Abstract
The c-Myc gene encodes an oncoprotein transcription factor which is frequently up-regulated in almost all cancer types, and is the subject of intense investigation for management of cancer because of its pleiotropic effects controlling a spectrum of cellular functions. However, due of its non-enzymatic nature, development of suitable strategies to block its protein-protein or protein-DNA interaction is challenging. Thus, c-Myc has been recognized as an elusive molecular target for cancer control and various approaches are in development to inhibit c-Myc-transcriptional activity. We observed that wedelolactone (WDL), an anti-inflammatory botanical compound, severely down-regulates the expression of c-Myc mRNA in prostate cancer cells. Moreover, WDL dramatically decreases the protein level, nuclear localization, DNA-binding, and transcriptional activities of c-Myc. c-Myc is a transforming oncogene widely expressed in prostate cancer cells and is critical for maintaining their transformed phenotype. Interestingly, WDL was found to strongly affect the viability of Myc-activated prostate cancer cells, and completely blocks their invasion as well as soft-agar colony-formation in vitro. WDL was also found to down-regulate c-Myc in vivo in nude mice xenografts. Moreover, WDL synergizes with enzalutamide to decrease the viability of androgen-sensitive prostate cancer cells via induction of apoptosis. These findings reveal a novel anticancer mechanism of the natural compound, WDL, and suggest that the oncogenic function of c-Myc in prostate cancer cells can be effectively down-regulated by WDL for the development of a new therapeutic strategy against Myc-driven prostate cancer.
Keywords: Wedelolactone, c-Myc, 5-Lipoxygenase, Enzalutamide, Prostate cancer, Apoptosis
Introduction
The c-Myc oncogene undergoes frequent deregulation during neoplastic transformation, and plays a causal role both in the development as well as progression phases of cancer. It is up-regulated in almost all cancer types, and is the subject of intense investigation because of its pleiotropic effects controlling a broad spectrum of functions including cell proliferation, metabolism, differentiation, sensitization to apoptotic stimuli, and genetic instability, which are events intimately associated with initiation, promotion and progression phases of cancer (1,2). Myc is a basic helix-loop-helix leucine zipper transcription factor that dimerizes with its binding partner MAX and associates with gene-promoters containing the E-box motifs (CACGTG or CACATG) to induce gene transcription (2,3). Because of its central role in oncogenesis, Myc has emerged as a promising, stand-alone molecular target for therapy of cancers afflicted with cells addicted to the c-Myc oncogene. Though Myc has been identified more than thirty years ago and anti-Myc agents, such as antisense-oligonucleotides, small-interfering RNA (siRNA), or phosphorodiamidate morpholino-oligomers (PMO) have been developed which induce tumor cell growth arrest, differentiation, or trigger apoptosis (4–7), development of direct Myc-targeting agents has yielded very limited success for clinical use, underscoring that novel therapeutic approaches should be developed to more-effectively block the over-activity of c-Myc in cancer cells. However, due to its action as a transcription factor, c-Myc enjoys the benefit of elusiveness because common approaches of active site-targeting do not apply to its non-enzymatic nature. This challenge is compounded by the fact that Myc regulates a plethora of target genes the combined action of which encompass cellular activities starting from cell proliferation to metabolic control, including cellular senescence, thus keeping both normal as well as cancer cells in its area of activity. Myc has also been well-recognized to promote androgen-independent prostate cancer cell growth, suggesting that Myc-driven signaling plays a major role in advanced stages of prostate cancer (8,9). Since, targeting c-Myc to block its protein-protein or protein-DNA interaction is a massive challenge, exploration of newer avenues should be encouraged which may yield effective Myc-targeting agents and bring Myc back into the realm of “druggable-targets.”
Natural products from terrestrial and aquatic plants have been a rich source of compounds for drug discovery. However, technical barriers in high-throughput assays and challenges associated with characterization of products against molecular targets have hindered proper use of these compounds in the past two decades. However, recently growing appreciation of functional assays and phenotypic screenings have contributed to the revival of interests in studying natural products for discovery and development of new drugs (10). Earlier, we reported that Wedelolactone (WDL), a plant-derived natural product, strongly inhibits the growth and survival characteristics of prostate cancer cells, but spares non-cancer cells in the same experimental conditions (11). The PI3K-Akt pathway is known as promoter of cell survival and preventer of cell death (12–16). Interestingly, it was observed that WDL kills prostate cancer cells without affecting the function of Akt, but does so via down-regulating the activity of PKC-epsilon (11). Not only these findings provided indication about the involvement of a new mechanism in the anti-cancer action of WDL, but also suggested about the existence of an Akt-independent, PKC-epsilon-dependent mechanism of survival which appears to be fundamental to the viability of prostate cancer cells. Various formulations of the source plant of WDL, Eclipta alba, have been used as remedies of a number of human ailments (17–22). Interestingly, the pure compound, WDL, has been reported to effectively inhibit the activity of 5-lipoxygenase (5-Lox) in some cells (23,24), and 5-Lox has been recently characterized to be a regulator of c-Myc oncogenic signaling in prostate cancer cells (25). However, details of the molecular mechanisms underlying the anti-cancer effects of WDL in prostate cancer cells are unknown, and possible involvement of the c-Myc oncogene as a down-stream target of WDL’s action has never been addressed before.
It is interesting to note that prostate cancer cells show several fold increase in the expression of c-Myc gene and a multi-fold increase in c-Myc mRNA compared to normal cells, such as lymphocytes (26–29). Ectopic expression of c-Myc in human androgen-dependent prostate cancer cells leads them to grow without androgen-stimulation and to keep their tumorigenic activity in androgen-depleted conditions (8,29,30), suggesting that Myc is a bona fide target for management of advanced castration-resistant prostate cancer. It has been documented that down-regulation of c-Myc slows the growth of prostate cancer cells, suggesting a physiological role for c-Myc in prostate cancer and a possible use of c-Myc as a stand-alone target for therapeutic intervention (7–9). However, c-Myc being a transcription factor, poses great difficulty to be targeted by agents to block its protein-protein or protein-DNA interactions. To get an insight into the molecular effects of WDL in prostate cancer cells, we have found that WDL dramatically decreases the mRNA and protein levels of c-Myc in prostate cancer cells in a clear dose- and time-dependent manner. Moreover, the transcriptional activity of c-Myc as well as the invasive and soft-agar colony forming abilities of prostate cancer cells are also down-regulated upon treatment with WDL. We reported earlier that WDL inhibits PKC-epsilon while inducing apoptosis in prostate cancer cells (11), and that PKC-epsilon has emerged as a newly characterized signaling intermediate in the 5-oxoETE/OXER1-driven prostate cancer cell-survival pathway (31,32). PKC-epsilon has been found to phosphorylate and activate Stat3 (33,34), and inhibition of Stat3 down-regulates expression of c-Myc gene in prostate cancer cells (25), suggesting that the inhibitory effect of WDL on c-Myc function in prostate cancer cells may be mediated via inhibition of PKC-epsilon and Stat3-mediated transcription of the c-Myc gene. Altogether, these findings revealed a new mechanism of action of WDL in prostate cancer cells, and since c-Myc is intimately associated with aggressive features in prostate cancer cells, our recent findings suggest that WDL may turn-out to be a novel agent to down-regulate c-Myc oncogenic signaling for development of an effective strategy for prostate cancer therapy.
Materials and Methods
2.1. Cell culture and reagents
LNCaP and PC3 human prostate cancer cells were purchased from American Type Culture Collection (Manassas, VA) and certified by STR analysis (2012). Cells were grown either in RPMI medium 1640 (Invitrogen, Carlsbad, CA). All the media were supplemented with 10% FBS and antibiotics. Antibodies against c-Myc and survivin were purchased from R and D Systems (Minneapolis, MN), and antibodies against, TMPRSS2, cyclin D1, CDK4, ATF3, TNF-alpha, GADD45-alpha and nucleoporin were from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-c-Myc antibodies were obtained from Abcam (Cambridge, MA). Polyclonal antibody against Aurora kinase was purchased from Cell Signaling Technology (Danvers, MA). Monoclonal anti-beta-actin antibody, wedelolactone, 10058-F4, MG132, calpain inhibitor, and ibuprofen were purchased from Sigma Chemical CO (St. Louis, MO). Enzalutamide was purchased from Selleck Chemicals (Houston, TX).
2.2. Cell viability assay
Cell viability was measured by MTS/PES One Solution Cell Titer Assay (Promega Corp, Madison, WI) as described before (11,25).
2.3. Microscopy
Cells (~3 × 105) were plated overnight in RPMI medium 1640 supplemented with 10% FBS onto 60 mm diameter tissue culture plates (Falcon) and allowed to grow for 48 hours. On the day of experiment, the spent culture medium was replaced with 2 ml fresh RPMI medium and the cells were treated with inhibitors. Control cells were treated with solvent (DMSO). Photographs were taken with a Nikon digital camera attached to a LEICA microscope at ×400. Image acquisition and data processing were done with a Dell computer attached to the microscope using Q-Capture 7 software.
2.4. Real-time quantitative polymerase chain reaction (qPCR)
LNCaP cells were plated and treated with 30 µM WDL at 37°C. Then, the cells were harvested, washed and total RNA was isolated from exponentially growing cells using RN-Easy Mini Kit from Qiagen (Valencia, CA). For the real-time qPCR, one microgram of total RNA was used from treated and untreated samples for the RT reaction using high capacity cDNA-RT kit from ABI/Life Technologies. Then the qPCR reactions were performed in triplicates using TaqMan gene expression assay kits from ABI/Life Technologies using ABI-7500 Fast real-time qPCR machine.
2.5. Western blot
LNCaP or PC3 cells (~3 × 105) were plated in 60 mm diameter plates and allowed to grow for 48 hours. The old medium was then replaced with 2 ml fresh RPMI medium and the cells were treated with inhibitors. After treatment, cells were harvested, washed, and lysed in lysis buffer (50 mM HEPES buffer, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 1% NP-40, and a cocktail of protease inhibitors). Proteins were separated by 12% SDS–PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat-milk and then blotted with appropriate primary antibody followed by peroxidase-labeled secondary antibody. Bands were visualized by enhanced chemiluminescence detection kit from Pierce Biotech (Rockford, IL) and analyzed with a densitometer using Kodak imaging software. Unless otherwise mentioned, protein blots were analyzed in three independent experiments.
2.6. DNA-binding assay
LNCaP cells were treated with WDL and nuclei were isolated using a kit from Sigma Chemical CO (St. Louis, MO). Localization of c-Myc was detected by Western blot using nucleoporin-A as a control. DNA-binding activity of c-Myc was measured using an ELISA kit from Active Motif (Carlsbad, CA) using 4 micrograms of nuclear extracts per assay. Free wild type (Wt) and mutated (Mut) E-box DNA sequences were used in parallel as positive and negative controls for assay validation.
2.7. Luciferase assay
LNCaP cells were transfected with lentiviral E-box-luciferase constructs (>90% cells transfected), expanded, and re-plated in 96 well culture plates in triplicates. Cells were then treated with MK591 and the luciferase activity was measured by a luciferase assay kit from Promega Corporation (Madison, WI). Ibu (ibuprofen) and F4 (10058-F4, a Myc-Max binding inhibitor) were used as negative and positive controls in parallel assays.
2.8. Invasion assay
Invasion assay was done in matrigel-coated Boyden transwell chambers from BD Biosciences. Transwell chambers were soaked in 50 µl serum-free RPMI medium for 30 minutes at RT and then ~40,000 cells (in RPMI medium containing 0.1% BSA) were placed into the upper chambers with or without drugs. These chambers were then placed in 24 well plate (one per well) on top of 500 µl RPMI medium containing 3% fetal serum as chemo-attractant. Inhibitors were added directly to the medium and mixed. Then the cells were incubated at 37°C in the CO2 incubator for 16 hours. Non-invaded cells along with matrigel in the upper chambers were scraped with a cotton tip applicator and then the membranes were fixed in methanol, stained with 0.25% crystal violet, and observed under a Leica microscope at ×200.
2.9. Soft-agar colony formation assay
Colony formation assays were performed in six well plates by placing ~15,000 LNCaP cells in 0.5 ml of 0.3% soft-agar on top of a 2 ml base layer of 0.6% agar. Plates were allowed to settle and then the wells were covered with 2 ml fresh RPMI medium containing 10% FBS with or without inhibitors. Plates were incubated at 37°C in the CO2 incubator for a maximum period of three weeks. Cell growth medium and inhibitors were exchanged every fourth day. At the end of incubation, cells were stained with 0.25% crystal violet. Then pictures were taken and colonies were counted under a Leica microscope at ×150.
2.10. Tumor xenograft and Immunohistochemistry
Balb/c nude (Nu/Nu) mice were purchased from Charles River Laboratories and injected subcutaneously with 2×106 LNCaP cells. They were randomized into two groups when tumors grew to ~100mm3, and then treated either with the solvent or with WDL at 200mg/kg/day five days a week for four weeks. Tumors were measured once a week with slide calipers and volumes were calculated using the formula a(b)2/2. Formalin-fixed paraffin-embedded sections from treated and untreated LNCaP prostate tumors were de-paraffinized and soaked in graded concentrations of ethanol. Then the sections were treated with antigen-retrieval buffer in the microwave (Power setting: High) for 1 minute. After washing, sections were blocked in 10% horse serum for 1 hour at RT and then treated either with control rabbit-IgG or with primary rabbit polyclonal anti-c-Myc or anti-survivin or anti-cyclin D1 antibody (1:100) overnight at 4°C, followed by secondary anti-rabbit IgG labeled with HRP. After washing, slides were stained for 30 seconds using DAB as substrate and then counter-stained with hematoxylin (Harris) for 30 seconds. Blocking, antibody treatment and color development were done using Vectastain Elite Impression kit [Cat.# MP-7401; Vector Laboratories, Burlingame, CA]. Photographs were taken with a Nikon digital camera attached to a Leica microscope at ×600.
Results
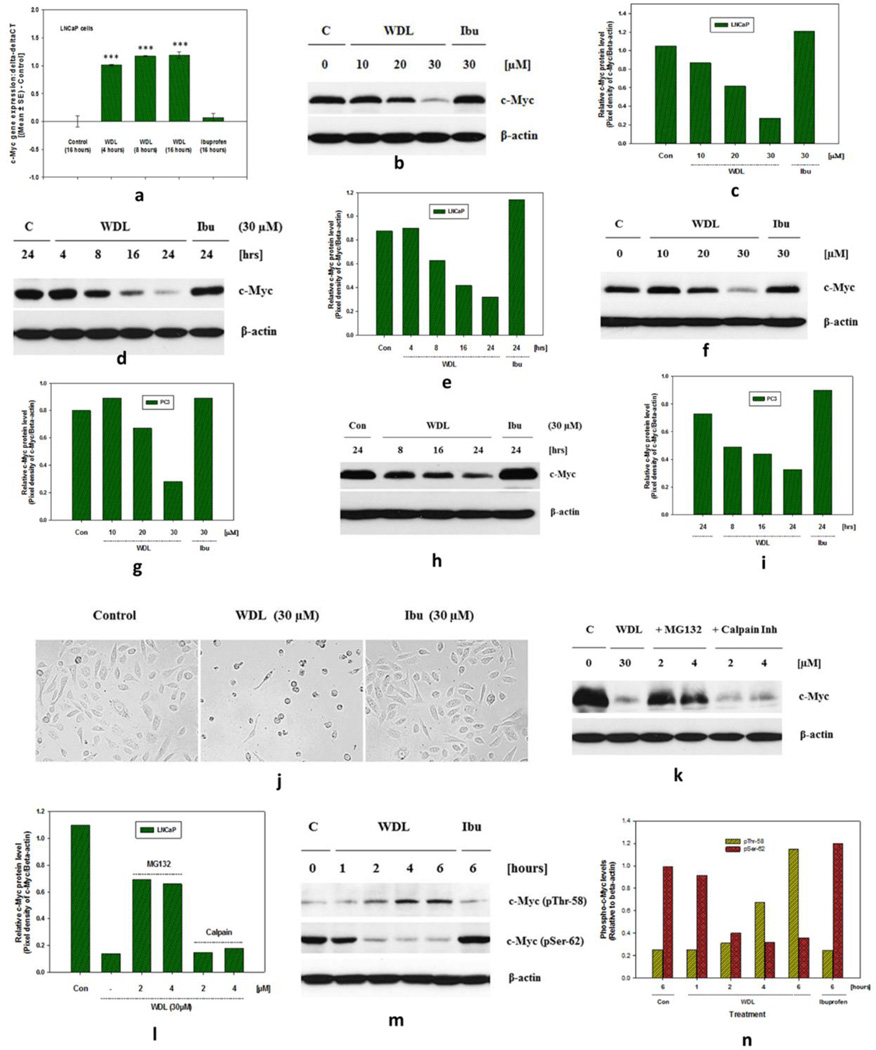
3.1. WDL dramatically decreases the mRNA and protein levels of c-Myc in prostate cancer cells
By real-time PCR analysis we found that treatment with WDL decreases the levels of c-Myc mRNA in prostate cancer cells which is clearly detectable as early as four hours post treatment (Figure 1a). We also found that WDL dramatically decreases the protein level of c-Myc in a clear dose- and time-dependent manner (Figures 1b–e). Similar effects of WDL were found in the androgen-independent PC3 human prostate cancer cells (Figures 1f–j). To understand the biochemical nature of the rapid protein loss, we found that WDL treatment-induced decrease in c-Myc protein is inhibited by MG132 (a proteasome inhibitor), but not by calpain inhibitor, suggesting that a proteasome-mediated degradation of c-Myc protein is triggered upon WDL treatment (Figures 1k,l). We also found that WDL treatment induces a rapid increase in the phosphorylation of c-Myc at Thr-58 and a decrease in the phosphorylation of c-Myc at Ser-62 (Figures 1m,n), events which are known to occur before ubiquitination and degradation of the c-Myc protein (2,35,36).
Figure 1. Effects of WDL treatment on mRNA and protein levels of c-Myc in prostate cancer cells.
In (a), LNCaP cells (1 × 106) were plated in 100mm diameter plates and allowed to grow for 48 hours. Then, the cells were treated with 30 µM WDL (please Figure S1 for chemical structure) at 37°C for 4–16 hours and total RNAs were isolated. The c-Myc gene expression was analyzed by qPCR in triplicates. Data presented as fold change in gene expression (delta-deltaCT) post treatment which were obtained by subtracting GAPDH-normalized values of inhibitor-treated samples with the corresponding control solvent (0.2% DMSO)-treated samples. Data presented as mean values of triplicate determination of each data point ± Standard Error (SE). *** p = <0.0005. Note: Ibuprofen (a cyclooxygenase inhibitor) was used as a negative control. In (b–e), 3 × 105 LNCaP cells, and in (f–i), 3 × 105 PC3 cells, were plated in 60mm diameter plates and treated with doses of drugs for 24 hours or as indicated. Dose and time-dependent changes in the levels of c-Myc proteins by treatment with WDL or ibuprofen are shown by Western blot. (j) Shows representative pictures of PC3 prostate cancer cells after WDL treatment. (k,l) Show WDL treatment-induced degradation of c-Myc protein is inhibited by proteasome inhibitor. LNCaP cells were plated and pretreated either with a proteasome inhibitor (MG132) or a calpain inhibitor (MG101) for 30 minutes at indicated concentrations. Then the cells were treated with WDL as shown and incubated for 16 hours at 37°C. c-Myc protein level in cell lysates were detected by Western blot. In (m,n), time-dependent changes in the amount of phosphorylation of c-Myc at Thr-58 and Ser-62 were detected by Western blot using corresponding phospho-specific antibodies.
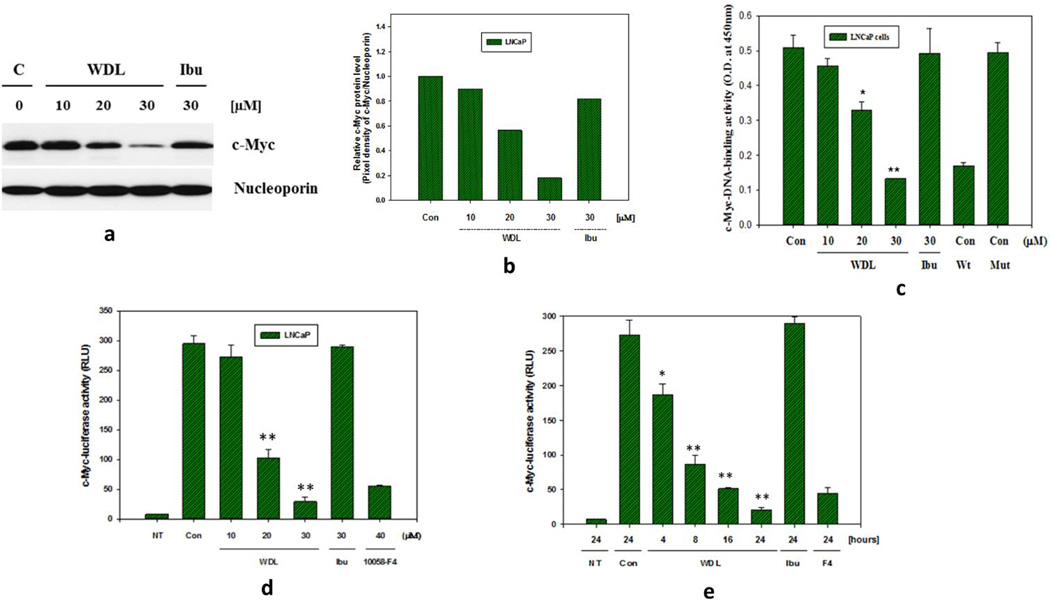
3.2. WDL down-regulates nuclear accumulation, DNA-binding, and transcriptional activities of c-Myc
Since, the protein product of c-Myc is a transcription factor, we examined whether inhibition of 5-Lox affects the nuclear accumulation and DNA-binding activity of c-Myc. Results are depicted in Figures 2a,b showing significant decrease in the nuclear accumulation of c-Myc upon treatment with WDL in a dose-dependent manner. We also observed that the DNA-binding activity of c-Myc in nuclear extracts is decreased accordingly in WDL-treated cells (Figure 2c). Moreover, we transfected prostate cancer cells with lentiviral constructs of the consensus c-Myc-binding sequence (E-box-Luciferase) to measure the transcriptional activity of c-Myc which revealed that the transcriptional activity of c-Myc is reduced by WDL in a dose and time-dependent manner (Figure 2d,e). Ibuprofen (a cyclooxygenase inhibitor) and 10058-F4 (a Myc-Max-binding inhibitor) were used as negative and positive controls respectively to verify measurement of Myc-specific effects.
Figure 2. Effects of WDL on down-regulation of c-Myc function in LNCaP cells.
In (a,b), LNCaP cells (3 × 105) were plated in 60 mm diameter plates and allowed to grow for 48 hours. Then the cells were treated with varying doses of WDL (10–30µM) at 37°C for 24 hours. Nuclear accumulation of c-Myc was determined by isolation of nuclei followed by detection of c-Myc proteins by Western blot using nucleoporin as loading control. In (c), DNA-binding activity of c-Myc in nuclear extracts was determined by ELISA using 4µg of nuclear extract proteins per assay. Note: Wild type (Wt) E-box consensus sequence DNA neutralizes c-Myc-DNA binding activity whereas the mutated (Mut) version of the sequence does not. In (d and e), dose- and time-dependent effects of WDL on c-Myc transcriptional activity (in terms of Relative Luciferase Unit or RLU) was detected by treating E-box-luciferase construct-transfected LNCaP cells with doses of inhibitors as indicated. Plates were incubated for 24 hours in (d), and in (e) 30µM of WDL or ibuprofen was used. Note: F4 (10058-F4), an inhibitor of Myc-Max binding (40µM), was used as positive control. NT represents values obtained with same number of non-transfected LNCaP cells under similar experimental conditions. These experiments were performed two to three times, and the results are shown as mean values of each data point ± standard error (n= 3). * p = <0.05 and ** p = <0.005.
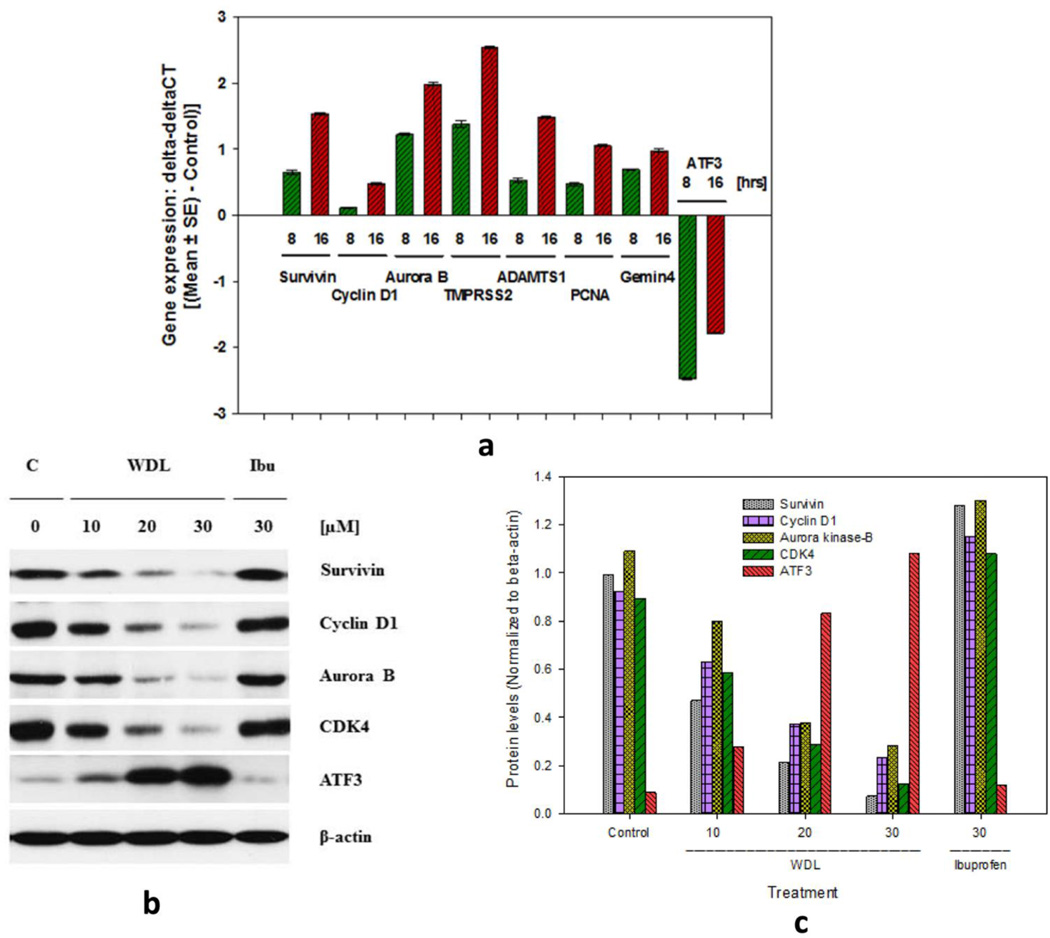
3.3. Treatment with WDL decreases the expression of c-Myc-target genes in prostate cancer cells
c-Myc is well-known for its strong oncogenic activity regulating transcription of a set of genes that in turn regulate important cell functions such as, cell survival, proliferation, invasion, and metastasis. Thus, we analyzed expression of some well-characterized c-Myc target genes after WDL treatment. Since, the oncogenic function of c-Myc involves promotion of cell division via activation of cyclins and CDKs, and prevention of cell death via activation of anti-apoptotic proteins, we examined the function of c-Myc by analyzing the regulation of well-known c-Myc targets. We observed that WDL dramatically decreases mRNA expression (delta-CT higher) of survivin, cyclin D1, Aurora kinase, TMPRSS2, ADAMTS1, PCNA and Gemin4, whereas it increases expression of the pro-apoptotic gene ATF3 (delta-CT lower) in a time-dependent manner (Figure 3a). We also observed that treatment with WDL decreases protein levels c-Myc targets (survivin, cyclin D1, aurora kinase, CDK4, and increases the level of ATF3 (Figures 3b,c).
Figure 3. Effects if WDL on expression of c-Myc-target genes in LNCaP cells.
In (a), LNCaP cells (~1 × 106 cells per plate) were plated in 100 mm diameter plates and allowed to grow for 48 hours. Then the cells were treated with MK591 (30µM) for 8 or 16 hours and time-dependent changes in the expression of c-Myc target genes were analyzed by real-time PCR. Change in expression (delta-deltaCT) was calculated by deducting mean values of control for each gene using the same probe set. Normalization of gene expression was done by using GAPDH as internal control. Results presented as mean value of each data point ± SE. In (b,c), changes in the protein levels of c-Myc-targets in LNCaP cells 24 hours post WDL treatment were detected by Western blot. Note: Ibu = Ibuprofen, an inhibitor of cyclooxygenase (30µM), was used in parallel which showed no appreciable effects on protein levels of c-Myc targets.
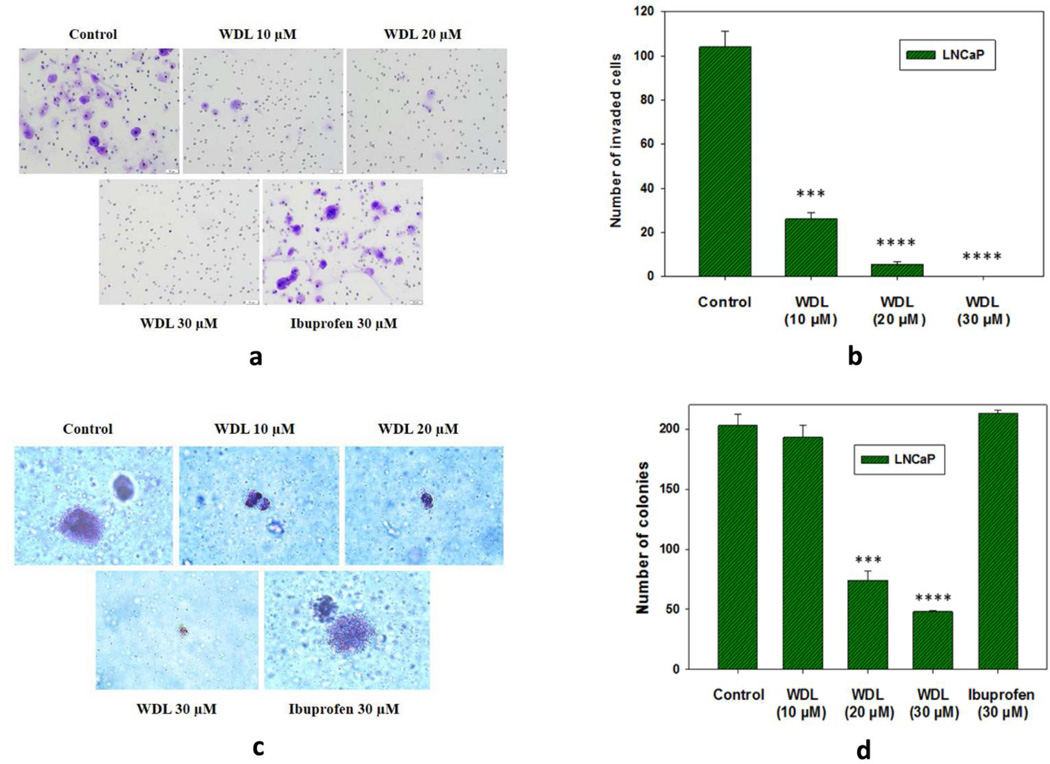
3.4. WDL blocks the in vitro invasion and soft-agar colony-forming abilities of prostate cancer cells
The c-Myc oncoprotein plays a pivotal role in promoting invasion and metastasis in various types of cancer, including prostate cancer (37–42). Thus, we analyzed the effect of WDL treatment on metastasis-related cell-functions which are mediated by c-Myc activity. We found that WDL decreases the matrigel-invasive ability of prostate cancer cells in a dose-dependent manner (Figures 4a,b). We also observed that WDL severely affects the soft-agar colony-forming abilities of prostate cancer cells, suggesting that the metastatic abilities of prostate cancer cells could be significantly inhibited by WDL treatment (Figures 4c,d). Ibuprofen, a cyclooxygenase inhibitor, was used as a negative control which was found to be completely ineffective to inhibit invasion or colony formation under the same experimental conditions.
Figure 4. Effects of WDL on in vitro invasion and soft-agar colony formation by LNCaP cells.
In (a), effect of WDL on invasive capability of LNCaP cells was assayed using matrigel-coated modified Boyden trans-well chambers as described in the “Methods” section. After incubation, cells were fixed and stained with crystal violet. Pictures were taken with a Leica microscope at ×200. (b) Shows quantitative measurements of the number of invaded cells with or without drug treatment. Results represent mean values of individual data point ± standard deviation (n=3). *** p = <0.0005; **** p = <0.00005. In (c), effects of WDL and ibuprofen on soft-agar colony formation by LNCaP cells are shown. Cells were plated on soft-agar in RPMI medium as described in the “Methods” section. After incubation for three weeks, cells were stained with crystal-violet and growing colonies were counted under microscope at ×150. In (d), growing colonies were counted, and the results are shown quantitatively as mean values of each data point ± standard deviation (n= 3). *** p = <0.0005; **** p = <0.00005.
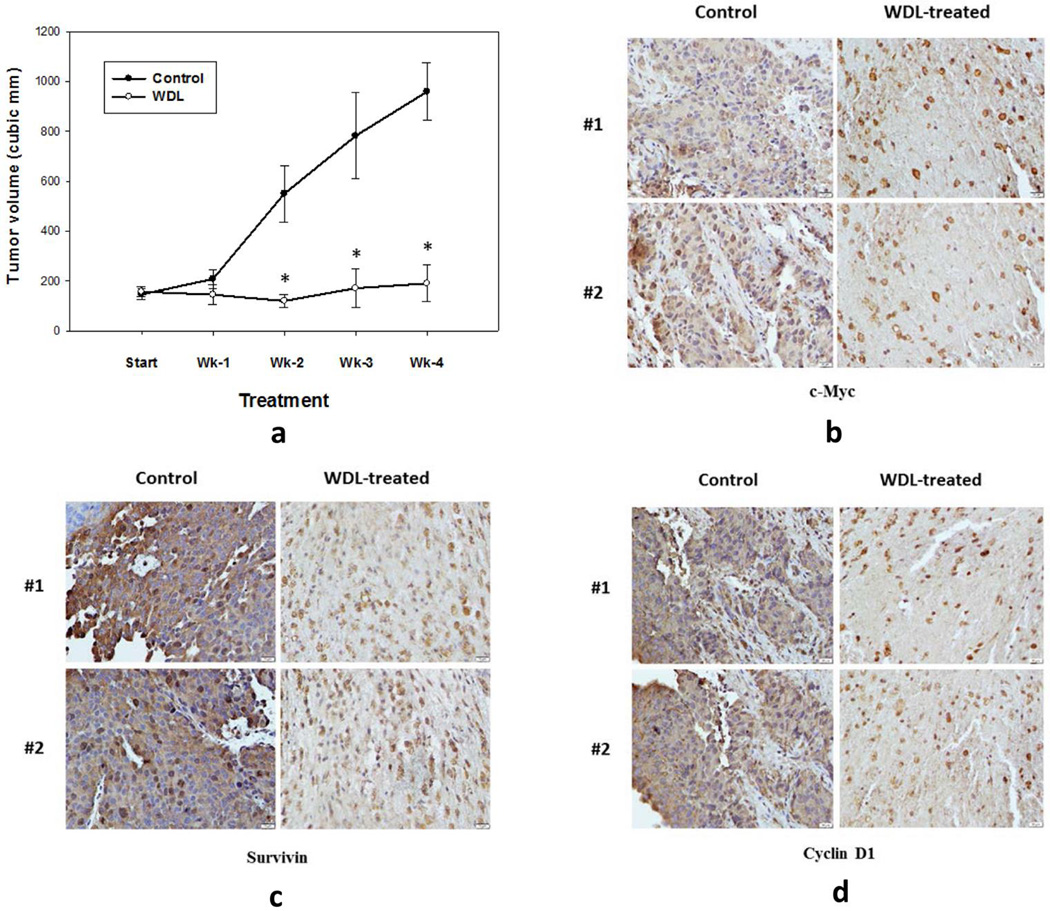
3.5. WDL decreases the protein levels of c-Myc and its targets in prostate tumor xenografts
In a pilot experiment, we tested the in vivo effects of WDL on tumor growth, and on protein levels of c-Myc and targets using LNCaP prostate tumor xenografts treating Balb/c nude (Nu/Nu) mice with WDL at a dose of 200mg/kg/day five days a week for four weeks via oral gavage. By immuno-histochemical analysis we found that WDL treatment significantly reduced tumor growth, and decreased the protein levels of c-Myc and its targets (survivin, cyclin D1) in prostate tumor xenografts (Figures 5a–d). Since, c-Myc and its targets are well-known to promote all stages of cancer, including tumor formation, tumor growth as well as metastasis, our findings suggest that WDL may turn out to be an effective agent in reducing prostate tumor-burden in vivo via down-regulation of c-Myc oncogenic signaling. Note: The dark-yellow color in cells of treated tumors is presumably due to excessive accumulation of WDL in them.
Figure 5. In vivo effects of WDL on protein levels of c-Myc and targets in prostate tumor xenografts.
Prostate tumor xenografts were developed by injecting LNCaP human prostate cancer cells (2 × 106 per site) to a size of ~100 mm3. Then, the tumor-bearing mice were randomly divided into three groups (n=3). Group 1 mice were treated with solvent only (1:1:8 mixture of DMSO:Cremophor:PBS) and Group 2 mice were treated with 200mg/kg/day of WDL orally for four weeks. At the end of treatment period, tumors were dissected and processed for immunohistochemistry as described in the “Materials and Methods” section. In (a), tumor volumes were measured by calipers and presented as mean values of each data point ± Standard Error. * p = <0.05. Expressions of c-Myc (b), survivin (c), and cyclin D1 (d), in tumors from treated and untreated mice were detected by corresponding rabbit polyclonal antibodies (1:100). Photographs were taken with a Leica microscope at ×600. Note: Data are presented with tumor samples obtained from two representative mice in each group (#1 and #2) for side-by-side comparison.
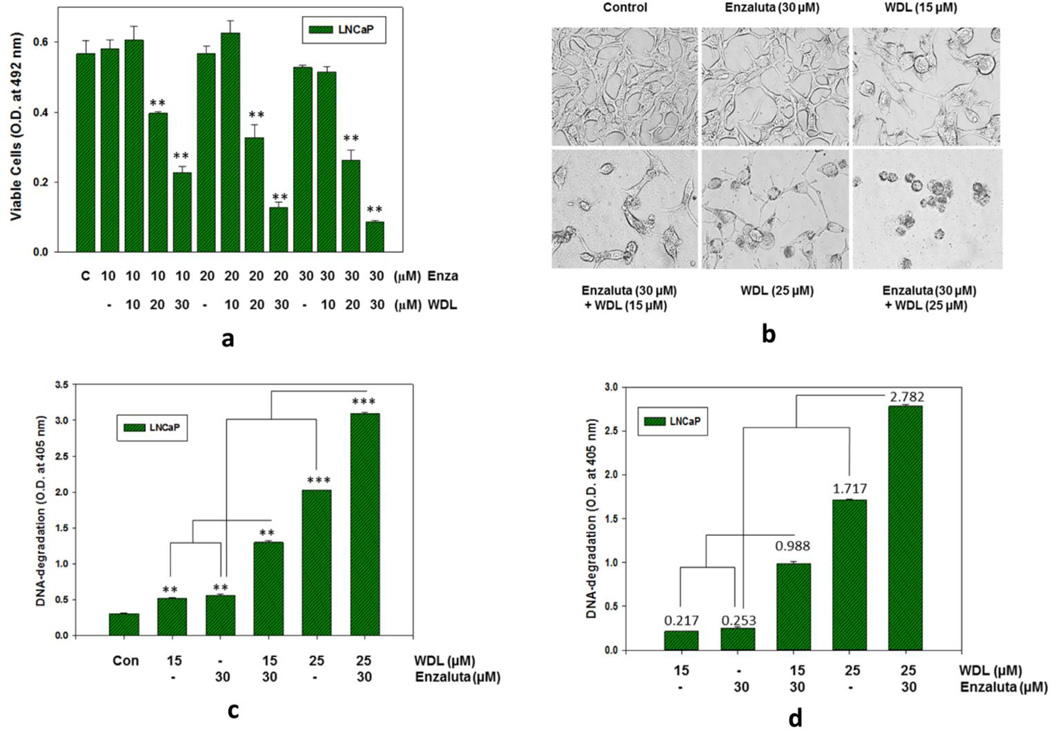
3.6. WDL synergizes with enzalutamide to inhibit prostate cancer cell viability via induction of apoptosis
The c-Myc oncogene is well-characterized to promote androgen-independent growth of prostate cancer cells, and over-activation of c-Myc is frequently observed to be associated with castration-resistant prostate tumors, suggesting that activated c-Myc supports the development of castration-resistant prostate cancer (8, 26–30). Thus, we wanted to test our hypothesis whether WDL (by virtue of its inhibition of c-Myc) may enhance the effects of enzalutamide, an FDA-approved inhibitor of androgen receptor function, which is commonly used in the clinic to treat disseminated prostate cancer that are not manageable by surgery or radiation therapy. Interestingly, we observed that WDL cooperates with enzalutamide to inhibit prostate cancer cell growth and viability (Figure 6a). Moreover, we observed that WDL and enzalutamide synergistically induce apoptosis to kill prostate cancer cells (Figures 6b,c,d). These findings indicate that co-administration of WDL may enhance the effects of enzalutamide to reduce prostate cancer cell viability, and suggest that a combination of WDL and enzalutamide may yield better clinical outcome to inhibit prostate tumor growth by killing prostate cancer cells via induction of apoptosis.
Figure 6. Cooperative and synergistic effects of WDL and enzalutamide on the viability and induction of apoptosis in prostate cancer cells.
In (a), LNCaP cells were plated in 96 well plates and treated with varying doses of enzalutamide (please see Figure S1 for chemical structure) with or without WDL for 72 hours. Control cells were treated with the solvent only (0.2% DMSO). At the end of incubation period, cell viability was measured by MTS/PES Cell Titer assay (Promega Corp, Madison, WI). In (b), LNCaP cells were plated and treated with doses of Enzalutamide and/or WDL as indicated for 48 hours. Then, the cells were photographed with a Leica microscope at ×300. In (c), apoptosis was quantitatively measured by detecting DNA degradation to nucleosomal fragments using Cell Death Detection ELISA Plus kit as described before (25,31). Results are shown as mean values of quadruplicate determination of each data point ± standard error. ** p = <0.005; *** p = <0.0005. In (d), quantitative values of apoptotic-DNA degradation in WDL and enzalutamide treated cells were used for calculation of synergy. For determination of synergy, the “Combination Index” method of Chou and Talalay (50) to calculate dose-effects of two drugs was used (CI) = (D)1/(Dx)1 + (D)2/(Dx)2. In our case, at ED50 level D1 and D2 individual effects were 0.217 and 0.253 respectively, and the combined effect is 0.988, and at ED90 level D1 and D2 individual effects were 1.717 and 0.253 respectively, and the combined effect is 2.782. So, in both cases the value of combined effects are higher than the total of individual values, and by calculating % effects of D1 and D2 at ED50 level we get 0,470, and calculating % effects of D1 and D2 at ED90 level we get 0,70, meaning the combination values in both cases are less than 1 and in the 0.3–0.7 range. Thus, WDL and Enzalutamide synergize to induce apoptosis in prostate cancer cells.
Discussion
We observed that WDL, a medicinal plant-derived coumestan compound, dramatically decreases the expression and function of c-Myc in prostate cancer cells. This is especially significant because the c-Myc oncoprotein (with many of its target-genes, encoding proteins that help maintain the transformed state) can initiate or promote almost all human cancers. Expression of c-Myc is frequently deregulated in a wide range of human cancers, including prostate cancer and is often associated with aggressive, poorly differentiated tumors (1–5,26–30). The importance of Myc as a cancer promoter stems from the fact that the Myc oncoprotein is a pleiotropic basic helix–loop–helix leucine zipper transcription factor which coordinates expression of diverse cellular programs that together regulates a variety of cellular processes including cell growth and proliferation, transcription, differentiation, cell motility and apoptosis (29,37–42). Myc coordinates a vast functionally diverse repertoire of genes that are required for orderly proliferation of cells and is functionally non-redundant. Interestingly, Myc is required by both normal and cancer cells for efficient proliferation. In normal cells Myc is turned on by growth factor signaling which instructs cell division cycle, however, its function in cancer cells is almost always compromised either by gene amplification or by mutation that promotes uncontrollable cell proliferation and tumor formation. Thus, Myc has repeatedly been recognized as an elusive molecular target for cancer therapy which triggered interest both in searching for its upstream and downstream regulators, and also in discovering agents that counteract the role it plays in transformation and therapy-resistance.
Previously, we reported that WDL reduces the viability of both androgen-sensitive (LNCaP) as well as androgen-independent (PC3, DU145) human prostate cancer cells, without affecting normal, non-cancer prostate epithelial cells (PrEC) in the same experimental conditions (11). Since many prostate cancer cells (such as LNCaP, PC3) bear Myc over-activation and are driven by Myc function for maintaining their cancer phenotype, we took advantage of using these cell culture models to address the question of the modulation of c-Myc by WDL. Our observation of the decrease in c-Myc protein level as well as its DNA-binding and E-box-luciferase activities suggest that c-Myc in prostate cancer cells can be effectively down-regulated by WDL (Figures 1,2). Moreover, the inhibition of c-Myc by WDL in prostate cancer cells is correlated with down-regulation of the expression of its target genes, such as survivin, aurora kinase, cyclin D1 (Figure 3). These findings opened up the possibility to control the expression and function of c-Myc in prostate (and possibly other types of) cancer cells by small molecule chemical inhibitors of 5-Lox, such as WDL. Ibuprofen, a cyclooxygenase inhibitor, was used as a negative control which was found to be ineffective. c-Myc has been well-characterized to play a pivotal role in promoting metastasis in various types of cancer cells through defined mechanisms (37–42). Thus, we analyzed the effect of WDL treatment on the invasive and soft-agar colony-forming abilities of prostate cancer cells. We observed that WDL effectively blocked both invasion and anchorage-independent colony formation by prostate cancer cells on soft-agar (Figure 4). Moreover, we observed that oral administration of WDL exerts significant in vivo effect in inhibiting growth of tumors and decreasing expression of c-Myc and its targets in nude mice xenografts (Figure 5), which suggest that WDL possesses all the desired characteristics of a standard chemotherapeutic agent, such as good solubility, bioavailability and in vivo efficacy.
Transition of prostate cancer from an androgen-dependent to an androgen independent phenotype is a complex process which presumably involves both the selection of pre-existing clones of androgen-independent cells as well as selection for genetic events that help the cancer cells survive and grow in an environment devoid of androgenic signaling. One molecular mechanism of developing androgen independence by which prostate cancer cells grow and proliferate independently of AR and androgen has been suggested to be based on Myc activation. c-Myc has been strongly implicated in the development and progression of castration-resistant prostate cancer. Several studies have detected a common amplicon during the conversion to androgen-independent prostate cancer in a short region spanning chromosome 8q and containing the c-Myc gene (26,27). In clinical samples, amplifications of c-Myc gene has been found in more than 70% of androgen-independent prostate tumors by fluorescence in situ hybridization and a significant increase in c-Myc amplification was observed after anti-androgen treatment (26–30). Bernard et al examined the effect of c-Myc in androgen-independent prostate cancer progression by using LNCaP cells treated with the antiandrogen Casodex, which showed that overexpression of c-Myc was sufficient to induce androgen-independent growth in Casodex treated cells (8). Interestingly, we observed that low-dose WDL synergizes with enzalutamide to reduce viability and to induce apoptosis in prostate cancer cells (Figure 6), suggesting that WDL may yield better results in prostate cancer therapy when used in combination with enzalutamide.
How WDL down-regulates a strong oncogene like c-Myc in prostate cancer cells is an intriguing but open question. Previous findings documented that WDL is a potent inhibitor of 5-lipoxygenase (IC50 = 2.5 µM), which inhibits the enzymatic activity of 5-Lox by an oxygen radical scavenger mechanism (23,24), though WDL also inhibits other molecules at higher concentrations (22,43). The enzyme 5-Lox is well known to process arachidonic acid to generate leukotrienes which plays a major role in asthma. Recent studies have demonstrated an important role of 5-Lox in the regulation of c-Myc oncogenic signaling in prostate cancer cells (25,44). Thus, 5-Lox has emerged as a potential molecular target for therapeutic development against Myc-driven prostate cancer. However, lingering problems with solubility and bioavailability of several available 5-Lox inhibitors have limited their use for prostate cancer therapy. Based on published reports about the 5-Lox inhibitory effect of WDL, we expected that WDL will inhibit c-Myc and induce apoptosis in prostate cancer cells as we recently observed with other 5-Lox inhibitors (31.32). Here, we observed that WDL induces dramatic loss of c-Myc in prostate cancer cells and this phenomenon is correlated with our previous observation of the inhibition of PKCε by WDL (11). PKCε activates Stat3 via phosphorylation at Ser-727 which has been found to be intimately linked with prostate cancer (33,34). Since Stat3 regulates c-Myc in prostate cancer cells (25), WDL may down-regulate c-Myc via inhibition of Stat3-mediated transcription.
Prostate cancer is the most common form of malignancy and second leading cause of cancer-related deaths in men in the United States (45). Though prostate cancer initially responds to anti-androgenic therapy, androgen-resistant disease almost always develops (46,47). Development of androgen-resistant metastatic prostate cancer always ends up with a fatal outcome because currently there is no effective therapy available for this type of prostate cancer. Thus, novel agents and strategies are urgently needed to improve treatment options for androgen-resistant prostate cancer. The universal deregulation of c-Myc gene expression in tumor cells makes inhibition of c-Myc an attractive pharmacological approach for treating diverse types of cancer, including prostate cancer, though direct targeting of c-Myc yielded no significant clinical benefit (48,49). Plus the enthusiasm of direct targeting of c-Myc has been muted by lack of evidence that c-Myc inhibition would be therapeutically efficacious, raising concerns that it would induce serious side effects by inhibiting normal, non-cancer cells where c-Myc is also functional. Under the circumstances, our findings revealed a novel strategy to silence c-Myc by WDL in prostate cancer cells which may open up new directions to monitor the oncogenic action of c-Myc, and thus, may help overcome practical difficulties in designing direct inhibitors of Myc. Based on the potency, solubility, and selectivity profile of WDL against prostate cancer cells in vitro and in vivo, it appears that WDL is a promising candidate drug, and should be tested further for development of a novel prostate cancer treatment strategy. Since, Myc plays a critical role both in the development and maintenance of transformed phenotype, our observations of the regulation of expression and function of c-Myc not only reveal an unknown mechanism of action of WDL, but also opens up a new avenue to utilize natural agents in prevention and treatment of prostate cancer, especially Myc-driven advanced prostate cancer.
Supplementary Material
Acknowledgments
We thank Dr. Zhou for his help with the qPCR experiments.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number RO1 CA 152334, the Department of Defense Prostate Cancer Research Program W81-XWH-05-1-0022, and the Henry Ford Health System internal grant A10203 to J. Ghosh.
List of abbreviations
- WDL
Wedelolactone
- Enza
Enzalutamide
- c-Myc
cellular-Myelocytoma gene
- 5-Lox
5-Lipoxygenase
- PARP
Poly-ADP ribose polymerase
- ELISA
Enzyme-linked immunosorbent assay
- FITC
Fluorescein isothiocyanate
Footnotes
Conflict of interest: None
References
- 1.Ponzielli R, Katz S, Barsyte-Lovejoy D, Penn LZ. Cancer therapeutics: targeting the dark side of Myc. Eur J Cancer. 2005;41:2485–2501. doi: 10.1016/j.ejca.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Meyer N, Penn LZ. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8:976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 3.Soucek L, Whitfield J, Martins CP, Finch AJ, Murphy DJ, Sodir NM, Karnezis AN, Swigart LB, Nasi S, Evan GI. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–683. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang YH, Liu S, Zhang G, Zhou CQ, Zhu HX, Zhou XB, Quan LP, Bai JF, Xu NZ. Knockdown of c-Myc expression by RNAi inhibits MCF-7 breast tumor cells growth in vitro and in vivo. Breast Cancer Res. 2005;7:R220–R228. doi: 10.1186/bcr975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura S, Maekawa T, Hirakawa K, Murakami A, Abe T. Alterations of c-Myc expression by antisense oligo-deoxynucleotides enhance the induction of apoptosis in HL-60 cells. Cancer Res. 1995;55:1379–1384. [PubMed] [Google Scholar]
- 6.Holt JT, Redner RL, Nienhuis AW. An oligomer complementary to c-Myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988;8:963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iversen PL, Arora V, Acker AJ, Mason DH, Devi GR. Efficacy of antisense morpholino oligomer targeted to c-Myc in prostate cancer xenograft murine model and a phase I safety study in humans. Clin Cancer Res. 2003;9:2510–2519. [PubMed] [Google Scholar]
- 8.Bernard D, Pourtier-Manzanedo A, Gil J, Beach DH. Myc confers androgen-independent prostate cancer cell growth. J Clin Invest. 2003;112:1724–1731. doi: 10.1172/JCI19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Schwartzman J, Gibbs A, Lisac R, Kleinschmidt R, Wilmot B, Bottomly D, Coleman I, Nelson P, McWeeney S, Alumkal J. Androgen receptor promotes ligand-independent prostate cancer progression through c-Myc upregulation. PLOS One. 2013;8:e63563. doi: 10.1371/journal.pone.0063563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey AL, Edrada-Ebel R, Quinn RJ. The re-emergence of natural products for drug discovery in the genomics era. Nat Rev Drug Discov. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- 11.Sarveswaran S, Gautam S, Ghosh J. Wedelolactone, a medicinal plant-derived coumestan, induces caspase-dependent apoptosis in prostate cancer cells via down-regulation of PKC-epsilon without inhibiting Akt. Intl J Oncol. 2012;41:2191–2199. doi: 10.3892/ijo.2012.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 14.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22:355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 15.Altomare DA, Testa JR. Perturbations of the Akt signaling pathway in human cancer. Oncogene. 2005;24:7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 16.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT Pathway for Cancer Drug Discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 17.Wagner H, Geyer B, Kiso Y, Hikino H, Rao GS. Coumestans as the main active principles of the liver drugs Eclipta alba and Wedelia calendulacea. Planta Med. 1986;52:370–374. [PubMed] [Google Scholar]
- 18.Singh B, Saxena AK, Chandan BK, Agarwal SG, Anand KK. In vivo hepatoprotective activity of active fraction from ethanolic extract of Eclipta alba leaves. Indian J Physiol Pharmacol. 2001;45:435–441. [PubMed] [Google Scholar]
- 19.Roy RK, Thakur M, Dixit VK. Hair growth promoting activity of Eclipta alba in male albino rats. Arch Dermatol Res. 2008;300:357–364. doi: 10.1007/s00403-008-0860-3. [DOI] [PubMed] [Google Scholar]
- 20.Diogo LC, Fernandes RS, Marcussi S, Menaldo DL, Roberto PG, Matrangulo PV, Pereira PS, Franca SC, Giuliatti S, Soares AM, Lourenco MV. Inhibition of snake venoms and phospholipases A2 by extracts from native and genetically modified Eclipta alba: Isolation of active coumestans. Basic Clin Pharmacol Toxicol. 2009;104:293–299. doi: 10.1111/j.1742-7843.2008.00350.x. [DOI] [PubMed] [Google Scholar]
- 21.Soares AM, Januario AH, Lourenco MV, Pereira AMS, Pereira PS. Neutralizing effects of Brazilian plants against snake venoms. Drugs Fut. 2004;29:1105–1109. [Google Scholar]
- 22.Kobori M, Yang Z, Gong D, Heissmeyer V, Zhu H, Jung Y, Angelica M, Gakidis M, Rao A, Sekine T, Ikegami F, Yuan Y, Yuan J. Wedelolactone suppresses LPS-induced caspase-11 expression by directly inhibiting the IKK Complex. Cell Death Differ. 2004;11:123–130. doi: 10.1038/sj.cdd.4401325. [DOI] [PubMed] [Google Scholar]
- 23.Wagner H, Fessler B. In vitro 5-lipoxygenase inhibition by Eclipta alba extracts and the coumestan derivative wedelolactone. Planta Med. 1986;52:374–377. [PubMed] [Google Scholar]
- 24.Werz O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007;73:1331–1357. doi: 10.1055/s-2007-990242. [DOI] [PubMed] [Google Scholar]
- 25.Sarveswaran S, Chakraborty D, Chitale D, Sears R, Ghosh J. Inhibition of 5-Lipoxygenase selectively triggers disruption of c-Myc signaling in prostate cancer cells. J Biol Chem. 2015;290:4994–4506. doi: 10.1074/jbc.M114.599035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visakorpi T, Kallioniemi AH, Syvanen AC, Hyytinen ER, Karhu R, Tammela T, Isola JJ, Kallioniemi OP. Genetic changes in primary and recurrent prostate cancer by comparative genomic hybridization. Cancer Res. 1995;55:342–347. [PubMed] [Google Scholar]
- 27.Nupponen NN, Kakkola L, Koivisto P, Visakorpi T. Genetic alterations in hormone-refractory recurrent prostate carcinomas. Am J Pathol. 1998;153:141–148. doi: 10.1016/S0002-9440(10)65554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaltz-Wittmer C, Klenk U, Glaessgen A, Aust DE, Diebold J, Lohrs U, Baretton GB. FISH analysis of gene aberrations (MYC, CCND1, ERBB2, RB, and AR) in advanced prostatic carcinomas before and after androgen deprivation therapy. Lab Invest. 2000;80:1455–1464. doi: 10.1038/labinvest.3780152. [DOI] [PubMed] [Google Scholar]
- 29.Gil J, Kerai P, Lleonart M, Bernard D, Cigudosa JC, Peters G, Carnero A, Beach D. Immortalization of primary human prostate epithelial cells by c-Myc. Cancer Res. 2005;65:2179–2185. doi: 10.1158/0008-5472.CAN-03-4030. [DOI] [PubMed] [Google Scholar]
- 30.Gil J. Molecular basis for androgen independency in prostate cancer. Cancer Therapy. 2006;4:143–152. [Google Scholar]
- 31.Sarveswaran S, Thamilselvan V, Brodie C, Ghosh J. Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epslilon. Biochim Biophys Acta. 2011;1813:2108–2117. doi: 10.1016/j.bbamcr.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarveswaran S, Ghosh J. OXER1, a G protein-coupled oxoeicosatetraenoid receptor, mediates the survival-promoting effects of arachidonate 5-lipoxygenase in prostate cancer cells. Cancer Lett. 2013;336:185–195. doi: 10.1016/j.canlet.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase C-ε interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3-Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 34.Aziz MH, Hafeez BB, Sand JM, Pierce DB, Aziz SW, Dreckschmidt NE, Verma AK. Protein kinase Cε mediates Stat3Ser727 phosphorylation, Stat3-regulated gene expression and cell invasion in various human cancer cell lines via integration with MAPK cascade (RAF-1, MEK1/2, and ERK1/2) Oncogene. 2010;29:3100–3109. doi: 10.1038/onc.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sears RC. The life cycle of C-myc: from synthesis to degradation. Cell Cycle. 2004;9:1133–1137. [PubMed] [Google Scholar]
- 36.Dang CV. Enigmatic MYC Conducts an Unfolding Systems Biology Symphony. Genes Cancer. 2010;1:526–531. doi: 10.1177/1947601910378742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfer A, Ramaswamy S. MYC and Metastasis. Cancer Res. 2011;71:2034–2037. doi: 10.1158/0008-5472.CAN-10-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfer A, Wittner BS, Irimia D, Flavin RJ, Lupien M, Gunawardane RN, Meyer CA, Eric S, Lightcap ES, Tamayo P, Mesirov JP, Liu XS, Shioda T, Toner M, Loda M, Brown M, Brugge JS, Ramaswamy S. MYC regulation of a "poor-prognosis" metastatic cancer cell state. Proc Natl Acad Sci USA. 2010;107:3698–3703. doi: 10.1073/pnas.0914203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rapp UR, Korn C, Ceteci F, Karreman C, Luetkenhaus K, Serafin V, Zanucco E, Castro I, Potapenko T. MYC is a metastasis gene for non-small-cell lung cancer. PLOS One. 2009;4:e6029. doi: 10.1371/journal.pone.0006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Liu X, Xu W, Zhou P, Gao P, Jiang S, Lobie PE, Zhu T. c-MYC-regulated miR-23a/24-2/27a cluster promotes mammary carcinoma cell invasion and hepatic metastasis by targeting Sprouty2. J Biol Chem. 2013;288:18121–18133. doi: 10.1074/jbc.M113.478560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang XY, DeSalle LM, Patel JH, Capobianco AJ, Yu D, Thomas-Tikhonenko A, McMahon SB. Metastasis-associated protein 1 (MTA1) is an essential downstream effector of the c-MYC oncoprotein. Proc Natl Acad Sci USA. 2005;102:13968–13973. doi: 10.1073/pnas.0502330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Syed SD, Deepak M, Yogisha S, Chandrashekar AP, Muddarachappa KA, D'Souza P, Agarwal A, Venkataraman BV. Trypsin inhibitory effect of wedelolactone and demethylwedelolactone. Phytother Res. 2003;4:420–421. doi: 10.1002/ptr.1153. [DOI] [PubMed] [Google Scholar]
- 44.Sarveswaran S, Ghosh R, Morisetty S, Ghosh J. MK591, a second generation leukotriene biosynthesis inhibitor, prevents invasion and induces apoptosis in the bone-invading C4-2B human prostate cancer cells: Implications for the treatment of castration-resistant, bone-metastatic prostate cancer. PLOS One. 2015;104:1–19. doi: 10.1371/journal.pone.0122805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 46.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marques RB, Dits NF, Erkens-Schulze S, Weerden WM, Jenster G. Bypass mechanisms of the androgen receptor pathway in therapy-resistant prostate cancer cell models. PLOS One. 2010;5:e13500. doi: 10.1371/journal.pone.0013500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo J, Parise RA, Joseph E, Egorin MJ, Lazo JS, Prochownik EV, Eiseman JL. Efficacy, pharmacokinetics, tissue distribution, and metabolism of the Myc–Max disruptor, 10058-F4 [Z,E]-5-[4-ethylbenzylidine]-2-thioxothiazolidin-4-one, in mice. Cancer Chemother Pharmacol. 2009;63:615–625. doi: 10.1007/s00280-008-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prochownik EV, Vogt PK. Therapeutic Targeting of Myc. Genes Cancer. 2010;1:650–659. doi: 10.1177/1947601910377494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.