Abstract
Introduction
The significant symptom overlap between progressive supranuclear palsy (PSP) and other parkinsonian neurodegenerative diseases frequently results in misdiagnosis. However, neuroimaging can be used to quantify disease-related morphological changes and specific markers. The cerebral peduncle angle (CPA) has been shown to differentiate clinically diagnosed PSP from other parkinsonian diseases but this result has yet to be confirmed in autopsy-proven disease.
Methods
Magnetic resonance imaging (MRI) scans were obtained for 168 patients representing 69 medical facilities. Following randomization, the images were divided into two groups (Type 1 and Type 2) based upon midbrain morphological differences. Two readers were blinded and independently measured the CPA of 146 patients with autopsy-proven progressive supranuclear palsy (PSP; n=54), corticobasal degeneration (n=16), multiple system atrophy (MSA; n=11) and Lewy body disease (n=65).
Results
Applying two separate measurement techniques revealed no statistically significant differences in CPA measurements among any study groups regardless of classification measurement approach. The interobserver agreement showed significant differences in measurements using the Type 2 approach.
Conclusion
Measuring the CPA on MRI is not a reliable way of differentiating among patients with PSP, corticobasal degeneration, MSA, or Lewy body disease.
Keywords: magnetic resonance imaging, cerebral peduncle angle, neurodegenerative diseases, PSP-Richardson Syndrome, multiple system atrophy, Parkinson’s disease, corticobasal degeneration, Lewy body disease
Introduction
Clinically diagnosing progressive supranuclear palsy (PSP) and other parkinsonian-like neurodegenerative diseases poses a challenge to practitioners. Clinical heterogeneity within disease populations makes reliance on the presence of pathognomonic features highly unreliable. For example, the absence of down-gaze palsy has been reported to result in the misdiagnosis in up to 60% of pathologically confirmed PSP patients [1–3]. Significant treatment differences among parkinsonian diseases have motivated the search for objective evidence to differentiate these diseases. Gross neuropathological changes including atrophy of the superior cerebellar peduncles and midbrain tegmentum are present in PSP but absent in multiple system atrophy (MSA) and Parkinson disease (PD) [4–7]. Previous imaging studies have attempted to quantify these changes [8–19]. However, many of these measurement techniques are cumbersome and time consuming [8–15]. The techniques that are more practical have been difficult to objectively quantify or have not been sufficiently confirmed with autopsy-proven subjects [17–18].
Atrophy of the midbrain tegmentum presents as changes in the interpeduncular cistern, which is the region between the cerebral peduncles. Yuki et al. observed that patients with PSP had interpeduncular cistern enlargement, which was later shown to be progressive [16, 21]. Fatterpekar and colleagues quantified midbrain atrophy by calculating an angle between the cerebral peduncles on magnetic resonance imaging (MRI). They showed the cerebral peduncle angle (CPA) to be significantly larger in clinically diagnosed PSP-Richardson Syndrome (PSP-RS) patients compared to MSA patients and PD patients and healthy controls [22]. Their study included patients diagnosed with MSA and PD according to Gilman et al. and Gelb et al., respectively [23, 24]. Given the difficulty of determining the clinical diagnosis of these diseases, their study groups may have consisted of heterogeneous conditions. We attempted to validate the CPA measurement technique for differentiating PSP from other neurodegenerative diseases by comparing magnetic resonance imaging (MRI) scans from autopsy-proven cases of PSP, corticobasal degeneration (CBD), MSA, and Lewy body disease (LBD).
Materials and Methods
Image Selection
Initial inclusion criteria required that all patients have pathologically confirmed disease. Medical records were acquired from 107 medical centers for 328 patients with autopsy-proven PSP (n=121), CBD (n=24), MSA (n=28) and LBD (n=155). Of these, MRIs of the brain were available for 168 patients; some had multiple MRIs, and in these cases, the most recent scan was used. T2-weighted imaging was the preferred MRI modality; however, in cases where T2-weighted imaging was not available, fluid attenuation inversion recovery (FLAIR) was preferred followed by Fast Spin Echo or Dual Echo. Axial images showing the midbrain were identified. In most cases, there were multiple levels at which the midbrain was visible. Slice level limits were used to specify and standardize the midbrain level for assessment. In the caudal direction, images where the pons was visible were excluded. In the rostral direction, images containing mammillary bodies were excluded. Images were also excluded if the midbrain interpeduncular cistern contour was too irregular for accurate measurement. Approval for this retrospective study was obtained from the Mayo Clinic institutional review board.
Measurement
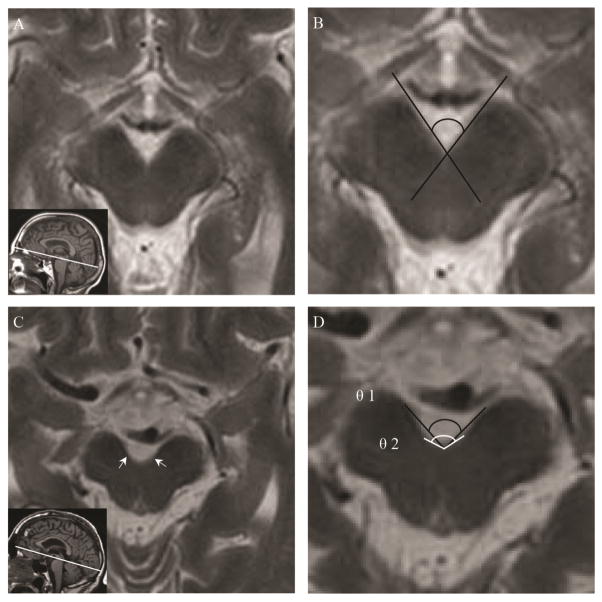
Despite the aforementioned rostral and caudal slice limits, there remained variability among axial slice levels resulting in contour variation of the medial borders of the midbrain peduncles. While rostral images consistently have linear medial peduncular borders, the contour becomes increasingly curvilinear as the slice level moves caudally. Therefore, a method of classification was developed to direct the measurement approach. Midbrain sections were classified based on the presence of bilateral inflection points (where the degree of curvature visibly changes) on the medial sides of the cerebral peduncles. Midbrain sections without a bilateral inflection point (i.e. those with a unilateral inflection point, or those with no inflection point on either side) were classified as Type 1 (Figure 1A, B) and those with a bilateral inflection point were classified as Type 2 (Figure 1C, D). Rostral images had the tendency to meet the Type 1 criteria, while caudal images tended to meet Type 2 criteria. CPAs were obtained for Type 1 sections using an intersecting line method. Two lines were drawn by independent readers using a function of the QREADS imaging software (Mayo Clinic). Each line was parallel to a medial border of the midbrain peduncle, resulting in an angle (Figure 1B). Due to the curvilinear nature of caudal slices (Type 2) two angles were obtained using three points for each angle (Figure 1D). Type 2 θ1 was formed with a vertex at the posteriormost midline point of the interpeduncular cistern and the two remaining points on the anteriormost medial aspect of the cerebral peduncles. Type 2 θ2 was formed using the same vertex as θ1 and the bilateral inflections points on the medial aspect of the peduncles. Measurements were made at magnification of 200%. Two readers independently measured all images meeting inclusion criteria. Readers applied the techniques described above. Readers were blinded to the patient’s diagnosis and the other reader’s measurements. Reader 1 was a neurology trainee and reader two was a practicing neurologist. Readers practiced measuring the CPA together for several months prior to makes measurements for this study. Both readers agreed upon the type classification of each midbrain slice. The measuring approach was supervised and approved by a fellowship-trained practicing neuroradiologist.
Fig. 1.
Measurement technique. A, T2-weighted MR image showing midbrain classified as Type 1. Inset is a T1-weighted MR image showing axial plane (white line). B, Magnified image A showing Type 1 measurement technique (black lines). C, T2-weighted MR image showing midbrain classified as Type 2. White arrows pointing to inflection points. Inset is a T1-weighted MR image showing axial plane (white line). D, Magnified image C showing Type 2 measurement technique. θ1 (black line), θ2 (white line).
Analysis
Data for both readers was analyzed separately. Mean angle measurements were calculated for each autopsy-proven neurodegenerative disease group and segregated by midbrain classification (Type 1, Type 2 θ1, Type 2 θ2). Mean angles were compared by an analysis of variance (ANOVA). The mean ages of each disease group were compared by ANOVA, and a Student’s t-test was used for paired group comparison. We used the Bland-Altman method for interobserver analysis. All statistical analyses were performed using Microsoft Excel and JMP (SAS Institute Inc., Cary, NC, USA).
Results
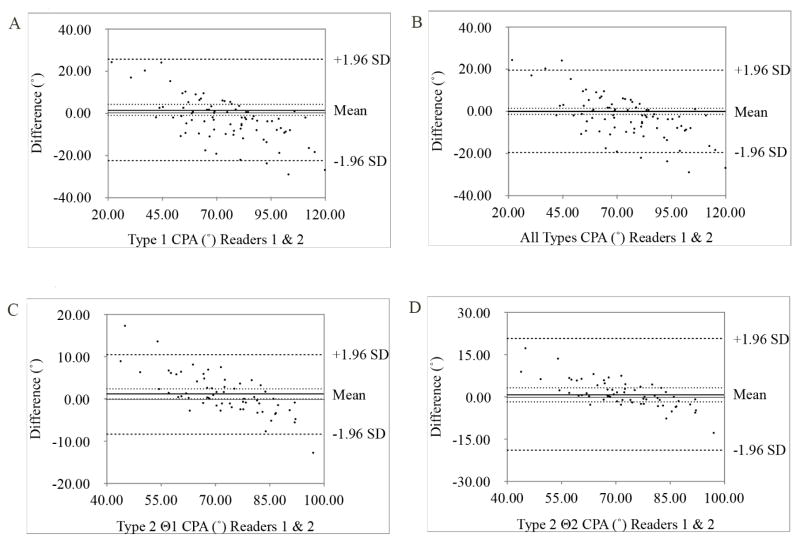
Of the 168 patients with MRI scans, 22 were excluded for the following reasons: 17 did not have an axial image with a slice level rostral to the pons and caudal to the mammillary bodies, and five had poor image quality. The 146 patients analyzed were stratified by diagnosis: PSP (n=54), CBD (n=16), MSA (n=11) and LBD (n=65). The results from the ANOVA of the disease group ages revealed a significant difference (P = 0.027). Paired comparisons isolated this significance to only LBD vs. MSA (p =0.021) and LBD vs. CBD (P = 0.020). Patients with PSP had a mean age of 71 years, which was not significantly different (P <0.05) from any other disease group. Slice thickness and spacing was obtained for every patient except one for whom slice spacing was not available. The mean slice thickness was 4.31 mm (standard deviation [SD]: 0.91 mm) and mean slice spacing was 0.39 mm (SD: 1.02 mm). Among study participants, 83 and 63 midbrain sections were measured using either the Type 1 and Type 2 approach, respectively. Table 1 displays the measurements for autopsy-confirmed groups of PSP, CBD, MSA, and LBD patients. No significant differences existed between the means. A Bland Altman plot was constructed for each measurement type to assess interobserver agreement between the two readers (Figure 2). There was no significant difference in interobserver measurements for Type 1, Type 2 θ2, or both types as a whole; however, the measurements for Type 2 θ1 were significantly different (p <0.044).
Table 1.
Cerebral Peduncle Angle Measurements
| Reader | PSP (n=54) | CBD (n=16) | MSA (n=11) | LBD (n=65) | P | |
|---|---|---|---|---|---|---|
| Type 1 (n=83) | 1 | 67.7° (14.9°) | 64.8° (15.6°) | 73.6° (22.9°) | 70.2° (18.2°) | 0.7 |
| 2 | 70.1° (14.0°) | 65.4° (19.2°) | 72.9° (20.0°) | 71.5° (17.0°) | 0.8 | |
| Average | 68.9° (14.4°) | 65.1° (16.9°) | 73.2° (20.8°) | 70.8° (18.0°) | 0.5 | |
|
| ||||||
| Type 2 θ1 (n=63) | 1 | 75.4° (7.3°) | 79.5° (4.2°) | 71.9° (5.5°) | 73.4° (9.4°) | 0.3 |
| 2 | 72.7° (8.8°) | 78.2° (5.7°) | 71.7° (5.5°) | 73.1° (10.1°) | 0.5 | |
| Average | 74.1° (8.1°) | 78.9° (4.9°) | 71.8° (4.9°) | 73.2° (9.6°) | 0.1 | |
|
| ||||||
| Type 2 θ2 (n=63) | 1 | 107.7° (12.7°) | 102.3° (7.1°) | 95.1° (8.5°) | 103.2° (13.2°) | 0.3 |
| 2 | 106.3° (12.0°) | 104.1° (12.7°) | 101.1° (7.4°) | 101.0° (12.0°) | 0.5 | |
| Average | 107° (12.2°) | 103.2° (10.0°) | 98.1° (7.8°) | 102.1° (13.0°) | 0.1 | |
Fig. 2.
Interobserver agreement. Graphs show Bland-Altman limits of agreement for measurement of cerebral peduncle angle (CPA) between readers 1 and 2. CPAs from both observers are plotted against the difference between readers’ measurements. Thin black lines represent zero difference, thick black lines represent the mean difference, dotted lines represent 95% confidence interval of the mean, dashed lines represent 95% limits of agreement. Interobserver variability for A, Type 1 1.5° (−1.2°, 4.1°) B, All Types 0.09° (−1.3°, 1.4°) C, Type 2 θ1 1.3 (0.0°, 2.4°) and D, Type 2 θ2 1.0° (−1.5°, 3.5°).
Discussion
All of our study subjects had pathologically confirmed disease, and the number of subjects was larger than that of the Fatterpekar et al (PSP-RS, n=15; MSA, n=15; PD, n=22) [22]; however, we could not verify the utility of the CPA measurement for differentiating PSP from other neurodegenerative diseases. Although atrophy occurs in the tegmentum of the midbrain and the superior cerebellar peduncles in PSP patients [4, 5], it is unclear if the CPA widens as atrophy progresses. If the pattern of tegmental atrophy was spatially heterogenous, then the resultant midbrain morphology could be heterogenous. Moreover, if the atrophy was more pronounced in the lateral tegmentum, then a widening CPA could be expected; however, if atrophy was mostly in the medial tegmentum, then the CPA may decrease. As tegmental atrophy progresses, the cerebral peduncles seem to invaginate toward the tegmentum, which may result in a narrower CPA. In fact, it has been suggested that the interpeduncular cistern enlarged toward the aqueduct as a result of this invagination in pathologically confirmed PSP patients [21]. Serial evaluation of the CPA in each patient would be warranted to demonstrate actual temporal changes of the CPA in PSP.
A major strength of our study is the large data set that consists entirely of pathologically confirmed cases of neurodegenerative disease. When assessing the quality of a new diagnostic metric it is absolutely necessary to compare it against the gold standard, which in this case is the histological assessment of these parkinsonian/neurodegenerative cases.
Our study was limited by the image criteria we used. Fatterpekar et al. reformatted and standardized T1-weighted axial images along the anterior commissure-posterior commissure line [22]. To maximize external validity, we used unstandardized axial MRI images on several sequences; however, the less stringent image criteria allowed differences in axial plane level and subsequently cerebral peduncular contour heterogeneity. These criteria forced us to classify CPAs into two categories according to midbrain morphology. Significant interobserver differences suggest that Type 2 θ1 is an unreliable metric for assessing the CPA. In type 2 θ1, the selection of two points on the anteriormost medial aspect of the cerebral peduncles was subjective due to the peduncular contour irregularities. This low precision measurement might produce a significant difference in measuring type 2 θ1 between two readers (Fig. 2). However, the lack of stringent image standardization techniques is representative of what clinicians have at their disposal. This real world testing increases the generalizability of our results. Another limitation was the point at which the MRI was taken. We used the most recent MRI scans assuming midbrain atrophy was more prominent with disease progression. However, the interval between the onset of disease and the time of MRI evaluation may be variable among study subjects. The CPA of a subject whose MRI was taken early in the disease may not reflect midbrain atrophy to the degree of one with more advanced disease. Last, unlike Fatterpekar et al. we did not separate patients with PSP-RS from our PSP group. It is possible that patients with PSP-RS could demonstrate a difference in CPA measurements compared to those who have CBD, MSA, or LBD.
Conclusion
Our results indicate that CPA measurements are unreliable and impractical for differentiating PSP from other neurodegenerative diseases.
Highlights.
There is variability in cerebral peduncular morphology on non-standardized MRI.
Two techniques for measuring the CPA are proposed to address this variability.
The CPA is not reliable in differentiating PSP from other parkinsonian-like diseases.
Acknowledgments
We thank Audrey Strongosky for coordinating the study and ensuring adherence to the institutional review board standards. We also thank Kelly Viola for her editorial and submission assistance.
Footnotes
Author Contributions
PT measured CPA in all images, analyzed the data, and authored much of the manuscript. TK measured all CPA images and authored much of the manuscript. DB supervised all measurements and edited the manuscript. DD provided autopsy-confirmed disease data. ZW supervised the project and edited the manuscript.
Previous Presentations: This study was presented in the form of a poster at the Mayo Clinic Young Investigators Research Symposium on March 19, 2016.
Financial Disclosure/Conflicts of Interest
P. Tipton reports no disclosures relevant to the manuscript. T. Konno received research support from the JSPS Postdoctoral Fellowship for Research Abroad and is partially supported by the gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. D. Broderick reports no disclosures relevant to the manuscript. D. Dickson is supported by the NIH P50 NS072187, P50 AG16574, The Robert E. Jacoby Professorship in Alzheimer’s Research and the Mangurian Foundation Lewy Body Dementia program at Mayo Clinic. Z. Wszolek is supported by the NIH P50 NS072187, Mayo Clinic Center for Regenerative Medicine, Mayo Clinic Center for Individualized Medicine, Mayo Clinic Neuroscience Focused Research Team (Cecilia and Dan Carmichael Family Foundation), and The Sol Goldman Charitable Trust.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Philip W. Tipton, Email: tipton.philip@mayo.edu.
Takuya Konno, Email: konno.takuya@mayo.edu.
Daniel F. Broderick, Email: Broderick.daniel.f@mayo.edu.
Dennis W. Dickson, Email: dickson.dennis@mayo.edu.
References
- 1.Collins SJ, Ahlskog JE, Parisi JE, Maraganore DM. Progressive supranuclear palsy: neuropathologically based diagnostic clinical criteria. J Neurol Neurosurg Psychiatry. 1995;58:167–173. doi: 10.1136/jnnp.58.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birdi S, Rajput AH, Frenton M, Donat JR, Rozdilsky B, Robinson C, Macaulay R, George D. Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord. 2002;17:1255–1264. doi: 10.1002/mds.10211. [DOI] [PubMed] [Google Scholar]
- 3.Daniel SE, de Bruin VM, Lees AJ. The clinical and pathological spectrum of Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy): a reappraisal. Brain. 1995;118:759–70. doi: 10.1093/brain/118.3.759. [DOI] [PubMed] [Google Scholar]
- 4.Tsuboi Y, Slowinski J, Josephs KA, Honer WG, Wszolek ZK, Dickson DW. Atrophy of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2003;60:1766–1769. doi: 10.1212/01.wnl.0000068011.21396.f4. [DOI] [PubMed] [Google Scholar]
- 5.Aiba I, Hashizume Y, Yoshida M, Okuda S, Murakami N, Ujihira N. Relationship between brainstem MRI and pathological findings in progressive supranuclear palsy: study in autopsy cases. J Neurol Sci. 1997;152:210–217. doi: 10.1016/s0022-510x(97)00166-4. [DOI] [PubMed] [Google Scholar]
- 6.Nicoletti G, Fera F, Condino F, Auteri W, Gallo O, Pugliese P, Arabia G, Morgante L, Barone P, Zappia M, Quattrone A. MR imaging of middle cerebellar peduncle width: differentiation of multiple system atrophy from Parkinson disease. Radiology. 2006;239:825–830. doi: 10.1148/radiol.2393050459. [DOI] [PubMed] [Google Scholar]
- 7.Wenning GK, Colosimo C, Geser F, Poewe W. Multiple system atrophy. Lancet Neurol. 2004;3:93–103. doi: 10.1016/s1474-4422(03)00662-8. [DOI] [PubMed] [Google Scholar]
- 8.Cosottini M, Ceravolo R, Faggioni L, Lazzarotti G, Michelassi MC, Bonuccelli U, Murri L, Bartolozzi C. Assessment of midbrain atrophy in patients with progressive supranuclear palsy with routine magnetic resonance imaging. Acta Neurol Scand. 2007;116:37–42. doi: 10.1111/j.1600-0404.2006.00767.x. [DOI] [PubMed] [Google Scholar]
- 9.Gröschel K, Hauser TK, Luft A, Patronas N, Dichgans J, Litvan I, Schulz JB. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage. 2004;21:714–724. doi: 10.1016/j.neuroimage.2003.09.070. [DOI] [PubMed] [Google Scholar]
- 10.Quattrone A, Nicoletti G, Messina D, Fera F, Condino F, Pugliese P, Lanza P, Barone P, Morgante L, Zappia M, Aguglia U, Gallo O. MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology. 2008;246:214–221. doi: 10.1148/radiol.2453061703. [DOI] [PubMed] [Google Scholar]
- 11.Hussl A, Mahlknecht P, Scherfler C, Esterhammer R, Schocke M, Poewe W, Seppi K. Diagnostic accuracy of the magnetic resonance parkinsonism index and the midbrain-to-pontine area ratio to differentiate progressive supranuclear palsy from Parkinson’s disease and the Parkinson variant of multiple system atrophy. Mov Disord. 2010;25:2444–2449. doi: 10.1002/mds.23351. [DOI] [PubMed] [Google Scholar]
- 12.Paviour DC, Price SL, Stevens JM, Lees AJ, Fox NC. Quantitative MRI measurement of superior cerebellar peduncle in progressive supranuclear palsy. Neurology. 2005;64:675–679. doi: 10.1212/01.WNL.0000151854.85743.C7. [DOI] [PubMed] [Google Scholar]
- 13.Schulz JB, Skalej M, Wedekind D, Luft AR, Abele M, Voigt K, Dichgans J, Klockgether T. Magnetic resonance imaging-based volumetry differentiates idiopathic Parkinson’s syndrome from multiple system atrophy and progressive supranuclear palsy. Ann Neurol. 1999;45:65–74. [PubMed] [Google Scholar]
- 14.Seppi K, Schocke MF, Esterhammer R, Kremser C, Brenneis C, Mueller J, Boesch S, Jaschke W, Poewe W, Wenning GK. Diffusion-weighted imaging discriminates progressive supranuclear palsy from PD, but not from the Parkinson variant of multiple system atrophy. Neurology. 2003;60:922–927. doi: 10.1212/01.wnl.0000049911.91657.9d. [DOI] [PubMed] [Google Scholar]
- 15.Schrag A, Good CD, Miszkiel K, Morris HR, Mathias CJ, Lees AJ, Quinn NP. Differentiation of atypical parkinsonian syndromes with routine MRI. Neurology. 2000;54:697–702. doi: 10.1212/wnl.54.3.697. [DOI] [PubMed] [Google Scholar]
- 16.Righini A, Antonini A, De Notaris R, Bianchini E, Meucci N, Sacilotto G, Canesi M, De Gaspari D, Triulzi F, Pezzoli G. MR imaging of the superior profile of the midbrain: differential diagnosis between progressive supra-nuclear palsy and Parkinson disease. AJNR Am J Neuroradiol. 2004;25:927–932. [PMC free article] [PubMed] [Google Scholar]
- 17.Oba H, Yagishita A, Terada H, Barkovich AJ, Kutomi K, Yamauchi T, Furui S, Shimizu T, Uchigata M, Matsumura K, Sonoo M, Sakai M, Takada K, Harasawa A, Takeshita K, Kohtake H, Tanaka H, Suzuki S. New and reliable MRI diagnosis for progressive supranuclear palsy. Neurology. 2005;64:2050–2055. doi: 10.1212/01.WNL.0000165960.04422.D0. [DOI] [PubMed] [Google Scholar]
- 18.Warmuth-Metz M, Naumann M, Csoti I, Solymosi L. Measurement of the midbrain diameter on routine magnetic resonance imaging: a simple and accurate method of differentiating between Parkinson disease and progressive supranuclear palsy. Arch Neurol. 2001;58:1076–1079. doi: 10.1001/archneur.58.7.1076. [DOI] [PubMed] [Google Scholar]
- 19.Brooks JD, Seppi K Neuroimaging Working Group on MSA. Proposed neuroimaging criteria for the diagnosis of multiple system atrophy. Mov Disord. 2009;24:949–964. doi: 10.1002/mds.22413. [DOI] [PubMed] [Google Scholar]
- 20.Hotter A, Esterhammer R, Schocke MF, Seppi K. Potential of advanced MR imaging techniques in the differential diagnosis of parkinsonism. Mov Disorder. 2009;24:S711–S720. doi: 10.1002/mds.22648. [DOI] [PubMed] [Google Scholar]
- 21.Yuki N, Sato S, Yuasa T, Ito J, Miyatake T. Computed tomographic findings of progressive supranuclear palsy compared with Parkinson’s disease. Jpn J Med. 1990;29:506–511. doi: 10.2169/internalmedicine1962.29.506. [DOI] [PubMed] [Google Scholar]
- 22.Fatterpekar GM, Dietrich A, Pantano P, Saba L, Knopp EA, Piattella MC, Raz E1. Cerebral Peduncle Angle: An Objective Criterion for Assessing Progressive Supranuclear Palsy Richardson Syndrome. AJR Am J Roentgenol. 2015;205:386–391. doi: 10.2214/AJR.14.12724. [DOI] [PubMed] [Google Scholar]
- 23.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Dürr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]