Zaucke for providing the anti-TSP4 antibody.
References
- 1.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell B. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Barnsley L, Lord SM, Wallis BJ, Bogduk N. The prevalence of chronic cervical zygapophysial joint pain after whiplash. Spine. 1995;20(1):20–25. doi: 10.1097/00007632-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Bouhassira D, Lanteri-Minet M, Attal N, Laurent B, Touboul C. Prevalence of chronic pain with neuropathic characteristics in the general population. Pain. 2008;136(3):380–387. doi: 10.1016/j.pain.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 6.Christopherson KS, Ullian EM, Stokes CCA, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120(3):421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Crosby ND, Zaucke F, Kras JV, Dong L, Luo ZD, Winkelstein BA. Thrombospondin-4 and excitatory synaptogenesis promote central sensitization after painful mechanical joint injury. Exp Neurol. 2015;264:111–120. doi: 10.1016/j.expneurol.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crosby ND, Gilliland TM, Winkelstein BA. Early afferent activity from the facet joint after painful trauma to its capsule potentiates neuronal excitability and glutamate signaling in the spinal cord. Pain. 2014;155(9):1878–1887. doi: 10.1016/j.pain.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui JG, O’Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73(1):87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 10.Dong L, Smith JR, Winkelstein BA. Ketorolac reduces spinal astrocytic activation and PAR1 expression associated with attenuation of pain following facet joint injury. J Neurotraum. 2013;30:818–825. doi: 10.1089/neu.2012.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers MD. Synapse formation: astrocytes spout off. Curr Biol. 2005;15(4):R134–R137. doi: 10.1016/j.cub.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Emsley JG, Macklis JD. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2006;2(3):175–186. doi: 10.1017/S1740925X06000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eroglu C, Allen NJ, Susman MW, O’Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, Green EM, Lawler J, Dolmetsch R, Garcia KC, Smith J, Luo ZD, Rosenthal A, Mosher DF, Barres BA. Gabapentin receptor a2d-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fyffe REW, Perl ER. Is ATP a central synaptic mediator for certain primary afferent fibers from mammalian skin? Proc Natl Acad Sci USA. 1984;81:6890–6893. doi: 10.1073/pnas.81.21.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikeda H, Miyatake M, Koshikawa N, Ochiai K, Yamada K, Kiss A, Donlin MJ, Panneton WM, Churchill JD, Green M, Siddiqui AM. Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem. 2010;285(49):38415–38427. doi: 10.1074/jbc.M110.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ippolito DM, Eroglu C. Quantifying synapses: an immunohistochemistry-based assay to quantify synapse number. J Vis Exp. 2010;45:2270. doi: 10.3791/2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaken RJP, Joosten EAJ, Knuwer M, Miller R, van der Meulen I, Marcus MAE, Deumens R. Synaptic plasticity in the substantia gelatinosa in a model of chronic neuropathic pain. Neurosci Lett. 2010;469(1):30–33. doi: 10.1016/j.neulet.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 18.Jeremic A, Jeftinija K, Stevanovic J, Glavaski A, Jeftinija S. ATP stimulates calcium-dependent glutamate release from cultured astrocytes. J Neurochem. 2001;77(2):664–675. doi: 10.1046/j.1471-4159.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 19.Kallakuri S, Singh A, Lu Y, Chen C, Patwardhan A, Cavanaugh JM. Tensile stretching of cervical facet joint capsule and related axonal changes. Eur Spine J. 2008;17:556–563. doi: 10.1007/s00586-007-0562-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawasaki Y, Kohno T, Zhuang ZY, Brenner GJ, Wang H, van der Meer C, Befort K, Woolf CJ, Ji RR. Ionotropic and metabotropic receptors, protein kinase A, protein kinase C, and Src contribute to C-fiber-induced ERK activation and cAMP response element-binding protein phosphorylation in dorsal horn neurons, leading to central sensitization. J Neurosci. 2004;24(38):8310–8321. doi: 10.1523/JNEUROSCI.2396-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DS, Li KW, Boroujerdi A, Yu YP, Zhou CY, Deng P, Park J, Zhang X, Lee J, Corpe M, Sharp K, Steward O, Eroglu C, Barres B, Zaucke F, Xu ZC, Lu ZD. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J Neurosci. 2012;32(26):8977–8987. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kras JV, Weisshaar CL, Quindlen JA, Winkelstein BA. Brain-derived neurotrophic factor is upregulated in the cervical DRG & spinal cord and contributes to the maintenance of pain from facet joint injury. J Neurosci Res. 2013;91:1312–1321. doi: 10.1002/jnr.23254. [DOI] [PubMed] [Google Scholar]
- 23.Lane EB, Pekny M. IF Stress Models. In: Omary MB, editor. Intermediate Filament Cytoskeleton. San Diego: Elsevier Academic Press; 2004. p. 253. [Google Scholar]
- 24.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee KE, Winkelstein BA. Joint distraction magnitude is associated with different behavioral outcomes and substance P levels for cervical facet joint loading in the rat. J Pain. 2009;10:436–445. doi: 10.1016/j.jpain.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Lee KE, Davis MB, Mejilla RM, Winkelstein BA. In vivo cervical facet capsule distraction: mechanical implications for whiplash and neck pain. Stapp Car C. 2004;48:373–395. doi: 10.4271/2004-22-0016. [DOI] [PubMed] [Google Scholar]
- 27.Li KW, Kim DS, Zaucke F, Luo ZD. Trigeminal nerve injury-induced thrombospondin-4 up-regulation contributes to orofacial neuropathic pain states in a rat model. Eur J Pain. 2014;18(4):489–495. doi: 10.1002/j.1532-2149.2013.00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu Y, Chen C, Kallakuri S, Patwardhan A, Cavanaugh JM. Neural response of cervical facet joint capsule to stretch: a study of whiplash pain mechanisms. Stapp Car C. 2005;49:49–65. doi: 10.4271/2005-22-0003. [DOI] [PubMed] [Google Scholar]
- 29.Matsuka Y, Ono T, Iwase H, Mitrirattanakul S, Omoto KS, Cho T, Lam YYN, Snyder B, Spigelman I. Altered ATP release and metabolism in dorsal root ganglia of neuropathic rats. Mol Pain. 2008;4(1):66. doi: 10.1186/1744-8069-4-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WJ, Leventhal I, Scarsella D, Haydon PG, Janmey PA, Meaney DF. Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness. J Neurotrauma. 2009;26:789–797. doi: 10.1089/neu.2008-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palkovits M, Láng T, Patthy A, Elekes I. Distribution and stress-induced increase of glutamate and aspartate levels in discrete brain nuclei of rats. Brain Res. 1986;373(1):252–257. doi: 10.1016/0006-8993(86)90339-2. [DOI] [PubMed] [Google Scholar]
- 32.Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31(3):170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roehr B. New report details uphill battle to solve the U.S.’s pain problem. Scientific American. 2011 Jul; [Google Scholar]
- 34.Seltzer Z, Cohn S, Ginzburg R, Beilin B. Modulation of neuropathic pain behavior in rats by spinal disinhibition and NMDA receptor blockade of injury discharge. Pain. 1991;45:69–75. doi: 10.1016/0304-3959(91)90166-U. [DOI] [PubMed] [Google Scholar]
- 35.Sheng M, Lin JW. eLS. John Wiley & Sons Ltd; Chichester: 2001. Glutamatergic synapses: molecular organization. [Google Scholar]
- 36.Silva E, Quiñones B, Freund N, Gonzalez LE, Hernandez L. Extracellular glutamate, aspartate and arginine increase in the ventral posterolateral thalamic nucleus during nociceptive stimulation. Brain Res. 2001;923(1):45–49. doi: 10.1016/s0006-8993(01)03195-x. [DOI] [PubMed] [Google Scholar]
- 37.Tran MD, Neary JT. Purinergic signaling induces thrombospondin-1 expression in astrocytes. Proc Natl Acad Sci USA. 2006;103(24):9321–9326. doi: 10.1073/pnas.0603146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399(1):85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 39.Vetter G, Geisslinger G, Irmgard T. Release of glutamate, nitric oxide and prostaglandin E 2 and metabolic activity in the spinal cord of rats following peripheral nociceptive stimulation. Pain. 2001;92(1):213–218. doi: 10.1016/s0304-3959(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 41.Xie W, Strong JA, Meij JTA, Zhang J, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yonezawa T, Hattori S, Inagaki J, Kurosaki M, Takigawa T, Hirohata S, Miyoshi T, Ninomiya Y. Type IV collagen induces expression of thrombospondin-1 that is mediated by integrin α1β1 in astrocytes. Glia. 2010;58(7):755–767. doi: 10.1002/glia.20959. [DOI] [PubMed] [Google Scholar]
- 43.Zeng J, Kim D, Li KW, Sharp K, Steward O, Zaucke F, Luo ZD. Thrombospondin-4 contributes to spinal cord injury-induced changes in nociception. Eur J Pain. 2013;17(10):1458–1464. doi: 10.1002/j.1532-2149.2013.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]