Abstract
Objective
In 2014, the US experienced an outbreak of enterovirus D68 (EV-D68) associated with severe respiratory illness. The clinical characteristics associated with severe illness from EV-D68 during this outbreak compared with the 2009 H1N1 influenza virus outbreak are unknown.
Design and Setting
In this retrospective cohort study, we characterized the clinical features of children with EV-D68 admitted to the pediatric ICU between August 1-November 1, 2014 and compared them with critically-ill children infected with H1N1 influenza during the pandemic admitted between May 1, 2009-January 31, 2010.
Patients
pediatric ICU patients
Interventions
none
Measurements and Main Results
Ninety-seven severely-ill children with EV-D68 infections were compared with 68 children infected with H1N1 influenza during the 2009 pandemic. Children with EV-D68 were more likely to have asthma (62% vs 23%, P< 0.001) and present with reactive airway disease exacerbations, with greater receipt of albuterol (94% vs 49%) and steroids (89% vs 40%, P< 0.0001 for both). While more children with EV-D68 were admitted to the ICU compared with H1N1 influenza, they had a shorter hospital length of stay (4 vs 7 days, P< 0.0001), with lower intubation rates (7% vs 44%), vasopressor use (3% vs 32%), ARDS (3% vs 24%), shock (0% vs 16%) and death (0% vs 12 %, P< 0.05 for all). Compared with children with other enteroviruses and rhinoviruses, children with EV-D68 were more likely to have a history of asthma (64% vs 45%) or multiple prior wheezing episodes (54% vs 34%, P < 0.01 for both).
Conclusions
Critically-ill children with EV-D68 were more likely to present with reactive airway disease exacerbations, whereas children with H1N1 influenza were more likely to present with pneumonia. Compared to the pandemic H1N1 influenza outbreak, the EV-D68 outbreak resulted in more children requiring admission to the ICU, but was associated with less severe outcomes.
Keywords: enterovirus-D68, respiratory tract illness, influenza virus
Introduction
Enteroviruses (EVs) have been implicated in a variety of illnesses, including hand, foot, and mouth disease, herpangina, conjunctivitis, hepatitis, myocarditis, neonatal sepsis, meningitis, encephalitis, and acute flaccid paralysis (1). Less commonly, EVs have been associated with respiratory disease. EV-D68 is a member of one of four enterovirus species that affect humans. EV-D68 was first isolated in 1962 (2), but until recently it had been reported infrequently in the United States. Small clusters of EV-D68 associated with respiratory illness, however, were reported in the United States, Europe, and Asia from 2008-2010 (3). From August to November 2014, a widespread outbreak of EV-D68 in the United States was associated with severe respiratory illness, leading to an increase in pediatric hospitalizations (4). The Centers for Disease Control and Prevention (CDC) have reported a total of 1153 cases of EV-D68-associated respiratory illness in 49 states and the District of Columbia with confirmed EV-D68 infection (5), although the number of cases were likely to be grossly underestimated due to a lack of widely available testing. It was the largest EV-D68 outbreak ever reported.
In order to characterize the clinical presentation and disease burden of EV-D68 during the most recent outbreak, we studied the demographics, clinical characteristics and hospital course of hospitalized children admitted to the intensive care unit (ICU) with respiratory samples that were positive for EV-D68 and compared them with children admitted to the ICU during the 2009 H1N1 pandemic. We also compared the clinical characteristics of children with EV-D68 infection with children with other entero/rhinovirus infection. The H1N1 influenza cohort was selected as the comparison group given that it was also an emerging respiratory pathogen at the time of the pandemic (6, 7), was associated with significant morbidity, has been well-characterized (8-10), and has global public health significance (11).
Materials and Methods
Study population and design
We conducted a retrospective cohort study of children admitted with EV-D68 to Children’s Hospital Colorado (CHCO). CHCO is an academic, tertiary care hospital serving Colorado and surrounding states. The primary population is the Denver metropolitan area, which has a population of approximately 2.5 million people. The hospital has approximately 500 inpatient beds, 32 ICU beds, 18,000 inpatient admissions and 150,000 emergency department and urgent care visits per year.
Hospitalized children admitted to the ICU with a respiratory specimen positive by real-time polymerase chain reaction (RT-PCR) for EV-D68 from August 1, 2014 to November 1, 2014 were included. Patients during the same time period with respiratory specimens positive for other entero-rhinoviruses were included for comparison. Children with a respiratory specimen positive by PCR or direct fluorescent antibody (DFA) for the 2009 influenza A H1N1 virus admitted to the ICU from May 1, 2009 to January 31, 2010 were used for comparison. We focused our analysis on inpatients admitted to the ICU because they represented the most severely ill children and our ICU routinely orders respiratory viral testing on all children who are admitted with a respiratory illness.
For our EV-D68 cohort, an initial database of children who were positive by RT-PCR for rhinovirus or enterovirus from August to November 2014 was generated. The influenza H1N1 cohort utilized a dataset that was previously collected for a pandemic influenza study (12). Respiratory specimens submitted for virus testing for both cohorts included nasopharyngeal washes, tracheal aspirates and bronchoalveolar lavage specimens.
The electronic medical records of the study cohort were reviewed by the study team. Data collected included demographics, clinical characteristics on admission, laboratory and radiographic findings, and hospital course.
Testing
During the influenza pandemic period, respiratory specimens were extracted using viral RNA kits on a BioRobot EZ1 extractor (Qiagen, Valencia, CA) per manufacturer instructions. Respiratory specimens were tested using a multiplex PCR (xTag® respiratory virus Panel [RVP], Luminex Molecular Diagnostics, Austin, TX), which identifies 16 respiratory viruses and subtypes including influenza A (subtypes H1 and H3). During the EV-D68 outbreak, samples were initially tested by the FilmArray® Respiratory Panel (BioFire Diagnostics, Salt Lake City, UT), a multiplex PCR that detects 17 respiratory viruses and subtypes but cannot discriminate between rhinoviruses (RVs) and EVs (13). Respiratory samples positive for RV/EV were sent to the CDC Polio and Picornavirus Laboratory Branch for EV-D68 testing by an EV-D68 specific real time RT-PCR (14).
Definitions
A diagnosis of asthma was based on prior or current documentation of a diagnosis of asthma, history of inhaler use, multiple prior wheezing episodes, or wheezing in the absence of viral respiratory infections. Hypoxia was defined as the use of oxygen during hospitalization. Acute respiratory distress syndrome (ARDS) was defined as acute onset, non-cardiogenic edema with bilateral pulmonary infiltrates on chest radiograph, and a ratio of partial pressure of oxygen in arterial blood less than or equal to 200 mm Hg, or requiring high peak end expiratory pressure to maintain inspired oxygen levels at non-toxic levels (15). Chest radiograph findings were categorized as abnormal if the radiologist documented focal or diffuse findings, or normal if no focal or diffuse process was identified. Radiographic findings consistent with pneumonia were defined as the presence of consolidation, unilateral or bilateral infiltrates, effusion, empyema, abscess, or radiologist final interpretation of bacterial pneumonia. Underlying medical conditions included underlying chronic pulmonary conditions (including asthma), hemodynamically significant cardiac disease, immunosuppressive disorders or therapy, chronic renal or hepatic dysfunction, metabolic, rheumatologic or hematologic disease, neurological disorders compromising respiratory function, children with global developmental delay and children on long-term aspirin therapy (16). Respiratory distress was defined as the presence of tachypnea, retractions or accessory muscle use on examination.
The protocol was approved by the Colorado Multiple Institutional Review Board. Data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Colorado (17).
Statistical analysis
The characteristics of children with EV-D68 infection admitted to the ICU were compared with those of children infected with pandemic H1N1 influenza and non EV-D68 EV or RV infection. For categorical variables, the frequency and percentages of children in each category were calculated. Fisher’s exact or chi-square tests were used to compare categorical data; the Wilcoxon rank-sum test was used for continuous variables with non-normal distributions. Multivariate logistic regression was used to evaluate the associations between patient characteristics and EV-D68 infection. Covariables thought to be clinically important were chosen a priori. Adjusted odds ratios and 95% confidence intervals were estimated. Analyses were conducted using SPSS, V 22 (IBM, Armonk, New York) and SAS software, version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
There were 222 inpatients admitted to the pediatric intensive care unit (ICU) with respiratory specimens that were positive for EV/RV from July 1 to October 30, 2014. Of these, 97 (43%) tested positive for EV-D68, representing 37% of patients admitted to the ICU with an acute respiratory illness during the same time period. From May 1, 2009 to January 30, 2010, 68 children were admitted to the ICU with a respiratory specimen positive for H1N1 influenza virus, representing 21.5% of children admitted to the ICU during the same time period with an acute respiratory illness. The epidemiologic curves for these two cohorts displaying number of cases by week are displayed in Figure 1.
Figure 1.
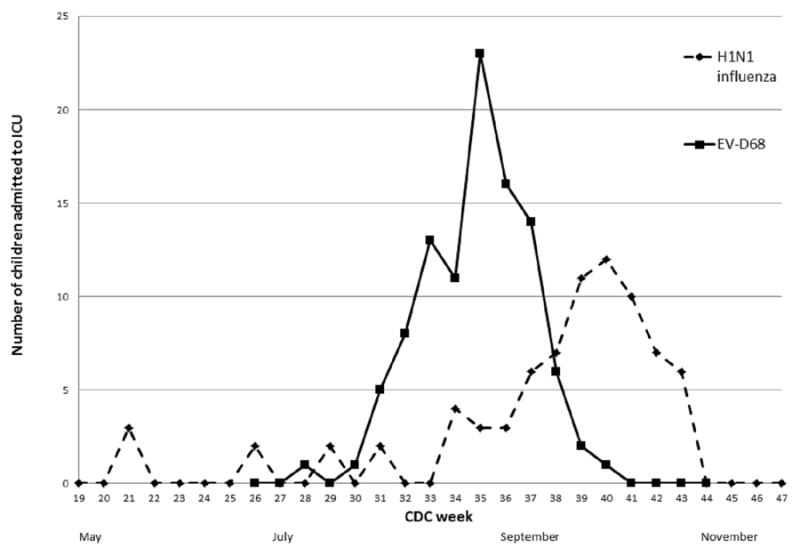
Children with EV-D68 (2014) and H1N1 influenza (2009-2010) virus admitted to the ICU at Children’s Hospital Colorado by date of illness onset.
y axis is number of children admitted to the ICU, x-axis is CDC week; black line represents children with EV-D68 and dotted line represents children with H1N1 influenza.
The CDC week is the week of the epidemiologic year for which the National Notifiable Diseases Surveillance System (NNDSS) disease report is assigned by the reporting local or state health department for the purposes of disease incidence reporting and publishing. Values for CDC week range from 1 to 53, although most years consist of 52 weeks.
Demographics and co-morbidities
There was a slight male preponderance of children with EV-D68 or H1N1 influenza, with a similar median age for both groups. Children with EV-D68 were more likely to have asthma compared with children with H1N1 influenza (64% vs 19%), and less likely to have other underlying medical conditions excluding asthma (15% vs 65%, P < 0.0001 for both) (Table 1).
Table 1.
Sociodemographic and clinical characteristics, laboratory and radiographic findings of children admitted to Children’s Hospital Colorado ICU with EV-D68 from August to November, 2014 compared with H1N1 Influenza May 2009 to January 2010 (N = 165)
| EV-D68 N = 97 | H1N1 influenza N = 68 | P valuea | |
|---|---|---|---|
| Demographics | |||
| Male sex, n (%) | 63 (65) | 44 (65) | 0.97 |
| Age, years; median, (IQR) | 7 (4, 12) | 8 (4, 13) | 0.66b |
| Co-morbidities | |||
| Asthma, n (%) | 62 (64) | 13 (19) | <.0001 |
| Anyc excluding asthma, n (%) | 15 (15) | 51 (65) | <.0001 |
| Symptoms | |||
| Number of days of symptoms prior to admission, days; median (IQR) | 1 (1, 2) | 2.5 (1, 5) | 0.007d |
| Fever, n (%) | 35 (36) | 54 (79) | <.0001 |
| Nausea, n (%) | 3 (3) | 13 (19) | 0.0006 |
| Abdominal Pain, n (%) | 2 (2) | 7 (10) | 0.03b |
| Shortness of breath or increased work of breathing, n (%) | 87 (90) | 41 (60) | <.0001 |
| Cough, n (%) | 86 (89) | 50 (74) | 0.01 |
| Sore throat, n (%) | 20 (21) | 7 (10) | 0.08 |
| Seizures, n (%) | 5 (5) | 11 (16) | 0.02 |
| Mental Status changes, n (%) | 3(3) | 13 (19) | 0.0006 |
| Signs | |||
| Respiratory distress, n (%) | 92 (95) | 49 (72) | <.0001 |
| Wheezing, n (%) | 75 (77) | 17 (25) | <.0001 |
| Hypoxia, n (%) | 91 (94) | 52 (76) | 0.0013 |
| Tachypnea, n (%) | 72 (74) | 40 (59) | 0.04 |
| Diminished breath sounds, n (%) | 75 (77) | 30 (44) | <.0001 |
| Laboratory values | |||
| White cell count > 10 × 103 cells/μL, n (%) (n=109) | 35 (74) | 19 (31) | <.0001 |
| Platelets ≤ 100 × 103 cells/μL, n (%) (n=108) | 1 (2) | 9 (15) | 0.04b |
| C-Reactive Protein ≥ 4 mg/dL, n (%) (n=42) | 2 (22) | 16 (48) | 0.26b |
| Another virus on respiratory PCR | 13 (13) | 11 (16) | 0.84 |
| Blood culture positivee | 9 (9) | 17 (25) | 0.01 |
| Bacterial culture positive (excluding blood culture) | 1 (1) | 5 (7) | 0.08 |
| Radiographic findings, n (%) | |||
| Abnormal CXR, n (%) | 72 (92) | 58 (89) | 0.52 |
| Airways disease, n (%) | 50 (69) | 15 (26) | <.0001 |
| Findings consistent with pneumoniaf, n (%) (n=130) | 28 (39) | 39 (67) | 0.0013 |
Chi Square unless otherwise specified
Wilcoxon Rank Sum Test
any comorbidity includes: underlying chronic pulmonary conditions, hemodynamically significant cardiac disease, immunosuppressive disorders or therapy, chronic renal or hepatic dysfunction, metabolic, rheumatologic or hematologic disease, neurological disorders compromising respiratory function, children with global developmental delay and children on long-term aspirin therapy.
Fisher’s Exact test
Excluding organisms commonly associated with contamination
Findings consistent with pneumonia were defined as: consolidation, unilateral, bilateral infiltrates, effusion, pneumonia, empyema, abscess
Clinical presentation
Children with EV-D68 were more likely to be admitted due to respiratory distress, compared with children with H1N1 influenza. In addition, they tended to present earlier in their illness course (median of 1 versus 2.5 days of symptoms prior to admission, P = 0.007). Children with EV-D68 had higher rates of shortness of breath/increased work of breathing (90% vs 60%) and cough (89% vs 74%) than children with H1N1 influenza (P < 0.05 for both). Children with EV-D68 were also more likely to present with wheezing (77% vs 25%), hypoxia (94% vs 76%) and diminished breath sounds (77% vs 44%), compared to children with H1N1 influenza (P< 0.005 for all). Children with EV-D68, however, were less likely to present with seizures (5% vs 16%, P = 0.02) and mental status changes (3% vs 19%, P = 0.0006) (Table 1). All cases of EV-D68 in our cohort were community acquired, and there were two cases of nosocomially-acquired H1N1.
Laboratory values and Radiographic findings
A larger proportion of children with EV-D68 had white cell counts over 10 × 103 cells/μL (74% vs 31%, P < 0.0001), with most children having normal C-reactive proteins in both groups. There was a lower proportion of children with EV-D68 infection with positive blood cultures (9% vs 25%, P = 0.01). A higher proportion of children with EV-D68 had radiographic findings consistent with reactive airways disease (69% vs 26%, P < 0.0001), with lower rates of consolidation, infiltrates, pleural effusions and other features consistent with pneumonia (39% vs 67%, P = 0.001) (Table 1).
Treatment and Hospital Course
There was higher steroid and bronchodilator use among children with EV-D68 (89% vs 40% and 94% vs 49% respectively), and lower antibiotic use compared with children with H1N1 influenza (37% vs 84%, P <0.0001 for all). Children with EV-D68 were more likely to receive bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP) (70% vs 31%), and were less likely to be intubated (7% vs 44%), receive ECMO (0% vs 6%), have hypotension requiring pressors (3% vs 32%), ARDS (3% vs 24%) and shock (0% vs 16%), P < 0.05 for all). Children with EV-D68 had shorter median lengths of hospital and ICU stay (4 days vs 7 days, and 2 vs 3 days respectively, P < 0.0001 for both). There was no mortality among children with EV-D68 in our cohort, whereas 12% of children with H1N1 influenza died (Table 2).
Table 2.
Treatment and hospital course for children admitted to the Children’s Hospital Colorado ICU with EV-D68 from August to November, 2014 compared with H1N1 Influenza, May 2009 to January 2010 (N=165)
| EV-D68 N = 97 | H1N1 influenza N = 68 | P valuea | |
|---|---|---|---|
| Treatment | |||
| Oxygen therapy duration, days; median (IQR) (n=136) | 3 (2, 5) | 7 (3,13) | <.0001b |
| Antibiotics, n (%) | 36 (37) | 57 (84) | <.0001 |
| Steroids, n (%) | 86 (89) | 27 (40) | <.0001 |
| Bronchodilators, n (%) | 91 (94) | 33 (49) | <.0001 |
| Hospital Course | |||
| Length of stay, days; median (IQR) | 4 (3, 6) | 7 (4, 23) | <.0001b |
| PICU length of stay, days; median (IQR) | 2 (1, 3) | 3 (2, 10) | <.0001b |
| Hypotension requiring pressors, n (%) | 3 (3) | 22 (32) | <.0001 |
| Intubation, n (%) | 7 (7) | 30 (44) | <.0001 |
| BiPAP or CPAPc, n (%) (n=164) | 68 (70) | 21 (31) | <.0001 |
| ECMOd, n (%) | 0 (0) | 4 (6) | 0.03e |
| Cardiovascular complications other than hypotension, n (%) | 1 (1) | 4 (6) | 0.16e |
| Neurological complications, n (%) | 2 (2) | 2 (3) | 1.0e |
| ARDSf, n (%) | 3 (3) | 16 (24) | <.0001 |
| Shock - any organs involved in hypoperfusion, n (%) | 0 (0) | 11 (16) | <.0001e |
| Death, n (%) | 0 (0) | 8 (12) | 0.0006e |
Chi Square unless otherwise specified
Wilcoxon Rank Sum Test
BiPAP – bilevel positive airway pressure, CPAP – Continuous positive airway pressure
ECMO- Extracorporeal membrane oxygenation
Fisher’s Exact test
ARDS- Acute respiratory distress syndrome
Multivariate analyses
In adjusted analyses, there were higher odds of wheezing, shortness of breath or increased work of breathing (among children with asthma) (OR 15.3, [95% CI 1.3-1179.4]), CPAP/BiPAP use (OR 5.1, [95% CI 2.6-10.1]), as well as bronchodilator (OR 9.0 [95% CI 3.3-24.6]) and steroid use (OR 6.74, [95% CI 2.8-16.0]) associated with EV-D68 infection. There were lower odds of mental status changes (OR 0.12, [95%CI 0.04-0.6]) and antibiotic use (OR 0.2, [95% CI 0.1-0.6]) with EV-D68 infection. Additionally, children with EV-D68 had a shorter length of stay in the ICU compared with children with H1N1 influenza when holding asthma, underlying medical conditions and findings consistent with pneumonia constant (Table 3).
Table 3.
Multivariate associations between children admitted to Children’s Hospital Colorado Intensive Care Unit, August to November 2014 with EV-D68 infection compared with H1N1 influenza infection (N=165)
| Unadjusted Odds Ratios (95% Confidence Intervals) | Adjusted Odds Ratios (95% Confidence Intervals) | |
|---|---|---|
| Symptoms | ||
| Days of symptoms prior to admissiona | 0.9 (0.8-1.0) | 1.1 (1.0-1.2) |
| Shortness of breath or increased work of breathing among those with history of asthmab (n=75) | 38.1 (3.9-369.0) | 15.3 (1.3-179.4) |
| Shortness of breath or increased work of breathing among those without history of asthmab (n=90) | 1.9 (0.8-4.9) | 1.8 (0.6-4.8) |
| Seizuresc | 0.3 (0.1-0.9) | 0.9 (0.2-3.5) |
| Mental status changesd | 0.1 (0.04-0.5) | 0.2 (0.04-0.6) |
| Signs | ||
| Wheezinge | 10.2 (4.9-21.1) | 7.4 (3.1-17.7) |
| Hypoxiaf | 4.7 (1.7-12.7) | 3.4 (1.0-11.4) |
| Treatment | ||
| Oxygen therapy durationg (n=110) | 0.9 (0.8-0.9) | 0.9 (0.9-1.0) |
| Antibioticsg (n=130) | 0.1 (0.1-0.2) | 0.2 (0.1-0.6) |
| Steroidsh | 11.9 (5.4-26.3) | 6.7 (2.8-16.0) |
| Bronchodilatorsh | 16.1 (6.2-41.7) | 9.0 (3.3-24.6) |
| BiPAP or CPAPi (n=164) | 5.1 (2.6-10.1) | 6.1 (2.5-14.9) |
| Hospital coursej | ||
| Hospital length of stay, days (n=130) | ||
| 3-4 days vs 1-2 days | 0.9 (0.3-2.6) | 1.7 (0.5-6.6) |
| 5-6 days vs 1-2 days | 0.5 (0.1-1.8) | 0.6 (0.1-2.7) |
| ≥1 week vs 1-2 days | 0.2 (0.1-0.5) | 0.3 (0.1-1.3) |
| ICU length of stay, daysl (n=125) | ||
| 3-4 days vs 1-2 days | 0.4 (0.2-1.0) | 0.4 (0.1-1.1) |
| 5-6 days vs 1-2 days | 0.7 (0.2-2.7) | 0.7 (0.2-3.1) |
| ≥1 week vs 1-2 days | 0.1 (0.05-0.3) | 0.2 (0.1-0.7) |
Adjusted for UMC, asthma, fever
History of asthma modifies the relationship between SOB/WOB and diagnosis, so stratum-specific estimates adjusted for non-asthma UMC are shown.
Adjusted for history of seizures
Adjusted for neurological disorder
Adjusted for age, asthma
Adjusted for asthma, UMC
Adjusted for UMC, asthma, CXR findings consistent with pneumonia
Adjusted for asthma
BiPAP – bilevel positive airway pressure, CPAP – Continuous positive airway pressure
Adjusted for asthma, underlying medical condition not asthma and findings consistent with pneumonia
Comparison of children with EV D-68 vs. other enteroviruses or rhinoviruses
A separate univariate analysis comparing children with EV-D68 with children with other EV/RV infections was conducted, limited to those with respiratory symptoms admitted to the ICU (Table 4). Children with EV-D68 were more likely to have a history of asthma (64% vs 45%), multiple prior wheezing episodes (54% vs 34%), or a history of inhaler use (64% vs 45%, P < 0.01 for all). Children with non-EV-D68 EV/RV were more likely to have a family member with asthma than children with EV68 (55% vs 34%), yet were less likely to have a prior history of wheezing (47% vs 69%, P < 0.05 for both). Children with non EV-D68 EV/RV admitted to the ICU were more likely to be intubated (21% vs 5%, P =0.002). Children with EV-D68 were also more likely to have received bronchodilators (95% vs 71%), continuous albuterol (83% vs 43%), terbutaline (17% vs 5%), magnesium (61% vs 28%) or steroids (89% vs 69%) (P < 0.01 for all).
Table 4.
Demographics, clinical characteristics and treatment of children with respiratory symptoms admitted to Children’s Hospital Colorado ICU with EV-D68 compared with other enterovirus/rhinovirus infections from August to November 2014 (N=181)
| Other EV/RV N = 86 | EV-D68 N = 95 | P valuea | |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age, years; median (IQR) | 3 (1, 8) | 7 (4,12) | <.0001b |
| Male sex | 51 (59) | 62 (65) | 0.41 |
| Risk factors, n (%) | |||
| Personal history asthma | 39 (45) | 61 (64) | 0.01 |
| Eczema | 1 (1) | 3 (3) | 0.62c |
| Allergies | 4 (5) | 3 (3) | 0.71c |
| Family history asthma | 47 (55) | 32 (34) | 0.005 |
| First degree relative | 30 (35) | 40 (42) | 0.32 |
| Not first degree relative | 5 (6) | 18 (19) | 0.008 |
| No history of wheezing | 46 (53) | 29 (31) | 0.002 |
| Prior wheezing before 3 years of age | 13 (15) | 13 (14) | 0.78 |
| Multiple prior wheezing episodes | 29 (34) | 51 (54) | 0.007 |
| Wheezing apart from respiratory illness | 6 (7) | 5 (5) | 0.63 |
| History of inhaler use (n=177) | 38 (45) | 59 (64) | 0.0095 |
| Treatment/intervention/Outcomes, n (%) | |||
| Hospital length of stay, days; median (IQR) (n=180) | 4 (3, 6) | 4 (3, 5) | 0.30b |
| ICU length of stay, days; median (IQR) | 2 (1, 3) | 2 (1, 3) | 0.14b |
| Intubated | 18 (21) | 5 (5) | 0.002 |
| BiPAPd | 36 (42) | 43 (45) | 0.65 |
| CPAPe | 37 (43) | 47 (49) | 0.38 |
| HHFO2f | 35 (41) | 24 (25) | 0.03 |
| Bronchodilators | 61 (71) | 90 (95) | <.0001 |
| Continuous albuterol | 37 (43) | 79 (83) | <.0001 |
| Terbutaline | 4 (5) | 16 (17) | 0.009 |
| Magnesium | 24 (28) | 58 (61) | <.0001 |
| Antibiotics | 39 (45) | 34 (36) | 0.19 |
| Steroids | 59 (69) | 85 (89) | 0.0005 |
Chi Square unless otherwise specified
Fisher’s Exact test
Wilcoxon Rank Sum Test
BiPAP – bilevel positive airway pressure
CPAP – Continuous positive airway pressure
HHFO2- Heated High Flow Oxygen
Discussion
Our study of children admitted to the ICU with EV-D68 infection demonstrated a higher volume of patients, and a higher proportion of children presenting earlier in their illness course with acute reactive airway disease exacerbations compared with influenza H1N1 during the 2009 pandemic. While their presentation was severe, and more patients with EV-D68 were admitted to the ICU, they had decreased morbidity, with lower rates of pneumonia, ARDS, intubation, vasopressor use, ECMO use, and shorter median length of stay in the ICU and hospital. There was no mortality associated with these patients. Our findings indicate substantial morbidity associated with EV-D68, but alert the clinician to a different presentation and course from H1N1, requiring alternative treatment and resource considerations.
In 2014, clinicians, public health officials and the media made several comparisons between the EV-D68 outbreak and the 2009 H1N1 influenza pandemic (18, 19): both were caused by novel respiratory pathogens resulting in nationwide outbreaks, occurring over a relatively short period of time with a high disease burden (11, 20). Many facilities implemented strategies that had been recommended for influenza pandemic preparedness, including enhanced precautions, visitor restrictions, staff surges and additional triage mechanisms (21). As these two outbreaks had many features in common and similar approaches were instituted, we compared the characteristics of critically ill children between these two cohorts. Despite their perceived similarities, however, our findings demonstrate marked differences between these two respiratory virus outbreaks, in terms of risk factors, presentation, and outcomes.
While children with asthma are considered at increased risk for influenza-related complications compared with healthy children (16), in our study in an ICU population, children with EV-D68 infections had a higher proportion of asthma diagnoses and earlier presentations with reactive airway disease than children with H1N1 influenza infections. Our study found that critically-ill children with EV68 were more likely to have and present with asthma than critically-ill children with H1N1, and that this association is more commonly observed in the setting of EV-D68 infection compared with other enteroviruses, as our study identified higher rates of asthma and albuterol use in children with EV-D68 compared with children with other EV or RV infections, similar to findings in another report (22).
EV-D68 shares characteristics with human RVs, which may account for its unique presentation compared with other EVs. Such characteristics include acid lability and growing more efficiently at lower temperatures (23). Human RVs are also a well-known trigger for asthma exacerbations, secondary to changes in airway responsiveness including stimulation of bronchial epithelial cells to produce pro-inflammatory chemokines and cytokines and increasing epithelial cell-derived nitric oxide synthesis (24, 25). Among the respiratory viruses detected in patients with asthma exacerbations, RVs are the most frequently implicated pathogens (26-30). The similarity between RVs and EV-D68 may explain the increased tendency for children in our EV-D68 cohort to present with airway exacerbations.
The association of EV-D68 among critically-ill children with asthma or reactive airways disease may also account for the more acute presentation in our cohort, and decreased overall morbidity. Compared to those with H1N1 influenza, children with EV-D68 had a shorter median duration of illness symptoms prior to admission, with a higher proportion presenting with acute respiratory features such as hypoxia, tachypnea, wheeze and diminished breath sounds. This may be due to more accelerated clinical deterioration from virus-triggered bronchospasm, and rapid reversibility from therapies received. Children with H1N1 influenza, in contrast, were more likely to develop secondary pneumonia, or ARDS, with a more gradual course and a mortality rate of 12%. As a consequence of these different disease manifestations, children with EV-D68 had shorter length of stay in the ICU and hospital, and were less likely to require intubation. In addition, they had lower rates of complications such as hypotension, mental status changes, pulmonary hemorrhage, ARDS, renal failure and shock in children with EV-D68 compared with H1N1 influenza. Thus, while their initial presentation appeared more severe, children with EV-D68 had faster recovery and fewer complications, most likely reflecting differences in underlying disease processes.
The differences in morbidity between the two cohorts might be explained, in part, by the differences in tropism between the two viruses. Animal models have demonstrated that H1N1 influenza infection is associated with an early and sustained inflammatory response (31), with evidence that lung injury caused by the virus increases susceptibility to further insult by bacterial superinfection (32). It has also been shown that a mutation in the hemagglutinin gene of H1N1 influenza leads to its ability to infect ciliated bronchial cells of the lower respiratory tract, and this cell tropism has the potential to increase the severity of illness (33-35). In contrast, EV-D68 has a higher affinity for receptors of the upper respiratory tract than the lower respiratory tract (36). The pathophysiology of severe infection with EV-D68 remains unknown, and most likely involves additional factors other than the distribution of viral receptors.
Our study has several limitations. Our data is from a single center, and does not represent the entire spectrum of disease, but is limited to those with more severe illness. Although we used a standardized data collection form, clinical evaluations were collected retrospectively and may have differed depending on the individual providers who were caring for these children. Some of our findings may represent changes in standard practice between the two study periods. Since our study population was focused on children admitted to the ICU, our sample size is small, precluding the adjustment for several confounders in our multivariate analyses, and the exploration of risk factors associated with ICU admission. We are also unable to determine rates of infection with EV-D68 in our population due to a lack of standardized testing among all hospitalized patients.
Our study provides detailed clinical characterization of severe EV-D68 infection in children and how it can be distinguished from influenza. Our findings have several important implications. Our study highlights that a singular approach to respiratory viral emerging diseases and outbreaks may not be sufficient, as during the EV-D68 outbreak there was a need to utilize different therapeutic approaches and preparedness strategies than those implemented for H1N1 influenza. This included increased need for staffing to administer medications such as albuterol, ensuring an adequate supply of respiratory equipment, in particular biPAP, CPAP, early steroid and albuterol use, and judicious use of antibiotics given the lower rates of pneumonia. Secondly, our findings support targeted parent and patient education efforts during such an outbreak, such as stressing asthma control and hygienic measures. Finally, if EV-D68 becomes endemic and/or continues to cause periodic outbreaks, given the high morbidity and burden of disease documented in this study, further research will be needed in the areas of therapeutic interventions, including development of antivirals, and preventive strategies such as vaccine development.
Keypoints.
Our study demonstrated a high volume of morbidity and reactive airway disease exacerbations among children infected with EV-D68, but the spectrum of illness was less severe compared to illness in influenza A H1N1-infected children during the pandemic period.
Acknowledgments
We thank Garrett Breazeale, Kristen Pretty, Randy Hengartner, and the CHCO Clinical Microbiology Laboratory for their assistance with diagnostic testing. We thank the staff at CHCO who expertly cared for these children during this outbreak.
Funding source: This work was supported by a grant from the National Institutes of Health/National Center for Research Resources (Colorado Clinical and Translational Sciences Institute grant number UL1 TR001082).
Abbreviations
- BiPAP
bi-level positive airway pressure
- CPAP
continuous positive airway pressure
- ICU
intensive care unit
- EV-D68
Enterovirus D68
Footnotes
Conflict of Interest: The authors have indicated that there are no conflicts of interest relevant to this article to disclose.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Contributor Information
Suchitra Rao, Department of Pediatrics (Hospital Medicine and Infectious Diseases), University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
Kevin Messacar, Department of Pediatrics (Hospital Medicine and Infectious Diseases), University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
Michelle R Torok, Department of Pediatrics (Hospital Medicine), University of Colorado School of Medicine; Adult and Child Center for Health Outcomes Research and Delivery Science, Aurora, CO, USA.
Anne-Marie Rick, Department of Pediatrics, University of Colorado School of Medicine, and Children’s Hospital Colorado, Aurora, CO, USA.
Jeffrey Holzberg, Department of Pediatrics, University of Colorado School of Medicine, and Children’s Hospital Colorado, Aurora, CO, USA.
Aaron Montano, University of Colorado School of Medicine, Aurora, CO, USA.
Dayanand Bagdure, Department of Pediatrics (Critical Care), University of Maryland School of Medicine, MD, USA.
Donna J Curtis, Department of Pediatrics (Infectious Diseases), University of Colorado School of Medicine, and Children’s Hospital Colorado, Aurora, CO, USA.
M Steven Oberste, Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
W Allan Nix, Division of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Gina de Masellis, Department of Pediatrics (Critical Care), University of Colorado School of Medicine, and Children’s Hospital Colorado, Aurora, CO, USA.
Christine C Robinson, Department of Microbiology (Virology), Children’s Hospital Colorado, Aurora, CO, USA.
Samuel R Dominguez, Department of Pediatrics (Infectious Diseases), University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora, CO, USA.
References
- 1.American Academy of Pediatrics Committee on Infectious Diseases Red Book - Enterovirus Infections Chapter. Elk Grove Village, IL: 2012. [Google Scholar]
- 2.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. American journal of epidemiology. 1967;85(2):297–310. doi: 10.1093/oxfordjournals.aje.a120693. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease, Control and Prevention. Clusters of acute respiratory illness associated with human enterovirus 68--Asia, Europe, and United States, 2008-2010. MMWR Morbidity and mortality weekly report. 2011;60(38):1301–4. [PubMed] [Google Scholar]
- 4.Midgley CM, Jackson MA, Selvarangan R, Turabelidze G, Obringer E, Johnson D, et al. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR Morbidity and mortality weekly report. 2014;63(36):798–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JE, Heffer RW, et al. The PedsQL Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2011;20(1):45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 6.Novel Swine-Origin Influenza AVIT. Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. The New England journal of medicine. 2009;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 7.Glatman-Freedman A, Portelli I, Jacobs SK, Mathew JI, Slutzman JE, Goldfrank LR, et al. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PloS one. 2012;7(11):e50228. doi: 10.1371/journal.pone.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan PA, Mermel LA, Andrea SB, McCulloh R, Mills JP, Echenique I, et al. Distinguishing characteristics between pandemic 2009-2010 influenza A (H1N1) and other viruses in patients hospitalized with respiratory illness. PloS one. 2011;6(9):e24734. doi: 10.1371/journal.pone.0024734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dominguez-Cherit G, Namendys-Silva SA, de la Torre A, Macias AE. Cordova-Villalobos JA. H1N1 Influenza Pandemic of 2009 Compared With Other Influenza Pandemics: Epidemiology, Diagnosis, Management, Pulmonary Complications, and Outcomes. Current infectious disease reports. 2010;12(3):204–10. doi: 10.1007/s11908-010-0097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Writing Committee of the WHOCoCAoPI. Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. The New England journal of medicine. 2010;362(18):1708–19. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 11.Fineberg HV. Pandemic preparedness and response--lessons from the H1N1 influenza of 2009. The New England journal of medicine. 2014;370(14):1335–42. doi: 10.1056/NEJMra1208802. [DOI] [PubMed] [Google Scholar]
- 12.Bagdure D, Curtis DJ, Dobyns E, Glode MP, Dominguez SR. Hospitalized children with 2009 pandemic influenza A (H1N1): comparison to seasonal influenza and risk factors for admission to the ICU. PloS one. 2010;5(12):e15173. doi: 10.1371/journal.pone.0015173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poritz MA, Blaschke AJ, Byington CL, Meyers L, Nilsson K, Jones DE, et al. FilmArray, an automated nested multiplex PCR system for multi-pathogen detection: development and application to respiratory tract infection. PloS one. 2011;6(10):e26047. doi: 10.1371/journal.pone.0026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [July 7, 2015];Centers for Disease Control and Prevention, Enterovirus D68 2014 real-time RT-PCR assay. http://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM446784.pdf.
- 15.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. American journal of respiratory and critical care medicine; The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination; 1994. pp. 818–24. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Pediatrics Committee on Infectious Diseases Red Book- Influenza chapter. Elk Grove Village, IL: 2012. [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayhew M. DHHS responses to Enterovirus D68 and Ebola Virus questions for October 15, 2014 HHS interim committee meeting; [June 1, 2015]. url: http://www.maine.gov/legis/opla/HHSEnterovirus%20EbolaResponses.pdf. [Google Scholar]
- 19.Tetro J. What You Need To Know About the Fast-Spreading Respiratory Virus EVD68. [June 1, 2015];2014 url: http://www.huffingtonpost.ca/jason-tetro/evd-68_b_5896172.html.
- 20.Messacar K, H S, Rao S, Baker J, Pearce K, Mistry RD, DeMasellis G, Tong S, Milton J, Dominguez SR, Parker S. An Unexpected Strain: Resource Burden of the 2014 Enterovirus D68 Respiratory 2 Disease Outbreak at Children’s Hospital Colorado. Submitted to JAMA Pediatrics. 2015 doi: 10.1001/jamapediatrics.2015.3879. [DOI] [PubMed] [Google Scholar]
- 21.Oermann CM, Schuster JE, Conners GP, Newland JG, Selvarangan R, Jackson MA. Enterovirus D68: A Focused Review and Clinical Highlights from the 2014 United States Outbreak. Annals of the American Thoracic Society. 2015;12(5):775–81. doi: 10.1513/AnnalsATS.201412-592FR. [DOI] [PubMed] [Google Scholar]
- 22.Schuster JE, Miller JO, Selvarangan R, Weddle G, Thompson MT, Hassan F, et al. Severe enterovirus 68 respiratory illness in children requiring intensive care management. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2015;70:77–82. doi: 10.1016/j.jcv.2015.07.298. [DOI] [PubMed] [Google Scholar]
- 23.Oberste MS, Maher K, Schnurr D, Flemister MR, Lovchik JC, Peters H, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. The Journal of general virology. 2004;85(Pt 9):2577–84. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 24.Grunstein MM, Hakonarson H, Whelan R, Yu Z, Grunstein JS, Chuang S. Rhinovirus elicits proasthmatic changes in airway responsiveness independently of viral infection. The Journal of allergy and clinical immunology. 2001;108(6):997–1004. doi: 10.1067/mai.2001.120276. [DOI] [PubMed] [Google Scholar]
- 25.Yamaya M, Sasaki H. Rhinovirus and asthma. Viral immunology. 2003;16(2):99–109. doi: 10.1089/088282403322017857. [DOI] [PubMed] [Google Scholar]
- 26.Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. Bmj. 1993;307(6910):982–6. doi: 10.1136/bmj.307.6910.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh K, Ellis EF, Hoffman LS, Lybass TG, Eller JJ, Fulginiti VA. The association of viral and bacterial respiratory infections with exacerbations of wheezing in young asthmatic children. The Journal of pediatrics. 1973;82(4):578–90. doi: 10.1016/S0022-3476(73)80582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minor TE, Dick EC, DeMeo AN, Ouellette JJ, Cohen M, Reed CE. Viruses as precipitants of asthmatic attacks in children. Jama. 1974;227(3):292–8. [PubMed] [Google Scholar]
- 29.Horn ME, Brain EA, Gregg I, Inglis JM, Yealland SJ, Taylor P. Respiratory viral infection and wheezy bronchitis in childhood. Thorax. 1979;34(1):23–8. doi: 10.1136/thx.34.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Bmj. 1995;310(6989):1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ilyushina NA, Khalenkov AM, Seiler JP, Forrest HL, Bovin NV, Marjuki H, et al. Adaptation of pandemic H1N1 influenza viruses in mice. Journal of virology. 2010;84(17):8607–16. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kash JC, Walters KA, Davis AS, Sandouk A, Schwartzman LM, Jagger BW, et al. Lethal synergism of 2009 pandemic H1N1 influenza virus and Streptococcus pneumoniae coinfection is associated with loss of murine lung repair responses. mBio. 2011;2(5) doi: 10.1128/mBio.00172-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Childs RA, Matrosovich T, Wharton S, Palma AS, Chai W, et al. Altered receptor specificity and cell tropism of D222G hemagglutinin mutants isolated from fatal cases of pandemic A(H1N1) 2009 influenza virus. Journal of virology. 2010;84(22):12069–74. doi: 10.1128/JVI.01639-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mak GC, Au KW, Tai LS, Chuang KC, Cheng KC, Shiu TC, et al. Association of D222G substitution in haemagglutinin of 2009 pandemic influenza A (H1N1) with severe disease. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2010;15(14) [PubMed] [Google Scholar]
- 35.Topfer L, Menk M, Weber-Carstens S, Spies C, Wernecke KD, Uhrig A, et al. Influenza A (H1N1) vs non-H1N1 ARDS: analysis of clinical course. Journal of critical care. 2014;29(3):340–6. doi: 10.1016/j.jcrc.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Imamura T, Okamoto M, Nakakita S, Suzuki A, Saito M, Tamaki R, et al. Antigenic and receptor binding properties of enterovirus 68. Journal of virology. 2014;88(5):2374–84. doi: 10.1128/JVI.03070-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


