Abstract
Objectives
Patients in the intensive care unit (ICU) frequently receive proton pump inhibitors (PPIs) and have high rates of Clostridium difficile infection (CDI). PPIs have been associated with CDI in hospitalized patients, but ICU patients differ fundamentally from non-ICU patients and few studies have focused on PPI use exclusively in the critical care setting. We performed a retrospective cohort study to determine the associations between PPIs and healthcare facility–onset CDI in the ICU.
Methods
We analyzed data from all adult ICU patients at three affiliated hospitals (14 ICUs) between 2010–2013. Patients were excluded if they had recent CDI or an ICU stay of <3 days. We parsed electronic medical records for ICU exposures, focusing on PPIs and other potentially modifiable exposures that occurred during ICU stays. Healthcare facility–onset CDI in the ICU was defined as a newly positive PCR for the C. difficile toxin B gene from an unformed stool, with subsequent receipt of anti-CDI therapy. We analyzed PPIs and other exposures as time-varying covariates and used Cox proportional hazards modeling to adjust for demographics, comorbidities, and other clinical factors.
Results
Of 18,134 patients who met criteria for inclusion, 271 (1.5%) developed healthcare facility–onset CDI in the ICU. Receipt of antibiotics was the strongest risk factor for CDI (adjusted HR (aHR) 2.79; 95% confidence interval (CI), 1.50–5.19). There was no significant increase in risk for CDI associated with PPIs in those who did not receive antibiotics (aHR 1.56; 95% CI, 0.72–3.35), and PPIs were actually associated with a decreased risk for CDI in those who received antibiotics (aHR 0.64; 95% CI, 0.48–0.83). There was also no evidence of increased risk for CDI in those who received higher doses of PPIs.
Conclusions
Exposure to antibiotics was the most important risk factor for healthcare facility–onset CDI in the ICU. PPIs did not increase risk for CDI in the ICU regardless of use of antibiotics.
Keywords: Clostridium difficile infection, proton pump inhibitors, antibiotics, intensive care unit, critical illness, microbiome, pharmacoepidemiology, outcomes research
Introduction
Clostridium difficile infection (CDI) is a rising cause of healthcare-associated infections and is associated with worse outcomes (1, 2) and increased costs among hospitalized patients (3, 4). In the United States, there are an estimated 450,000 cases of CDI annually (5), and C. difficile causes 12% of all healthcare-associated infections (6). Patients hospitalized in intensive care units (ICUs) are at increased risk for CDI compared to other inpatients (7), and mortality rates for ICU patients with CDI exceed baseline ICU mortality rates (8).
Risk factors associated with the development of CDI have been studied extensively in the community (9–14) and among inpatients (15–21), but the risk factors for the onset of CDI among ICU patients have received less attention (22–26). Among hospitalized patients, established risk factors for incident CDI include older age and comorbid medical conditions such as impaired renal function and low serum albumin (27, 28). Potentially modifiable risk factors associated with hospital-onset CDI include receipt of antibiotics and receipt of proton pump inhibitors (PPIs) (29, 30).
Critically ill patients differ from patients hospitalized on a general medical or surgical floor. Clostridium difficile infection is the archetypal disease of the gastrointestinal microbiome, and loss of normal fecal microbial diversity often precedes CDI (31). Compared to other inpatients, ICU patients have very low fecal microbial diversity, which further declines during treatment in the ICU (32). Additionally, ICU patients are more likely to receive PPIs for stress ulcer prophylaxis or active gastrointestinal bleeding. In the ICU compared to other hospital locations, there is increased use of antibiotics, luminal penetration of antibiotics, gut wall edema, and derangements in gastrointestinal motility (33). For all of these reasons, traditional risk factors such as antibiotics and PPIs may have distinct relationships with risk for CDI when CDI arises in the ICU setting. We performed a retrospective cohort study to determine the crucial risk factors for healthcare facility–onset CDI in the ICU, focusing on PPIs. A priori, we hypothesized that PPIs are associated with increased risk for CDI in the ICU and that, when PPIs are given concomitantly with antibiotics, there is an additive or multiplicative increase in risk for CDI.
Methods
Study Population
We conducted a retrospective cohort study of all adult patients (≥ 18 years old at the time of admission) admitted to any one of 14 distinct ICUs at three affiliated university hospitals between January 1, 2010 and December 30, 2013. We chose 2010 for the study’s start date because it was the first full calendar year during which these institutions all utilized the stool PCR test for the C. difficile toxin B gene for the diagnosis of CDI. Patients were excluded from the study if they had an ICU stay of <3 days. Because the risk factors for recurrent CDI may differ from the risk factors for incident CDI, we also excluded patients if they had a positive stool test for C. difficile during the 90 days preceding ICU admission (19). If patients had multiple ICU admissions, only their first ICU admission was included in the analysis. Electronically available data obtained through the project Health Information Technology to Reduce Healthcare Associated Infections were used for cross-validation of our sample cohort. The study was approved by the institutional review boards at Columbia University Medical Center, the Allen Hospital, and Weill Cornell Medical College.
Healthcare facility-onset CDI in the ICU
CDI was defined as a newly positive stool test for C. difficile toxin B from an unformed stool followed by initiation of appropriate therapy (i.e. metronidazole or oral vancomycin) for CDI. For this study, the day of CDI onset was the day on which the positive stool sample was produced. Healthcare facility–onset CDI in the ICU was conservatively defined as CDI that occurred more than 3 days after admission to the ICU, consistent with current guidelines describing healthcare facility-onset infections as those occurring 48 to 72 hours after admission (34).
Risk Factors
We parsed electronic medical records and extracted risk factor information using automated queries. We examined the following potential risk factors for healthcare facility–onset CDI in the ICU: demographics; creatinine (continuous, mg/dL), white blood cell count (continuous, cells/μL and categorical, normal 3.5–9.1K vs. abnormal <3.5K or >9.1K) and albumin (continuous, g/dL) at the time of ICU admission; ICU type (medical, surgical, cardiac, neurological); ICU length of stay (days); and ICU therapies as categorical variables including mechanical ventilation, hemodialysis (including both continuous venovenous hemodialysis and intermittent hemodialysis), immunosuppressants (including systemic steroids at a minimum daily dose of 5 mg prednisone or equivalent, calcineurin inhibitors, anti-metabolites, anti-TNFα agents, or mycophenolic acid), antibiotics (classified as exposed/unexposed, number of individual antibiotics exposed, and additionally organized according to antibiotic class), H2 receptor antagonists (H2RAs, classified as exposed/unexposed) and PPIs. In the primary analysis, subjects were classified as exposed or not unexposed to PPIs based on receipt of an oral or intravenous PPI at any dose or duration. In an additional sensitivity analysis, we assessed for a dose-response effect between PPIs and healthcare facility-onset CDI in the ICU by classifying PPI exposure according to the average daily dose of PPIs received.
All medication exposures were classified based on when they were actually administered as opposed to when they were ordered; this information was retrieved from the computerized provider order entry system. Variables gathered at the time of ICU admission such as demographic information and comorbid conditions, were treated as time-fixed. Exposures that occurred during the ICU stay, including receipt of antibiotics and PPIs, were analyzed as time-varying covariates (classified as absent until the time of initial exposure following ICU admission and thereafter as present). This reflects the observation that risk for CDI remains steadily elevated for over 30 days following receipt of antibiotics or similar exposures (35).
Our electronic medical record uses standard International Classification of Diseases (ICD) coding to describe patient comorbidities. Our previous work has shown that ICD coding of patient comorbidities is often inaccurate compared with manual review (19) a problem that others have also described (36). To better capture patient comorbidities, we additionally used a natural language processing system that transforms clinical text pertaining to medical comorbidities into computable data, with methods we have described previously (37). This information was extracted in an automated manner as the components of the Charlson Comorbidity Index (CCI) (38).
Statistical Analysis
For continuous variables, we computed means (if data were normally distributed) or medians and interquartile ranges (IQRs), and compared these variables using Student’s t-test or the Mann Whitney U-test. We compared categorical variables using the chi-squared test. Our multivariable analysis was constructed using a Cox proportional hazards model with patients followed for 30 days or until death, ICU discharge, or the onset of CDI. To build the final model, we first tested for interactions between individual predictor variables and PPIs; when there was evidence of a significant interaction (P<0.05), we included the relevant variable along with an appropriate interaction term. Variables were then added stepwise to the final model and included if they had a significant relationship with healthcare facility–onset CDI in the ICU (P<0.05) or if they changed the β-coefficient representing PPIs by ≥10%. To assess for the possibility of death as a competing risk for CDI, we conducted a restriction analysis among the subset of patients who survived to ICU discharge or CDI. To determine whether inclusion of patients at risk for recurrent CDI would alter our findings, we conducted a sensitivity analysis including patients who were diagnosed with CDI within 90 days prior to ICU admission. Statistical analyses were performed using the statistical software Stata version 12. A P-value of <0.05 was considered statistically significant.
Results
Healthcare facility–onset CDI in the ICU
Of 39,535 unique adult patients admitted to intensive care during the study period, 18,134 met eligibility criteria and 271 of these (1.5%) developed healthcare facility–onset CDI in the ICU (Figure 1). The median interval between ICU admission and diagnosis of CDI was 7 days (IQR 5–11). Compared to ICU patients without CDI, those who developed CDI had increased mortality during the index ICU stay (23% vs 9% respectively, P<0.01) and the index hospital stay (30% vs 14% respectively, P<0.01).
Figure 1.
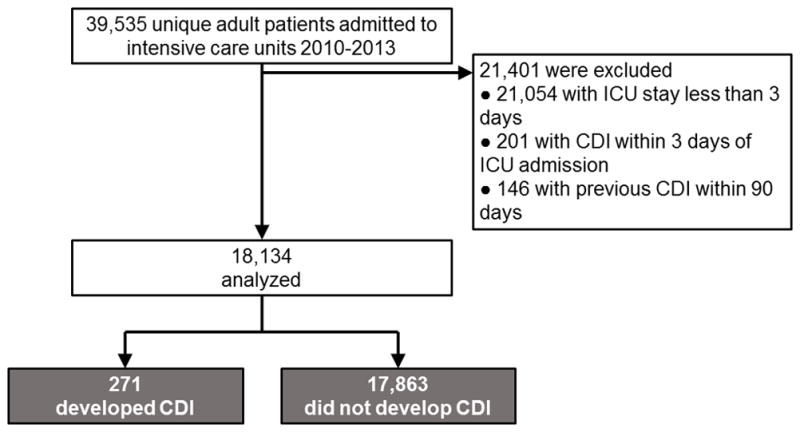
Flow of patients into the study
Baseline Risk Factors
At the time of admission to intensive care, those who later developed healthcare facility–onset CDI in the ICU had higher serum creatinine and lower serum albumin values than those who never developed CDI (P<0.01 for both, Table 1). CDI occurred more often in the neurological ICU than in other ICU types (P<0.01). The CCI score was significantly higher in those who developed CDI compared to those who did not (P<0.01), with cerebrovascular disease (CVD), chronic kidney disease, chronic obstructive pulmonary disease (COPD) and diabetes each associated with increased risk for CDI.
Table 1.
Characteristics at the time of admission to intensive care and risk for healthcare facility–onset Clostridium difficile infection in the ICU.
| Characteristic | All (%) | CDI (%) | No CDI (%) | P |
|---|---|---|---|---|
| Sex | 0.12 | |||
| Male | 10,160 (56.0) | 139 (51.3) | 10,021 (56.1) | |
| Female | 7,974 (44.0) | 132 (48.7) | 7,842 (43.9) | |
| Age* | 67 (55–78) | 66 (54–76) | 66 (54–76) | 0.25 |
| Race/ethnicity | 0.32 | |||
| White | 5,830 (32.2) | 86 (31.7) | 5,744 (32.2) | |
| Black | 1,252 (6.9) | 26 (9.6) | 1,226 (6.9) | |
| Hispanic | 1,938 (10.7) | 31 (11.4) | 1,907 (10.7) | |
| Other/Unknown | 9,114 (50.3) | 128 (47.2) | 8,986 (50.3) | |
| Admitted to ICU from hospital floor | 0.06 | |||
| Yes | 8,319 (45.9) | 109 (40.2) | 8,210 (46.0) | |
| No | 9,815 (54.1) | 162 (59.8) | 9,653 (54.0) | |
| Pre-ICU length of stay (days) | 2.1 ± 5.4 | 2.4 ± 5.3 | 2.1 ± 5.4 | 0.27 |
| ICU type | <0.01 | |||
| Medical | 4,292 (23.7) | 80 (29.5) | 4,212 (23.6) | |
| Surgical | 7,185 (39.6) | 82 (30.3) | 7,103 (39.8) | |
| Cardiac | 4,149 (22.9) | 50 (18.5) | 4,099 (23.0) | |
| Neurological | 2,508 (13.8) | 59 (21.8) | 2,449 (13.7) | |
| Serum creatinine (mg/dl) | 1.50 ±1.60 | 1.95 ± 2.19 | 1.50 ± 1.58 | <0.01 |
| Serum albumin (mg/dl) | 3.04 ± 0.68 | 2.94 ± 0.68 | 3.05 ± 0.68 | <0.01 |
| White blood cell count (x10−9/L) | 12.5 ± 11.0 | 13.4 ± 8.1 | 12.5 ± 11.0 | 0.20 |
| Abnormal (<3.5 or >9.1 x10−9/L) | 11,238 (67.7) | 185 (71.4) | 11,053 (67.6) | 0.19 |
| Normal (3.5–9.1 x10−9/L) | 5,373 (32.4) | 74 (28.6) | 5,299 (32.4) | |
| Comorbidities | ||||
| AIDS | 457 (2.5) | 11 (4.1) | 446 (2.5) | 0.10 |
| Cancer, local | 5,119 (28.2) | 71 (26.2) | 5,048 (28.3) | 0.45 |
| Cancer, metastatic | 1,125 (6.2) | 13 (4.8) | 1,112 (6.2) | 0.33 |
| Cerebrovascular accident | 4,508 (24.9) | 93 (34.3) | 4,415 (24.7) | <0.01 |
| Congestive heart failure | 6,587 (36.3) | 114 (42.1) | 6,473 (36.2) | 0.05 |
| Chronic kidney disease | 5,287 (29.2) | 113 (41.7) | 5,174 (29.0) | <0.01 |
| COPD | 3,898 (21.5) | 87 (32.1) | 3,811 (21.3) | <0.01 |
| Dementia | 698 (3.9) | 16 (5.9) | 682 (3.8) | 0.08 |
| Diabetes mellitus, uncomplicated | 3,594 (19.8) | 84 (31.0) | 3,510 (19.7) | <0.01 |
| Diabetes mellitus, with complications | 907 (5.0) | 18 (6.6) | 889 (5.0) | 0.21 |
| Hemiplegia | 1,677 (9.3) | 36 (13.3) | 1,641 (9.2) | 0.02 |
| Liver disease, mild | 942 (5.2) | 13 (4.8) | 929 (5.2) | 0.75 |
| Liver disease, moderate to severe | 827 (4.6) | 18 (6.6) | 809 (4.5) | 0.11 |
| Myocardial infarction prior | 4,889 (27.0) | 76 (28.0) | 4,813 (27.0) | 0.68 |
| Peptic ulcer disease | 880 (4.9) | 13 (4.8) | 867 (4.9) | 0.97 |
| Peripheral vascular disease | 2,729 (15.1) | 40 (14.8) | 2,689 (15.1) | 0.89 |
| Rheumatologic disease | 1,297 (7.2) | 13 (4.8) | 1,284 (7.2) | 0.13 |
| Charlson Comorbidity Index | 3.79 ± 3.08 | 4.60 ± 2.99 | 3.78 ± 3.08 | <0.01 |
| 0–2 | 7,217 (39.8) | 69 (25.5) | 7,148 (40.0) | <0.01 |
| 3+ | 10,917 (60.2) | 202 (74.5) | 10,715 (60.0) | |
Interval variables are reported as mean ± standard deviation, while counts are reported as count (percentage).
Age is reported as median (inter-quartile range).
ICU, intensive care unit; CDI, C. difficile infection.
Risk Factors During ICU Treatment
Mechanical ventilation, hemodialysis, and receipt of antibiotics were associated with healthcare facility–onset CDI in the ICU (Table 2). The antibiotic classes individually associated with significantly increased risk for CDI were β-lactam/β-lactamase inhibitors (including piperacillin-tazobactam, amoxicillin-clavulanate and ampicillin-sulbactam, combined aHR 1.71; 95% CI, 1.30–2.27) as well as intravenous (IV) vancomycin (aHR 1.42; 95% CI, 1.05–1.92). Fluoroquinolones were not significantly associated with CDI (aHR 1.03; 95% CI 0.67–1.59) nor were cephalosporins (aHR 0.77, 95% CI 0.56–1.07) (Supplementary Table 1). Rates of healthcare facility-onset CDI in the ICU increased as the number of concurrent antibiotics increased (0.5% in those who received no antibiotics, 1% in those who received only one antibiotic, and 2.6% in those who received two or more different antibiotics) but this trend was not significant when tested in our final model (p=0.71).
Table 2.
Characteristics during treatment in intensive care and risk for healthcare facility–onset Clostridium difficile infection in the ICU
| Risk Factors | All (%) | CDI (%) | No CDI (%) | P |
|---|---|---|---|---|
| Receipt of PPIs | 0.06 | |||
| Yes | 11,230 (61.9) | 155 (57.2) | 11,075 (62.0) | |
| No | 6,904 (38.1) | 116 (42.8) | 6,788 (38.0) | |
| Antibiotics | <0.01 | |||
| Yes | 12,239 (67.5) | 239 (88.2) | 12,000 (67.2) | |
| No | 5,895 (32.5) | 32 (11.8) | 5,863 (32.8) | |
| Mechanical ventilation | <0.01 | |||
| Yes | 8,791 (48.5) | 196 (72.3) | 8,595 (48.1) | |
| No | 9,343 (51.5) | 75 (27.7) | 9,268 (51.9) | |
| Hemodialysis | <0.01 | |||
| Yes | 1,528 (8.4) | 56 (20.7) | 1,472 (8.2) | |
| No | 16,606 (91.6) | 215 (79.3) | 16,391 (91.8) | |
| Immunosuppression | 0.55 | |||
| Yes | 4,744 (26.2) | 85 (31.4) | 4,659 (26.1) | |
| No | 13,390 (73.8) | 186 (68.6) | 13,204 (73.9) |
PPI, proton pump inhibitor; ICU, intensive care unit; CDI, C. difficile infection
The cumulative incidence of CDI was 0.4% in those who received no antibiotics or PPIs, 0.7% in those who received PPIs but no antibiotics, 2.7% in those who received antibiotics and no PPIs and 1.6% in those who received both antibiotics and PPIs (P<0.01, Figure 2). There was no evidence of a dose-based relationship between PPIs and CDI (Supplementary Table 2). Again, there was the suggestion that the relationship between PPIs and CDI depended in part on concurrent antibiotic exposure. Neither PPIs nor antibiotics were risk factors for worse outcomes after CDI as measured by 90-day mortality rates, which were 36% for those exposed to PPIs versus 33% for those not exposed (p=0.56) and 35% for those exposed to antibiotics versus 31% for those not exposed (p=0.66). Exposure to H2RAs without PPIs was rare in our cohort (6.4%) and there was no difference in rates of CDI between those who were and were not exposed to H2RAs (1.3% versus 1.8% respectively, p=0.27).
Figure 2.
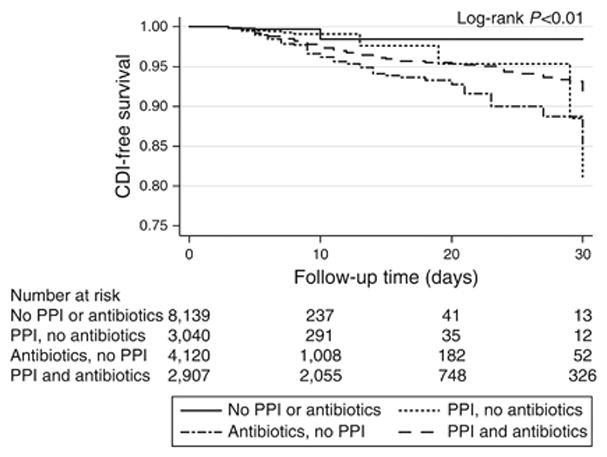
Kaplan-Meier plot showing CDI-free survival through 30 days, by antibiotic and PPI risk category
Multivariable Model
Our final multivariable model included ICU type, serum creatinine and serum albumin upon ICU admission, CCI, and receipt of ventilation, hemodialysis, antibiotics, and PPIs while in the ICU (Table 3). These relationships were similar when we analyzed only the subjects who survived to ICU discharge and also similar when we included in the analysis an additional 146 subjects who had been diagnosed with CDI within the 90 days prior to ICU admission. There was evidence of a significant interaction between antibiotics and PPIs (interaction term P=0.01) and PPIs were included in the multivariable model using an interaction term for antibiotics. There were no other significant interactions with PPIs. After stratifying by receipt of antibiotics, PPIs were not associated with increased risk for CDI in those who received antibiotics (aHR 0.64; 95% CI, 0.48–0.83) or in those who did not receive antibiotics (aHR 1.56; 95% CI, 0.72–3.35).
Table 3.
Final multivariable model of risk factors for healthcare facility-onset Clostridium difficile infection in the ICU.
| Risk Factors | Adjusted HR (95% CI) |
|---|---|
| ICU Type | |
| Medical | Ref |
| Surgical | 1.05 (0.76–1.46) |
| Cardiac | 0.89 (0.60–1.31) |
| Neurological | 1.50 (1.01–2.23) |
| Creatinine (per mg/dl increase) | 1.09 (1.02–1.16) |
| Albumin (per g/dl increase) | 0.93 (0.77–1.12) |
| Charlson comorbidity index | |
| 0–2 | Ref |
| 3+ | 1.34 (1.00–1.79) |
| Mechanical ventilation | 1.31 (0.96–1.77) |
| Hemodialysis | 1.14 (0.79–1.64) |
| Proton pump inhibitors | 1.29 (0.62–2.70) |
| Antibiotics | 2.79 (1.50–5.19) |
ICU, intensive care unit; HR, hazard ratio; CI, confidence interval. The model includes an interaction term for proton pump inhibitors and antibiotics.
Discussion
In a large, multi-center retrospective cohort of adult patients hospitalized in intensive care for 3 days or more, we found that serum creatinine at the time of ICU admission, admission to a neurological ICU, and receipt of antibiotics were associated with healthcare facility–onset CDI in the ICU. Receipt of PPIs was not significantly associated with CDI in the intensive care setting, and this remained true when we examined patients who received PPIs at higher total doses. There was evidence that antibiotics modified the PPI-CDI relationship.
Antibiotics predispose to colonization and infection by C. difficile through alterations in the intestinal flora (39). In our study, certain broad-spectrum antibiotics with anaerobic and Gram-negative coverage were associated with increased risk for CDI. Cephalosporins and fluoroquinolones, which have previously been associated with CDI, were not independently associated with CDI in this cohort (23–25, 40–43). In the ICU setting compared to other settings, luminal penetration of antibiotics and other factors may fundamentally change the relationship between certain antibiotic classes and risk for CDI. Interestingly, intravenous vancomycin, a relatively narrow spectrum antibiotic affecting Gram-positive organisms such as C. difficile, was associated with CDI. Previous studies in hospitalized patients have had similar findings (44). The dynamics of C. difficile proliferation are complex, and C. difficile proliferation may be promoted by relatively brief courses of antibiotics such as vancomycin that are subsequently discontinued (45). A recent study in mice demonstrated that treatment with oral vancomycin markedly disrupts the microbiome and enables colonization by nosocomial pathogens (46). Our study was not designed to address the dynamics of antibiotic use and risk for CDI in the ICU.
Antibiotics alter the colonic microbiome and reduce fecal microbial diversity. PPIs may also alter the colonic microbiome and thus may influence risk for CDI. Fecal microbial diversity is reduced in patients who subsequently develop CDI (31) and patients admitted to the ICU have extraordinarily low fecal microbial diversity and a high risk for CDI (32). However, the data regarding PPIs and fecal microbial diversity is mixed. A study using 16S rRNA sequencing showed lower total sequence counts for bacteria in fecal samples from patients taken after initiation of PPIs but no changes in fecal microbial diversity (47). Two larger studies found that PPIs were associated with decreased fecal microbial diversity (48, 49) and a population-based Dutch study found that PPIs explained a greater degree of inter-individual variability in the fecal microbiome than any other single medication (50). A prospective crossover trial of PPIs found no within-individual change in fecal microbial diversity after PPIs but did find changes in specific bacterial taxa that have previously been associated with CDI (51). In sum, most experimental evidence suggests that PPIs probably do change the colonic microbiome but that these changes are relatively subtle.
In this study, we found no evidence that PPIs increased risk for CDI in the ICU setting of low baseline colonic microbial diversity and we found an inverse association between PPIs and CDI among those on antibiotics. Overall, our data are consistent with experimental data showing that any effect of PPIs on the fecal microbiome and consequently on risk for CDI is modest compared to the effect of antibiotics. When patients are receiving concurrent antibiotics, the effect of PPIs on specific colonic bacteria may be overwhelmed. In fact, PPIs were associated with reduced risk for CDI in patients who were receiving antibiotics in our study. Because PPIs appear to increase predominantly Gram positive, aerobic organisms, these same bacteria may confer a protective effect against CDI after antibiotic-induced depletion of colonic anaerobes (51, 52). Future studies should test the hypothesis that the effect of PPIs on risk for CDI is mediated by the underlying colonic microbial composition.
In meta-analyses, PPIs have been associated with healthcare facility-onset CDI but there are little data focused exclusively on ICU patients, and the existing data are mixed. Several ICU-based studies found that PPIs were associated with CDI (53–55) whereas other studies did not not (22, 40). Although these studies adjusted for concurrent use of antibiotics, none of them performed a stratified analysis to test whether the nature of the relationship between PPIs and CDI differed in patients based on whether or not they received concurrent antibiotics. One study conducted in inpatients suggested that the risk for CDI associated with PPIs was more pronounced in those who were not taking antibiotics compared to those who were taking high-risk antibiotics (56). MacLaren et al used propensity score-adjusted models to compare patients who received PPIs to those who received H2RAs in the ICU and found a small but statistically significant 29% relative increase in risk for CDI associated with PPIs (55). In our data, ICU patients selected to receive H2RAs had lower rates for CDI than patients who received no acid suppression. Patients selected to receive H2RAs instead of PPIs in the ICU may have uniquely low risk for CDI because these patients may fundamentally differ from other ICU patients in ways that are difficult or impossible to adjust for adequately. We found no association between PPIs and risk for CDI in our study, which compared patients who received PPIs to all other patients.
Elevated serum creatinine and admission to a neurological ICU were associated with increased risk for CDI. Impaired renal function has been shown to induce alterations in tight junctions between intestinal epithelial cells, intestinal barrier function, and the gastrointestinal microbiome (57). Renal dysfunction has also been associated with CDI in the ICU (25, 43). Finally, our study found that ICU type may affect the risk for CDI. This may reflect purely local conditions—only two neurological ICUs were included in this study—or the effect of unmeasured neurological risk factors.
Our study has multiple strengths. It is the largest study of healthcare facility–onset CDI in the ICU to date, with data from several institutions and ICUs. We used automated capture of key exposure and demographic variables including patient comorbidities, which permitted a cohort rather than a case-control study design. Finally, we conducted time-varying analyses to assess exposures that occurred within the ICU, which best reflects current understanding of the mechanism linking PPIs and other exposures to CDI. Our study also has limitations. We were unable to assess for all potential covariates that may impact risk for CDI, including the presence or absence of non-C. difficile infections (40). We could not accurately ascertain the indications for particular antibiotics. Data to compute traditional scores for severity of illness in the ICU (e.g., APACHE or SAPS) were not readily accessible, and instead we used the Charlson Comorbidity Index to approximate the burden of comorbid diseases (58). Our ability to capture recent CDI at other institutions was limited. However, when we reanalyzed our data including patients with known prior CDI, there was no substantive difference in our findings.
In conclusion, in this large study of patients admitted to any one of 14 ICUs for > 3 days, exposure to antibiotics was the dominating risk factor associated with healthcare facility–onset CDI in the ICU. The relationship between PPIs and CDI was modified by exposure to antibiotics, but PPIs were not associated with significantly increased risk for CDI in either those who did or in those who did not receive antibiotics. Concern for increased risk for CDI should not play a role in the decision of whether or not to use PPIs in the ICU.
Supplementary Material
STUDY HIGHLIGHTS.
1. WHAT IS CURRENT KNOWLEDGE
Intensive care unit (ICU) patients who acquire Clostridium difficile infection (CDI) have high morbidity and mortality
Proton pump inhibitors (PPIs) are a modifiable risk factor for healthcare facility-onset CDI
2. WHAT IS NEW HERE
After adjusting for comorbidities and other factors, proton pump inhibitors did not increase the risk of CDI in a large cohort of ICU patients
Even among ICU patients who received the highest doses of PPIs, there was still no risk for healthcare facility-onset CDI associated with PPIs
Among antibiotic classes, β-lactam/β-lactamase inhibitor combination antibiotics were most strongly associated with increased risk for healthcare facility-onset CDI in the ICU
Acknowledgments
Financial support: Dr. Freedberg was supported by a Research Scholar Award from the American Gastroenterological Association and by the National Center for Advancing Translational Sciences, National Institutes of Health, (KL2 TR000081, formerly KL2 RR024157). Data was obtained in part through the project Health Information Technology to Reduce Healthcare Associated Infections (NIH-R01NR010822 to Dr. Larson). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: David M. Faleck, MD
Specific author contributions: Conception and design: DMF, EYF, JAA, DEF. Data collection: DMF, HS, ELL, DEF. Analysis and interpretation: DMF, HS, JAA, DEF. Drafting the manuscript for important intellectual content: DMF, HS, EYF, ELL, JAA, DEF. All authors have approved the final manuscript.
Potential competing interests: None.
References
- 1.Dubberke ER, Butler AM, Reske KA, et al. Attributable outcomes of endemic Clostridium difficile–associated disease in nonsurgical patients. Emerging infectious diseases. 2008;14:1031. doi: 10.3201/eid1407.070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lagu T, Stefan MS, Haessler S, et al. The impact of hospital-onset Clostridium difficile infection on outcomes of hospitalized patients with sepsis. Journal of Hospital Medicine. 2014;9:411–417. doi: 10.1002/jhm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghantoji S, Sail K, Lairson D, et al. Economic healthcare costs of Clostridium difficile infection: a systematic review. Journal of Hospital Infection. 2010;74:309–318. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Gabriel L, Beriot-Mathiot A. Hospitalization stay and costs attributable to Clostridium difficile infection: a critical review. J Hosp Infect. 2014;88:12–21. doi: 10.1016/j.jhin.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–34. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magill SS, Edwards JR, Bamberg W, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. New England Journal of Medicine. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobo LD, Dubberke ER. Recognition and prevention of hospital-associated enteric infections in the intensive care unit. Crit Care Med. 2010;38:S324–34. doi: 10.1097/CCM.0b013e3181e69f05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lofgren ET, Cole SR, Weber DJ, et al. Hospital-acquired Clostridium difficile infections: estimating all-cause mortality and length of stay. Epidemiology. 2014;25:570–5. doi: 10.1097/EDE.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins CE, Ayturk MD, Flahive JM, et al. Epidemiology and outcomes of community-acquired Clostridium difficile infections in Medicare beneficiaries. J Am Coll Surg. 2014;218:1141–1147. e1. doi: 10.1016/j.jamcollsurg.2014.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshpande A, Pasupuleti V, Thota P, et al. Community-associated Clostridium difficile infection and antibiotics: a meta-analysis. J Antimicrob Chemother. 2013;68:1951–61. doi: 10.1093/jac/dkt129. [DOI] [PubMed] [Google Scholar]
- 11.Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, Exposure to Medications, and the Risk of Community-Acquired Clostridium difficile Infection: A Systematic Review and Meta-analysis. Infect Control Hosp Epidemiol. 2015;36:132–41. doi: 10.1017/ice.2014.39. [DOI] [PubMed] [Google Scholar]
- 12.Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist. 2014;7:63–72. doi: 10.2147/IDR.S46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soes LM, Holt HM, Bottiger B, et al. Risk factors for Clostridium difficile infection in the community: a case-control study in patients in general practice, Denmark, 2009–2011. Epidemiol Infect. 2014;142:1437–48. doi: 10.1017/S0950268813002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taori SK, Wroe A, Hardie A, et al. A prospective study of community-associated Clostridium difficile infections: the role of antibiotics and co-infections. J Infect. 2014;69:134–44. doi: 10.1016/j.jinf.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365:1693–703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 16.Barbut F, Corthier G, Charpak Y, et al. Prevalence and pathogenicity of Clostridium difficile in hospitalized patients. A French multicenter study. Arch Intern Med. 1996;156:1449–54. [PubMed] [Google Scholar]
- 17.Campbell KA, Phillips MS, Stachel A, et al. Incidence and risk factors for hospital-acquired Clostridium difficile infection among inpatients in an orthopaedic tertiary care hospital. J Hosp Infect. 2013;83:146–9. doi: 10.1016/j.jhin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Dial S, Alrasadi K, Manoukian C, et al. Risk of Clostridium difficile diarrhea among hospital inpatients prescribed proton pump inhibitors: cohort and case-control studies. Cmaj. 2004;171:33–8. doi: 10.1503/cmaj.1040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedberg DE, Salmasian H, Friedman C, et al. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol. 2013;108:1794–801. doi: 10.1038/ajg.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao K, Micic D, Chenoweth E, et al. Poor functional status as a risk factor for severe Clostridium difficile infection in hospitalized older adults. J Am Geriatr Soc. 2013;61:1738–42. doi: 10.1111/jgs.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shivashankar R, Khanna S, Kammer PP, et al. Clinical factors associated with development of severe-complicated Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11:1466–71. doi: 10.1016/j.cgh.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Cai L, Yu R, et al. ICU-Onset Clostridium difficile infection in a university hospital in China: a prospective cohort study. PLoS One. 2014;9:e111735. doi: 10.1371/journal.pone.0111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ang CW, Heyes G, Morrison P, et al. The acquisition and outcome of ICU-acquired Clostridium difficile infection in a single centre in the UK. J Infect. 2008;57:435–40. doi: 10.1016/j.jinf.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Micek ST, Schramm G, Morrow L, et al. Clostridium difficile infection: a multicenter study of epidemiology and outcomes in mechanically ventilated patients. Crit Care Med. 2013;41:1968–75. doi: 10.1097/CCM.0b013e31828a40d5. [DOI] [PubMed] [Google Scholar]
- 25.Musa SA, Moran C, Thomson SJ, et al. Clostridium difficile-associated disease acquired in the cardiothoracic intensive care unit. J Cardiothorac Vasc Anesth. 2011;25:263–7. doi: 10.1053/j.jvca.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Bobo LD, Dubberke ER, Kollef M. Clostridium difficile in the ICU: the struggle continues. Chest. 2011;140:1643–53. doi: 10.1378/chest.11-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for Diagnosis, Treatment, and Prevention of Clostridium difficile Infections. Am J Gastroenterol. 2013;108:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 28.Balihar K, Kozak F, Kozeluhova J, et al. Clostridium difficile infection in hospitalized patients at a Czech tertiary center: analysis of epidemiology, clinical features, and risk factors of fulminant course. European journal of gastroenterology & hepatology. 2014;26:880–887. doi: 10.1097/MEG.0000000000000139. [DOI] [PubMed] [Google Scholar]
- 29.Kwok CS, Arthur AK, Anibueze CI, et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107:1011–9. doi: 10.1038/ajg.2012.108. [DOI] [PubMed] [Google Scholar]
- 30.Janarthanan S, Ditah I, Adler DG, et al. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107:1001–10. doi: 10.1038/ajg.2012.179. [DOI] [PubMed] [Google Scholar]
- 31.Vincent C, Stephens DA, Loo VG, et al. Reductions in intestinal Clostridiales precede the development of nosocomial Clostridium difficile infection. Microbiome. 2013;1:18. doi: 10.1186/2049-2618-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaborin A, Smith D, Garfield K, et al. Membership and behavior of ultra-low-diversity pathogen communities present in the gut of humans during prolonged critical illness. MBio. 2014;5:e01361–14. doi: 10.1128/mBio.01361-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ukleja A. Altered GI motility in critically Ill patients: current understanding of pathophysiology, clinical impact, and diagnostic approach. Nutr Clin Pract. 2010;25:16–25. doi: 10.1177/0884533609357568. [DOI] [PubMed] [Google Scholar]
- 34.CDC. Multidrug-Resistant Organism & Clostridium difficile Infection (MDRO/CDI) Module. 2015 [cited 2015 2 Feb]; Available from: http://www.cdc.gov/nhsn/PDFs/pscManual/12pscMDRO_CDADcurrent.pdf.
- 35.Hensgens MP, Goorhuis A, Dekkers OM, et al. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67:742–8. doi: 10.1093/jac/dkr508. [DOI] [PubMed] [Google Scholar]
- 36.Szeto HC, Coleman RK, Gholami P, et al. Accuracy of computerized outpatient diagnoses in a Veterans Affairs general medicine clinic. Am J Manag Care. 2002;8:37–43. [PubMed] [Google Scholar]
- 37.Salmasian H, Freedberg DE, Friedman C. Deriving comorbidities from medical records using natural language processing. J Am Med Inform Assoc. 2013;20:e239–42. doi: 10.1136/amiajnl-2013-001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology. 2014;146:1547–53. doi: 10.1053/j.gastro.2014.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawrence SJ, Puzniak LA, Shadel BN, et al. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28:123–30. doi: 10.1086/511793. [DOI] [PubMed] [Google Scholar]
- 41.Marra AR, Edmond MB, Wenzel RP, et al. Hospital-acquired Clostridium difficile-associated disease in the intensive care unit setting: epidemiology, clinical course and outcome. BMC Infect Dis. 2007;7:42. doi: 10.1186/1471-2334-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenneally C, Rosini JM, Skrupky LP, et al. Analysis of 30-day mortality for clostridium difficile-associated disease in the ICU setting. Chest. 2007;132:418–24. doi: 10.1378/chest.07-0202. [DOI] [PubMed] [Google Scholar]
- 43.Shaughnessy MK, Micielli RL, DePestel DD, et al. Evaluation of hospital room assignment and acquisition of Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:201–6. doi: 10.1086/658669. [DOI] [PubMed] [Google Scholar]
- 44.Dubberke ER, Reske KA, Yan Y, et al. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45:1543–9. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 45.Stein RR, Bucci V, Toussaint NC, et al. Ecological modeling from time-series inference: insight into dynamics and stability of intestinal microbiota. PLoS Comput Biol. 2013;9:e1003388. doi: 10.1371/journal.pcbi.1003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis BB, Buffie CG, Carter R, et al. Loss of microbiota-mediated colonization resistance to Clostridium difficile infection is greater following oral vancomycin as compared with metronidazole. J Infect Dis. 2015 doi: 10.1093/infdis/jiv256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seto CT, Jeraldo P, Orenstein R, et al. Prolonged use of a proton pump inhibitor reduces microbial diversity: implications for Clostridium difficile susceptibility. Microbiome. 2014;2:42. doi: 10.1186/2049-2618-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jackson MA, Goodrich JK, Maxan ME, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–56. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–8. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352:565–9. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freedberg DE, Toussaint NC, Chen SP, et al. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosen R, Hu L, Amirault J, et al. 16S community profiling identifies proton pump inhibitor related differences in gastric, lung, and oropharyngeal microflora. J Pediatr. 2015;166:917–23. doi: 10.1016/j.jpeds.2014.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barletta JF, Sclar DA. Proton pump inhibitors increase the risk for hospital-acquired Clostridium difficile infection in critically ill patients. Crit Care. 2014;18:714. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buendgens L, Bruensing J, Matthes M, et al. Administration of proton pump inhibitors in critically ill medical patients is associated with increased risk of developing Clostridium difficile–associated diarrhea. Journal of Critical Care. 2014;29:696.e11–696.e15. doi: 10.1016/j.jcrc.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 55.MacLaren R, Reynolds PM, Allen RR. Histamine-2 receptor antagonists vs proton pump inhibitors on gastrointestinal tract hemorrhage and infectious complications in the intensive care unit. JAMA Intern Med. 2014;174:564–74. doi: 10.1001/jamainternmed.2013.14673. [DOI] [PubMed] [Google Scholar]
- 56.Howell MD, Novack V, Grgurich P, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–90. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 57.Sabatino A, Regolisti G, Brusasco I, et al. Alterations of intestinal barrier and microbiota in chronic kidney disease. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu287. [DOI] [PubMed] [Google Scholar]
- 58.Christensen S, Johansen MB, Christiansen CF, et al. Comparison of Charlson comorbidity index with SAPS and APACHE scores for prediction of mortality following intensive care. Clinical Epidemiology. 2011;3:203–211. doi: 10.2147/CLEP.S20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


