Abstract
Background
Human Parainfluenza virus-3 (HPIV) is a common cause of respiratory infection in immunocompromised patients, and presently has no effective therapies. Virus-specific T-cell therapy has been successful for the treatment or prevention of viral infections in immunocompromised patients, but requires determination of T-cell antigens on targeted viruses.
Methods
HPIV3-specific T cells were expanded from peripheral blood of healthy donors using a rapid generation protocol targeting four HPIV3 proteins. Immunophenotyping was performed by flow cytometry. Viral specificity was determined by IFN-γ ELISpot, intracellular cytokine staining, and cytokine measurements from culture supernatants by Luminex assay. Cytotoxic activity was tested by 51Cr release and CD107a mobilization assays. Virus-specific T-cells targeting 6 viruses were then produced by rapid protocol, and the phenotype of HPIV3-specific T-cells was determined by immunomagnetic sorting for IFN-γ producing cells.
Results
HPIV3-specific T cells were expanded from 13 healthy donors. HPIV3-specific T-cells showed a CD4+ predominance (mean CD4:CD8 ratio 2.89), and demonstrated specificity for multiple HPIV3 antigens. The expanded T-cells were polyfunctional based on cytokine production, but only had a minor cytotoxic component. T cells targeting six viruses in a single product similarly showed HPIV3 specificity, with a predominant effector memory phenotype (CD3+/CD45RA-/CCR7-) in responder cells.
Discussion
HPIV3-specific T cells can be produced using a rapid ex vivo protocol from healthy donors and are predominantly CD4+ T-cells with Th1 activity. HPIV3 epitopes can also be successfully targeted alongside multiple other viral epitopes in production of 6-virus T-cells, without loss of HPIV3 specificity. These products may be clinically beneficial to combat HPIV3 infections by adoptive T-cell therapy in immune compromised patients.
Keywords: Immunodeficiency, immunotherapy, cytotoxic T-lymphocytes, antiviral therapy, Human Parainfluenza
Introduction
Human parainfluenza viruses (HPIVs) are enveloped, single-stranded negative sense RNA viruses in the Paramyxoviridae family with 4 major subtypes (HPIV 1-4) that cause acute respiratory infections (ARIs) in all ages, but notably in infants and young children.1 HPIV 1-3 account for 6.8% of all hospitalization for fever and/or ARI in children under 5 years of age in the United States, with HPIV3 accounting for the majority of these hospitalizations. HPIV 1 and 2 are more commonly associated with upper respiratory illnesses (URIs) such as croup while HPIV3 usually causes lower respiratory illnesses (LRIs) including pneumonia and bronchiolitis.2 Despite the prevalence of HPIV infection at a young age, reinfection is common.
While HPIV infections in healthy children most commonly cause self-limited, mild ARIs, children with immunodeficiency due to genetic disorders, solid organ transplantation or hematopoietic stem cell transplant (HSCT) are at increased risk for severe infections to HPIV.3,4 HPIV3 is the most common serotype isolated from immunocompromised patients with lower respiratory tract infections.1 One study showed that HPIV accounted for 47% of viral respiratory infections in pediatric HSCT patients with 41% developing pneumonia and 6% dying of this complication.5 In adult HSCT patients and patients with leukemia the development of HPIV infection results in mortality rates of 22-75%.1,6,7 Though typically thought of as a respiratory pathogen, HPIV has also been isolated from cerebrospinal fluid, pericardial fluid, liver, and myocardium of HSCT patients.8,9
Given the considerable morbidity and mortality associated with HPIV in the immune compromised host, there is significant interest in developing preventative and treatment strategies for this disease. While several vaccines are currently being tested in clinical trials, there has been limited success to date.1,10 Neuraminidase inhibitors have shown anti-HPIV activity in vitro but have not demonstrated clinical efficacy. High-titer pooled human immunoglobulin is often used for prevention and treatment of HPIV3 in immune compromised hosts but with limited evidence of efficacy. More recently, DAS181, a sialidase catalytic domain/amphiregulin glycosaminoglycan binding sequence fusion protein designed to prevent viral entry, has demonstrated in vivo efficacy in three immune compromised patients and is currently being investigated in clinical trials.11
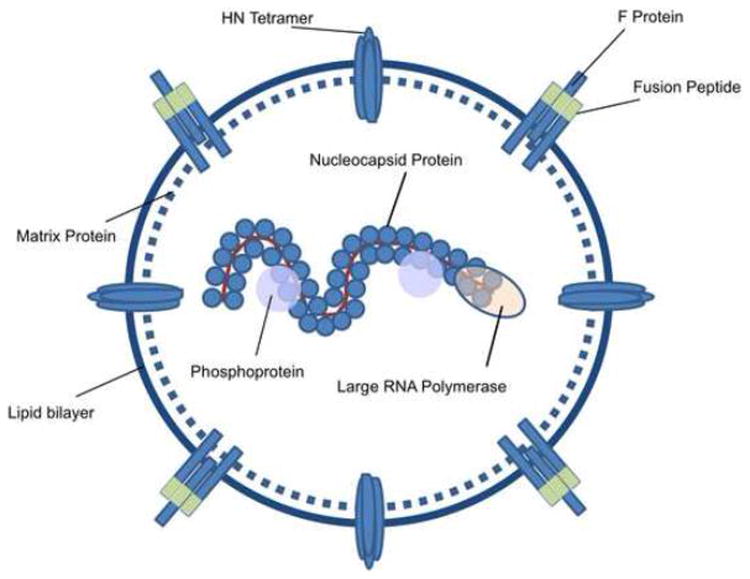
As delay in the recovery of T-cell immunity following HSCT is highly associated with viral reactivation and disease, adoptive cellular immunotherapy to restore antiviral immunity is an attractive treatment option.12 Adoptive transfer of virus-specific T-cells (VST) has proven to be a safe and effective strategy for prevention and treatment of Epstein Barr virus (EBV), cytomegalovirus (CMV), adenovirus (Adv), BK virus (BKV), and Human herpesvirus-6B (HHV6B) infections after HSCT.13-18 To date, adoptive T-cell therapy has never been attempted for prevention or treatment of HPIV3. To enable production of VST targeting HPIV3, the knowledge of viral antigens recognized by T-cells is necessary. The HPIV3 genome encodes only 6 known viral products, including two surface glycoproteins (hemagglutinin-neuramidase [HN] glycoprotein and the fusion protein [Fus]); a structural Matrix [Mat] protein, which is critical for virion assembly and budding; a nucleocapsid protein [NP]; and the P phosphoprotein and L protein, which interact to form the viral RNA polymerase (Figure 1).1 Data on T-cell determinants in HPIV are limited, but have been described to recognize the HN, P, and NP proteins of HPIV-1 and HPIV-3.19 In this study, we describe the generation of HPIV3-specific T-cells using a rapid ex vivo expansion protocol from healthy donors, and the characterization of the resulting cells, which may be useful in future trials of adoptive immunotherapy.
Figure 1. Human Parainfluenza 3 structure and viral proteins.
HPIV3 is an enveloped RNA virus. Surface proteins include hemaglutinnin-neuramidase (HN) and the Fusion (F) glycoprotein, which contains an extracellular fusion motif. Matrix protein permits virion budding, while nucleocapsid protein, phosphoprotein, and Large RNA polymerase are responsible for viral genome encapsidation and maintenance. Adapted from Fields Virology, Chapter 34, “Parainfluenza Viruses.”37
Methods
Subjects/Blood samples
Peripheral blood was collected from healthy donors who consented to the study at our institution, and peripheral blood mononuclear cells (PBMC) were isolated by Ficoll/Hypaque centrifugation.
Ex vivo expansion of HPIV3-specific VST
VST targeting HPIV3 were produced by a rapid protocol as previously described.20 Briefly, PBMC were pulsed with overlapping pools of 15-mer peptides encompassing the HPIV3 hemagglutinin/neuraminidase (HN), fusion (Fus), matrix (Mat), and nucleocapsid (NP) proteins at 0.5 nmol/peptide (JPT, Berlin, DE). Protein sequences from the HPIV3 Wash/47885/57 strain were utilized for peptide production.21 Pulsed PBMC were cultured in Grex-10 bioreactors (Wilson Wolf, New Brighton, MN) or 24-well plates, with medium containing 45% RPMI, 45% Click's medium, 10% fetal bovine serum, and supplemented with 2mM L-glutamine, IL-4 at 400IU/ml, and IL-7 at 10ng/ml (R&D Systems, Minneapolis, MN). VST were supplemented with cytokines at day 4 and medium at day 6-7, and were harvested between days 10-12.
Ex vivo expansion of 6-virus specific VST
VST targeting six viruses (CMV, EBV, Adv, HHV6B, BK virus, and HPIV3) were produced using the same methods as described above, but utilizing the following pepmixes for PBMC stimulation: CMV-pp65, CMV-IE1, EBV-LMP2, EBV-EBNA1, Adv-Hexon, Adv-Penton, HHV6B-U54, HHV6B-U90, BKV-Large T, BKV-VP1, HPIV3-NP, HPIV3-Fus (JPT). A pepmix mastermix was made using equimolar volumes of the 12 pepmixes so that PBMCs were pulsed with 0.5 nmol/peptide.
IFN-γ ELISpot
Ninety-six-well filtration plates (MultiScreen, MSIPS4W10; Millipore, Billerica, MA) were coated overnight with 10ug/ml anti-hu-IFN-mAb (capture mAB (1-DIK purified); Mabtech). VST (1×105 cells/well) or PBMC (2×105 cells/well) were plated in ninety-six well plates and stimulated with overlapping 15-mer peptide pools encompassing HPIV3 proteins (HN, Fus, Mat, and NP) at 0.5nmol/peptide/well. Each condition was run in duplicate or triplicate. Staphylococcal enterotoxin-B (SEB) was used as a positive control (1ug/ml). A 15-mer peptide pool encompassing the actin protein was used as a negative control. After 18–24 hours, the plates were washed and incubated with the secondary biotin-conjugated anti-human-IFNγ- mAb (detector mAb (7-B6-1 biotin); Mabtech, Cincinnati, OH) at 1ug/ml. After incubation with avidin:biotinylated HRP complex (Vectastain Elite ABC Kit (standard), PK6100; Vector Laboratories, Burlingame, CA), plates were developed with AEC substrate (A6926, Sigma-Aldrich, St Louis, MO), dried overnight, and quantified (Zellnet Consulting, New York, NY). The frequency of T-cells specific for each peptide was expressed as spot-forming cells (SFC) per 1×105 cells (or 2×105 cells for PBMC conditions). A correction for confluence was applied as follows: Well count + 2×(Well count × (% confluence / [1- %confluence])). Blocking of MHC class I and class II was performed by incubation of T-cells with mouse polyclonal antibodies to human HLA-A/B/C or HLA-DR/DP/DQ (Dako, Carpinteria, CA) for 1 hour prior to plating for ELISpot assay as described above. Responses that were at least 20 SFC/1 × 105 cells greater than background (unstimulated VSTs plated with media alone) were regarded as significant.
Flow cytometry
Staining of cell surface markers on PBMC and T-cells was performed with CD3-APC/Vio770, CD4-Vioblue, CD8-Viogreen, CD19-FITC, CD56-PE/Vio770, CD16-PE, CD62L-Vioblue, CD45RA-PE, CD45RO-APC, and CCR7-FITC (Miltenyi, San Diego, CA; BD, Franklin Lakes, NJ). All samples were acquired on a MACSQuant Cytometry (Miltenyi) and the data analyzed with Flow Jo (Treestar, Ashland, OR).
Intracellular cytokine staining
T-cells were rested with IL-2 (100U/ml) overnight and then stimulated with pepmixes encompassing viral or control proteins (JPT) at 0.5 nmol/ul, along with anti-CD49d/CD28 antibodies (BD) at 1:1000 for co-stimulation and Brefeldin A at 1ul/ml. SEB and Actin were utilized as controls. T-cells were stained with cell surface markers (CD3, CD4, CD8), followed by permeabilization with Cytofix/Cytoperm (BD FastImmune), washing, and staining with IFN-γ-APC (Biolegend, San Diego, CA), IL-2-FITC, and TNFα-PE (BD).
CD107A mobilization
For evaluation of CD107a mobilization, T-cells were stimulated with viral or control pepmixes (JPT) in the presence of Brefeldin A at 1ul/ml for 4 hours, followed by surface marker staining utilizing the antibodies listed above. Samples were acquired on MACSQuant Analyzer 10 (Miltenyi Biotec) and analyzed with Flow Jo (Treestar, Ashland, OR).
Interferon-γ secretion assay
Measurement of IFN-γ secretion by HPIV3-specific T-cells in 6-virus VST was performed in cells that were stimulated for 4 hours with or without 2.5 μl/ml HPIV3 pepmixes (Fus, NP) in presence of 1μl/ml CD49d/CD28 antibodies. Cell surface detection of IFN-γ secreted molecules was performed with an IFN-γ specific high affinity capture matrix, subsequently stained with PE-anti IFN-γ, and magnetically labeled with anti-PE microbeads following manufacturer recommendations (Miltenyi Biotec). PE-IFN-γ+ cells were enriched and analyzed with a MACSQuant Analyzer 10 (Miltenyi Biotec). Cell surface phenotype analysis was performed via CD4 and CD8 VioBlue; CD45RA-APC VioGreen; CCR7 APC; and CD62L-FITC mAbs (Miltenyi Biotec).
Cytotoxicity assay
Cytotoxicity was measured by standard 51Cr release assay. Briefly, autologous or allogeneic phytohemagglutinin (PHA)-stimulated lymphoblasts were produced, pre-incubated with IFNγ (100ng/ml) alone or with HPIV3 pepmixes (0.5nmol/peptide/ml of each protein) for 12 hours, and then pulsed with 51Cr (Perkin Elmer, Waltham, MA) at 10 uCi per 1×105 cells for 1 hour and washed. Effector T-cells were pre-incubated overnight with IL-2 (100u/ml) and then plated with radiolabeled target cells at multiple effector to target ratios. Maximum release was determined by lysis of radiolabeled targets with 1% Triton-X100 detergent (Sigma-Aldrich). Targets and effectors were incubated at 37°C for 4-6 hours, centrifuged, and supernatants transferred to a 96-well scintillation plate and allowed to dry. Plates were read on a MicroBeta2 LumiJet Scintillation counter (Perkin Elmer). Specific lysis was determined as follows: (Experimental counts per minute [CPM] − Background CPM) / (Maximum CPM − Background CPM). All conditions were performed in triplicate.
Multiplex cytokine assay
VST were plated on 96 well plates at 1×105 cell per well and stimulated with HPIV3 pepmixes or controls (SEB, Actin) at the same conditions as described for IFNγ ELISpot, and incubated overnight at 37°C, 5% CO2. Suspensions underwent centrifugation, and supernatants were removed for analysis. Cytokine analysis of the supernatants was performed using a custom Bio-Plex Pro kit (Bio-Rad, Hercules, CA) per manufacturer's protocol, and was analyzed on a Bio-Plex MAGPIX Multiplex Reader (Luminex, Madison, WI) using Bio-Plex Manager 6.1.
Data Analysis / Statistics
Results were evaluated using descriptive statistics (means, standard deviations, ranges). Comparative analysis between ELISpot and flow cytometry results for different epitopes was performed via one-way ANOVA. Comparisons between responses to viral peptides and irrelevant peptides were performed using a 2-sided unpaired T-Test. Analysis was performed in Graphpad Prism (GraphPad software, La Jolla, CA) and StatPlus (AnalystSoft, Walnut, CA).
Results
Ex vivo expanded CD4+ and CD8+ HPIV3 specific VSTs recognize multiple viral antigens
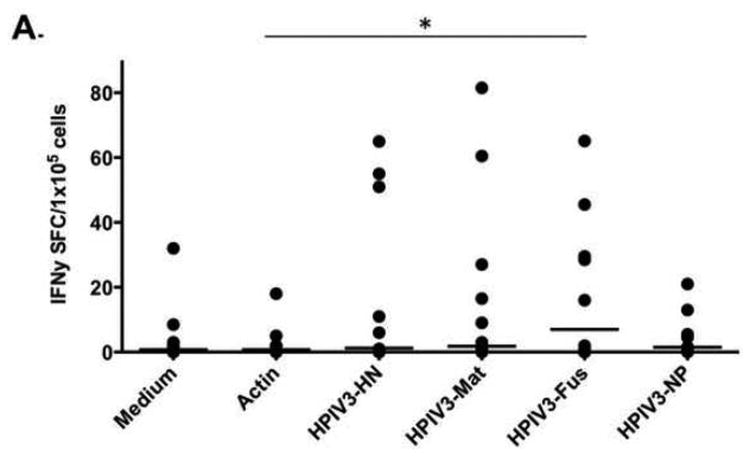
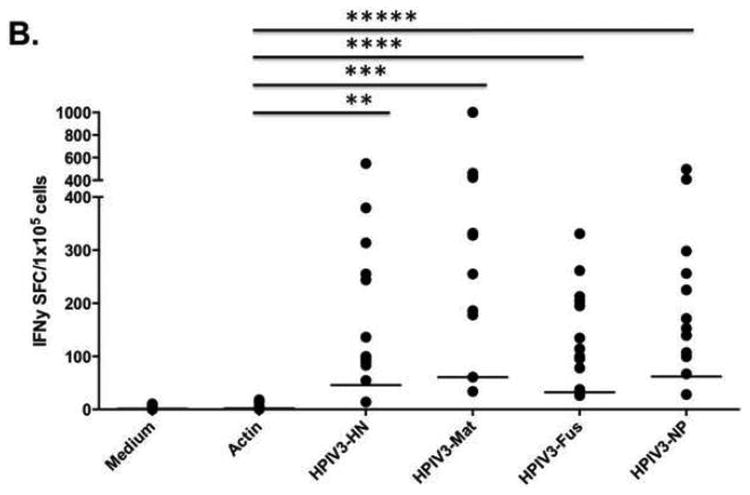
Immunity to HPIV3 was evaluated in donor PBMCs as baseline using IFNγ ELISpot assay. Only low-level responses to viral proteins in selected individuals were detected (Figure 2A). Compared with the Actin control, only the immune response to HPIV3-Fusion protein reached significance (p=0.047). Following stimulation with viral pepmixes and culture, VSTs expanded a mean of 4.41 fold (range 3.33 to 5.56). At day 10, donor VST showed significant responses to all viral pepmixes using IFNγ ELISpot assay (Figure 2B) (p≤0.00027, unpaired two-sided T-Test). Among the IFNγ responses to HPIV3 antigens, Matrix protein generated the highest spot counts (mean 333 SFC) compared to responses to the other antigens (mean SFC 173 [HN], 140 [Fus], 219 [NP], p=0.00181 [one-way ANOVA]).
Figure 2. IFNγ ELISpot responses to HPIV3 proteins.
A) Responses to HPIV3 protein from donor PBMCs at day 0 were seen in only 3 of 7 tested donors, and only reached statistical significance for Fusion protein (* p=0.047). B) At day 10 of culture, responses to HPIV3 were robust and uniform (n=13), with responses to each protein reaching statistical significance (**p=0.00005, ***p=0.0000002, ****p=0.00027, *****p=0.00022).
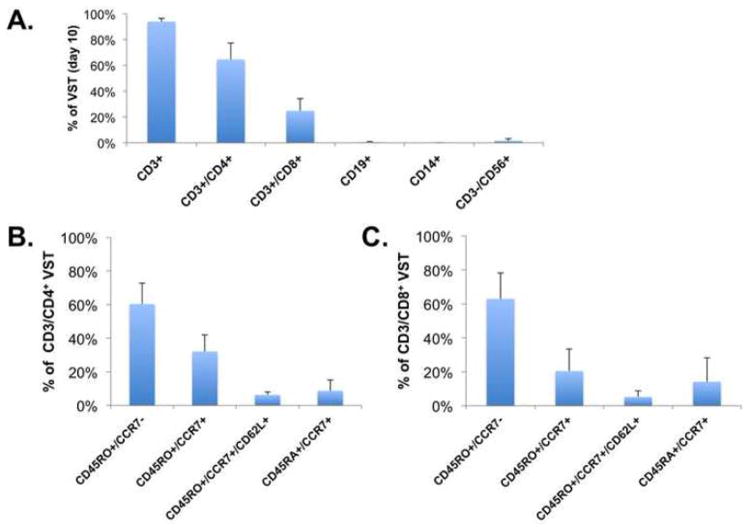
All of the final VST products were largely made up of T-cells (Figure 3A), with a strong CD4+ predominance (mean CD4:CD8 ratio 2.89, range 1.23-8.85). There was no B-cell outgrowth and minimal monocytes remaining in the final cultures. Natural killer cells were detectable at low level (mean 1.65% of lymphocytes, range 0.62-6.26%). Both CD4+ and CD8+ subsets consisted primarily of effector memory (60-63%) and central memory T cells (25-38%) with a small population of naïve T cells (Figures 3B and 3C) with no difference between CD4+ and CD8+ subsets. To determine whether the responses to HPIV3 pepmixes were class I and/or class II restricted, ELISPOT assays using MHC class I and II blocking antibodies were performed. As shown in Supplemental Figure 1A, following MHC class I and II antibody blockade, class II responses to the HPIV3 pepmixes via IFNγ ELISpot showed significantly attenuated responses to Fusion, and NP proteins in one HPIV3-VST product (p=0.05 and p=0.033, respectively). In a second HPIV3-VST product tested, both class I and class II restricted responses were observed (Supplemental Figure 1B).
Figure 3. Surface phenotyping of HPIV3-VST.
A) Surface marker phenotype of HPIV3-VST at day 10 (n=11, error bars: standard deviation) showed predominantly CD4+ T-cells (mean 68%). B) CD4+ T cell subsets and C) CD8+ T cell subsets showing predominantly effector memory (CD45RO+/CCR7-) phenotypes with smaller populations of central memory (CD45RO+/CCR7+/CD62L+) and naïve (CD45RA+/CCR7+) T cells.
HPIV3-VST products are polyfunctional in vitro
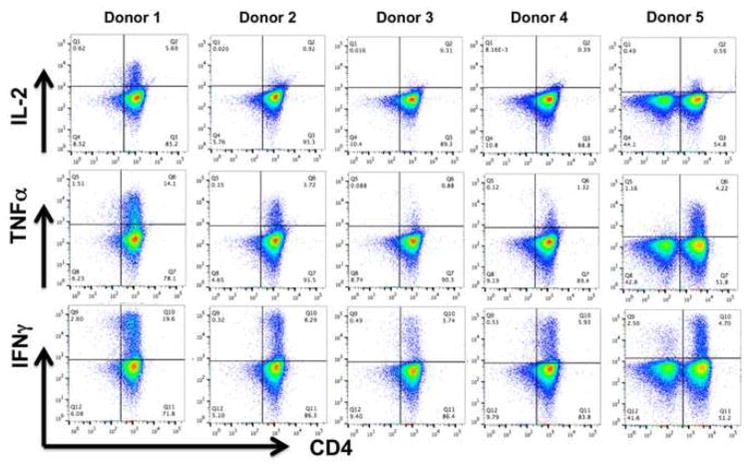
Intracellular cytokine assays were performed using flow cytometry on VST products at day 10 following restimulation with HPIV3 pepmixes. As shown in Figure 4, IFNγ and TNFα cytokine release was detected in the CD4+ T-cell populations. IL-2 production in response to HPIV3 pepmix was generally absent, with only one VST product (donor 1) showing a specific IL-2 response. No significant cytokine release was noted from the CD8+ population. Cytokine analysis of VST supernatants following restimulation with HPIV3 pepmixes also showed a response predominated by IFNγ and TNFα, with low-level production of IL-10, and undetectable IL-6 and IL-2 (Supplemental Figure 2).
Figure 4. Intracellular cytokine staining of HPIV3-VST.
Intracellular cytokine staining of HPIV3-VST following restimulation with viral pepmix showed polyfunctionality, with two cytokines (INFγ, TNFα) produced by VST from 4 donors, and one VST product producing IFNγ, TNFα, and IL-2.
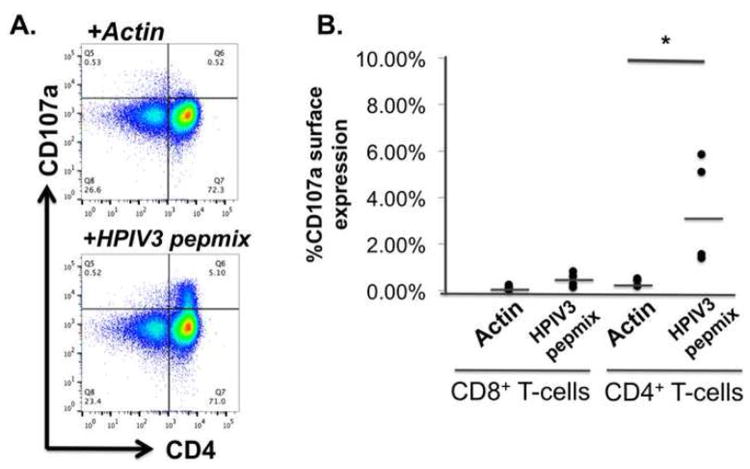
To evaluate the cytolytic capacity of the VST products, degranulation of VST via CD107a mobilization was evaluated following stimulation with viral or control pepmix. As shown in Figure 5A, a component of the VST population expressed CD107a in response to HPIV3 pepmixes, which reached significance only in the CD4+ fraction (Figure 5B)(p=0.039, unpaired two-way T-test). In addition, using 51Cr radiolabeled peptide-pulsed PHA blasts or EBV-LCL as targets, only minimal cytotoxicity was observed in a 6 hour assay consistent with a predominantly CD4+ mediated response (Supplemental 3).
Figure 5. Cytotoxicity testing of HPIV3-VST.
A) VST degranulation was noted in response to HPIV3 proteins by CD107a mobilization. B) Specific degranulation of VST was only noted in the CD4+ fraction (* p=0.039).
Ex vivo expanded multiviral VST products show specificity for six viruses including HPIV3
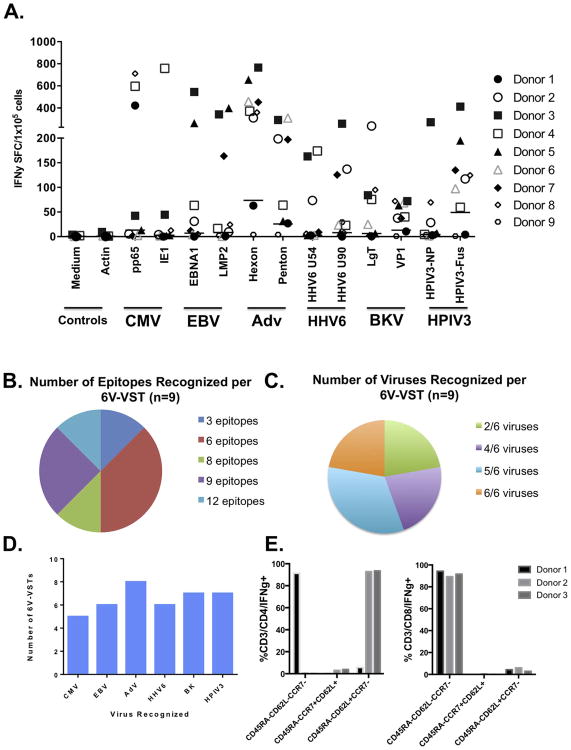
Following rapid 10-day ex vivo expansion against 12 viral pepmixes from 6 viruses (CMV [pp65, IE1], EBV [LMP2, EBNA1] Adv [Hexon, Penton], HHV6 [U54, U90], BKV [Large T, VP1] HPIV3 [Fus, NP]), VST were responsive to a median of 6 of 12 viral proteins (range 3-12) and 5 of 6 viruses (range 2-6) (Figures 6B-C). Seven of nine of the VST products showed responses to at least one HPIV3 peptide (Figures 6D). Testing of three of the 6V-VST products via immunomagnetic IFNγ capture assay showed that similar to the single virus targeted product, CD4+ and CD8+ T-cells were HPIV3-specific and predominantly of an effector memory phenotype (CD45RA-/CCR7-/CD62L+/-, Figure 6E and Supplemental 4).
Figure 6. Evaluation of 6V-VST by IFNγ ELISpot and Gamma capture.
A) ELISpot evaluation of 6V-VST (n=7) showed activity against included HPIV3 pepmixes (Fus, NP) in seven of nine 6V-VST products. B) 6V-VSTs recognized multiple epitopes and C) multiple viruses per product as determined by ELISpot evaluation. D) The majority of 6V-VST recognized at least one epitope from multiple viruses. E) 6V-VSTs isolated IFNγ capture following stimulation with HPIV3 pepmixes were predominantly CD45RA-CCR7-, but differed with regard to CD62L expression, with CD4+ T-cells expressing CD62L in two of three products tested, while CD8+ T-cells were uniformly CD62L-.
Discussion
HPIV3 is a common respiratory virus that causes appreciable morbidity and mortality in immunocompromised patients, yet currently no preventative and treatment strategies exist. Based on the previous success using multivirus-specific VSTs for the prophylaxis and treatment of other viral infections post-HSCT13,15,16,20,22-26, we aimed to develop a similar therapeutic targeting HPIV3 as this is the most frequent HPIV subtype to affect immunocompromised patients.
Here, we show that HPIV3-specfic VSTs can be expanded from healthy donors to a clinically useful cell volume using a straightforward strategy in less than two weeks. This rapid manufacturing timeframe ensures that HPIV3 VSTs potentially have wide clinical applicability, particularly as immunocompromised patients have prolonged shedding of HPIV3.27,28 All HPIV3-VST demonstrated specificity for multiple HPIV3 antigens, with 12 of 13 donors recognizing all four of the targeted antigens by IFN-γ ELISpot assay. Quantitatively, the response to Matrix protein was the most robust based on cytokine production. Though the ideal HPIV3 viral antigens to target when generating a product for clinical use are unknown, Matrix protein has been shown to be highly conserved across HPIV3 strains,21 which is not surprising given its role in viral packaging. Comparatively, mutational drift in the HN and Fusion glycoproteins has been described.29,30 However, the sequence homology between the Wash/47885/57 strain and other sequenced HPIV3 strains is relatively high for the 4 tested viral proteins (Supplemental 6). In practice, targeting of multiple viral proteins should improve VSTs clinical efficacy by reducing the risk of antigenic escape through mutations and strain variations. While previous studies using multivirus VSTs have demonstrated antigenic competition in which more immunogenic viral antigens such as CMVpp65 and CMV IE1 tend to dominate cultures,16 we have demonstrated that HPIV3 can be targeted in multiviral VSTs as 7 out of 9 multivirus specific T cell products showed HPIV3 specificity (Figures 6A, 6D). Of note, the only VST products that did not have HPIV3 specificity (from Donors 1 and 9) were only specific for 2 of 6 viruses while all others VSTs were specific for 4 or more viruses. One limitation of this study is that the serostatus of some donors was unknown, and thus, more antigenic competition may potentially be observed if more CMV and EBV seropositive donors are included. However, Donor 8 was known to be EBV and CMV seropositive and demonstrated virus-specific activity for 5 of 6 viruses (Figure 6A), including both HPIV3 NP and Fus, establishing that HPIV3 can be targeted alongside more immunogenic viral antigens such as CMV and EBV. Hence, the ability to target multiple common viruses that contribute to the mortality and morbidity of patients following HSCT within a single VST product could greatly broaden the applicability of this therapeutic strategy by avoiding the cost and time associated with generating multiple single virus-specific VST products.
In addition to targeting multiple HPIV3 antigens, the HPIV3-specific VSTs were also polyfunctional as demonstrated by intracellular staining (Figure 4) and cytokine production (Supplemental 2). The predominance of IFNγ and TNFα with comparatively low production of IL-10 is suggestive of a TH1 CD4+ phenotype. This is important since polyfunctional T-cells have been associated with improved cellular immunity and viral control in vivo.31
HPIV3 VSTs showed a strong predominance of CD4+ T cells compared to CD8+ T cells. Cytotoxic activity of the final VST cultures was not robust, and CD107a mobilization showed that degranulation occurred almost exclusively in CD4+ T-cells. This was not surprising given the predominance of CD4+ T-cells in the final VST cultures, and we have shown in other studies that in vitro cytotoxicity does not necessarily predict efficacy in vivo especially when products are predominantly CD4+.16,32 Moreover, a similar CD4+ T-cell predominance has also been described in previous studies targeting adenoviral-hexon protein,33,34 and likely reflects the importance of TH1 CD4+ T-cells in clearance of respiratory viruses. Additionally, Einsele et al. showed that infusion of CD4+ CMV T-cell products in post-HSCT patients with refractory CMV not only rendered CMV levels undetectable but also increased the in vivo recovery of CMV-specific CD8+ T-cells.35 Whether these cells may aid by recruitment of other T-cell and innate-like lymphocyte subsets, direct cytolytic activity, or initiation of a sterilizing B-cell response is not clear. Additionally, we observed a population of HPIV3-specific CD4+ cells in the 6V-VSTs that were CD45RA-CCR7- consistent with effector memory phenotype but with expression of CD62L, which suggests that this population may have been recently derived from the central memory or stem cell memory (Tscm) compartment. Given the presence of CD4+ and CD8+ central memory (CD45RO+CCR7+CD62L+) subsets in both HPIV3-VSTs and 6V-VSTs, which have been described to be essential for T-cell persistence,36 this approach could potentially elicit sustained antiviral immunity in vivo.
In summary, we have developed an efficient and reliable method to expand HPIV3-specific T-cells ex vivo from healthy donors, which may be valuable as a novel therapeutic option for the prophylaxis and treatment of HPIV3 infection in immune compromised patients. Given the successful use of peptide stimulation to produce multiviral VST with a similar rapid ex vivo expansion protocol using Good Manufacturing Practices,13 we are hopeful that this strategy will easily translate to future clinical trials to test the safety and efficacy of VST targeting HPIV3.
Supplementary Material
Supplemental Figure 1: IFNγ ELIspot responses with HLA class I / II blocking (n=2) showed a trend toward decreased responses to HN, Fusion, and NC proteins following HLA class II blocking in one HPIV3-VST tested (A) but not the second (B). These findings were statistically significant for responses to Fusion (*p= 0.050) and NP (**p=0.033) antigens as shown in Supplemental Figure 1A. Responses to Matrix protein were excluded from analysis due to complete confluence in wells.
Supplemental Figure 2: Cytokine measurements in culture supernatants by Bioplex showed production of IFNγ and TNFα, and low level production IL-10 in response to all targeted HPIV3 protein in comparison with controls (n=3, bars: mean concentrations, error bars: standard deviation).
Supplemental Figure 3: Cytotoxicity testing of VST by 51Cr release assay against autologous PHA blast targets (alone or pulsed with HPIV3 pepmix) showed a low level cytotoxic activity against pulsed targets.
Supplemental Figure 4: Phenotyping of HPIV3-specific cells isolated from 6-virus VST by IFNγ capture following pepmix-restimulation showed a predominant effector memory population of responders. IFNγ+ responders were predominantly T-cells in response to either HPIV3 pepmixes or the Staphylococcal enterotoxin B (SEB) control, with minimal IFNγ+ responders in the unstimulated VST product.
Supplemental Figure 5: Surface phenotyping of 6V-VSTs day 10 (n=9, error bars: standard deviation) showed a majority of CD4+ T-cells (mean: 63.8 ± 18.6%). CD3+ T cells were predominantly effector memory as shown by IFNγ capture (Figure 6E and Supplemental Figure 4) but also included smaller populations of central memory (CD3+CD45RA-/CCR7+/CD62L+, mean 4.12 ± 1.52%) and naïve (CD3+CD45RA+/CCR7+, mean 9.24 ± 1.52%) T cells.
Supplemental Figure 6: The sequence homology between the Wash/47885/57 strain and other sequenced HPIV3 strains is relatively high for the 4 tested viral proteins, with higher degree of conservation in the Matrix and Fusion proteins (Uniprot database, www.uniprot.org).
Highlights.
Human Parainfluenza Virus-3 (HPIV3) specific T-cells can be generated from healthy donors using a rapid ex vivo expansion protocol
HPIV3-specific T-cells have a CD4+ predominance and are polyfunctional based on cytokine production, with a minor cytotoxic component.
HPIV3 antigens can be targeted alongside other viruses during ex vivo expansion of multiple-specific T-cells without loss of HPIV3 specific activity.
Acknowledgments
The authors would like to thank our donors, Dr. Fahmida Hoq, Dr. David Jacobsohn, the Clinical and Translational Science Institute at Children's National Medical Center, the staffs of the Divisions of Blood and Marrow Transplantation, Allergy and Immunology, and the Program for Cell Enhancement and Technologies for Immunotherapy at Children's National Medical Center, and the Jeffrey Modell Foundation for their support of this research.
Funding: This research was funded by grants from the National Institutes of Health: U54 HL081007 (to CMB), National Cancer Institute PO1 CA148600e02 (to CMB), K12 HD001399 (to MDK), the Clinical and Translational Science Institute at Children's National (to MDK), and a Translational Research Grant from the Jeffrey Modell Foundation (to MDK)
Abbreviations
- HPIV3
Human Parainfluenza-3
- HSCT
hematopoietic stem cell transplantation
- EBV
Epstein Barr virus
- CMV
Cytomegalovirus
- Adv
Adenovirus
- BKV
BK virus
- HHV6B
Human herpesvirus-6B
- LCL
lymphoblastoid cell lines
- PBMC
peripheral blood mononuclear cells
- VST
virus-specific T-lymphocytes
- SFC
spot forming cells
- PHA
Phytohemagglutinin-stimulated lymphoblasts
Footnotes
COI Statement: The authors have no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003;16(2):242–64. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154(5):694–9. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Hutspardol S, Essa M, Richardson S, et al. Significant Transplantation-Related Mortality from Respiratory Virus Infections within the First One Hundred Days in Children after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2015;21(10):1802–7. doi: 10.1016/j.bbmt.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis. 2014;59(5):S344–51. doi: 10.1093/cid/ciu623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lujan-Zilbermann J, Benaim E, Tong X, Srivastava DK, Patrick CC, DeVincenzo JP. Respiratory virus infections in pediatric hematopoietic stem cell transplantation. Clin Infect Dis. 2001;33(7):962–8. doi: 10.1086/322628. [DOI] [PubMed] [Google Scholar]
- 6.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573–8. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 7.Lewis VA, Champlin R, Englund J, et al. Respiratory disease due to parainfluenza virus in adult bone marrow transplant recipients. Clin Infect Dis. 1996;23(5):1033–7. doi: 10.1093/clinids/23.5.1033. [DOI] [PubMed] [Google Scholar]
- 8.Baxter BD, Couch RB, Greenberg SB, Kasel JA. Maintenance of viability and comparison of identification methods for influenza and other respiratory viruses of humans. J Clin Microbiol. 1977;6(1):19–22. doi: 10.1128/jcm.6.1.19-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishaut M, Tubergen D, McIntosh K. Cellular response to respiratory viruses with particular reference to children with disorders of cell-mediated immunity. J Pediatr. 1980;96(2):179–86. doi: 10.1016/s0022-3476(80)80799-2. [DOI] [PubMed] [Google Scholar]
- 10.Englund JA, Karron RA, Cunningham CK, et al. Safety and infectivity of two doses of live-attenuated recombinant cold-passaged human parainfluenza type 3 virus vaccine rHPIV3cp45 in HPIV3-seronegative young children. Vaccine. 2013;31(48):5706–12. doi: 10.1016/j.vaccine.2013.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moscona A. Entry of parainfluenza virus into cells as a target for interrupting childhood respiratory disease. J Clin Invest. 2005;115(7):1688–98. doi: 10.1172/JCI25669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leen AM, Heslop HE, Brenner MK. Antiviral T-cell therapy. Immunol Rev. 2014;258(1):12–29. doi: 10.1111/imr.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Science translational medicine. 2014;6(242):242ra83. doi: 10.1126/scitranslmed.3008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013 doi: 10.1038/mt.2013.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leen AM, Christin A, Myers GD, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114(19):4283–92. doi: 10.1182/blood-2009-07-232454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]
- 17.Koehne G, Hasan A, Doubrovina E, et al. Immunotherapy with Donor T Cells Sensitized with Overlapping Pentadecapeptides for Treatment of Persistent Cytomegalovirus Infection or Viremia. Biol Blood Marrow Transplant. 2015;21(9):1663–78. doi: 10.1016/j.bbmt.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Reilly RJ, Small TN, Papadopoulos E, Lucas K, Lacerda J, Koulova L. Biology and adoptive cell therapy of Epstein-Barr virus-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol Rev. 1997;157:195–216. doi: 10.1111/j.1600-065x.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 19.Dave VP, Allan JE, Slobod KS, et al. Viral cross-reactivity and antigenic determinants recognized by human parainfluenza virus type 1-specific cytotoxic T-cells. Virology. 1994;199(2):376–83. doi: 10.1006/viro.1994.1135. [DOI] [PubMed] [Google Scholar]
- 20.Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther. 2012;20(8):1622–32. doi: 10.1038/mt.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes A, Tierney EL, Murphy BR, Hall SL. The complete nucleotide sequence of the JS strain of human parainfluenza virus type 3: comparison with the Wash/47885/57 prototype strain. Virus Res. 1992;25(1-2):91–103. doi: 10.1016/0168-1702(92)90102-f. [DOI] [PubMed] [Google Scholar]
- 22.Naik S, Nicholas SK, Martinez CA, et al. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2015.12.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanley PJ, Keller MD, Martin Manso M, et al. A Phase 1 Perspective: Multivirus-Specific T-cells from both Cord Blood and Bone Marrow Transplant Donors. Cytotherapy. 2016;18(6):S8. [Google Scholar]
- 24.Ma CK, Blyth E, Clancy L, et al. Addition of varicella zoster virus-specific T cells to cytomegalovirus, Epstein-Barr virus and adenovirus tri-specific T cells as adoptive immunotherapy in patients undergoing allogeneic hematopoietic stem cell transplantation. Cytotherapy. 2015;17(10):1406–20. doi: 10.1016/j.jcyt.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745–58. doi: 10.1182/blood-2012-08-448977. [DOI] [PubMed] [Google Scholar]
- 26.Micklethwaite KP, Clancy L, Sandher U, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112(10):3974–81. doi: 10.1182/blood-2008-06-161695. [DOI] [PubMed] [Google Scholar]
- 27.Lehners N, Tabatabai J, Prifert C, et al. Long-Term Shedding of Influenza Virus, Parainfluenza Virus, Respiratory Syncytial Virus and Nosocomial Epidemiology in Patients with Hematological Disorders. PLoS One. 2016;11(2):e0148258. doi: 10.1371/journal.pone.0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lima CR, Mirandolli TB, Carneiro LC, et al. Prolonged respiratory viral shedding in transplant patients. Transpl Infect Dis. 2014;16(1):165–9. doi: 10.1111/tid.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goya S, Mistchenko AS, Viegas M. Phylogenetic and molecular analyses of human parainfluenza type 3 virus in Buenos Aires, Argentina, between 2009 and 2013: The emergence of new genetic lineages. Infect Genet Evol. 2016;39:85–91. doi: 10.1016/j.meegid.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Mizuta K, Tsukagoshi H, Ikeda T, et al. Molecular evolution of the haemagglutinin-neuraminidase gene in human parainfluenza virus type 3 isolates from children with acute respiratory illness in Yamagata prefecture, Japan. J Med Microbiol. 2014;63(Pt 4):570–7. doi: 10.1099/jmm.0.068189-0. [DOI] [PubMed] [Google Scholar]
- 31.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–55. [PubMed] [Google Scholar]
- 33.Feuchtinger T, Richard C, Joachim S, et al. Clinical grade generation of hexon-specific T cells for adoptive T-cell transfer as a treatment of adenovirus infection after allogeneic stem cell transplantation. J Immunother. 2008;31(2):199–206. doi: 10.1097/CJI.0b013e31815ef862. [DOI] [PubMed] [Google Scholar]
- 34.Leen AM, Christin A, Khalil M, et al. Identification of hexon-specific CD4 and CD8 T-cell epitopes for vaccine and immunotherapy. J Virol. 2008;82(1):546–54. doi: 10.1128/JVI.01689-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Einsele H, R E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee H, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916–22. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 36.Busch DH, Frassle SP, Sommermeyer D, Buchholz VR, Riddell SR. Role of memory T cell subsets for adoptive immunotherapy. Semin Immunol. 2016 doi: 10.1016/j.smim.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karron RA, Collins PL. Parainfluenza Viruses. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 996–1023. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: IFNγ ELIspot responses with HLA class I / II blocking (n=2) showed a trend toward decreased responses to HN, Fusion, and NC proteins following HLA class II blocking in one HPIV3-VST tested (A) but not the second (B). These findings were statistically significant for responses to Fusion (*p= 0.050) and NP (**p=0.033) antigens as shown in Supplemental Figure 1A. Responses to Matrix protein were excluded from analysis due to complete confluence in wells.
Supplemental Figure 2: Cytokine measurements in culture supernatants by Bioplex showed production of IFNγ and TNFα, and low level production IL-10 in response to all targeted HPIV3 protein in comparison with controls (n=3, bars: mean concentrations, error bars: standard deviation).
Supplemental Figure 3: Cytotoxicity testing of VST by 51Cr release assay against autologous PHA blast targets (alone or pulsed with HPIV3 pepmix) showed a low level cytotoxic activity against pulsed targets.
Supplemental Figure 4: Phenotyping of HPIV3-specific cells isolated from 6-virus VST by IFNγ capture following pepmix-restimulation showed a predominant effector memory population of responders. IFNγ+ responders were predominantly T-cells in response to either HPIV3 pepmixes or the Staphylococcal enterotoxin B (SEB) control, with minimal IFNγ+ responders in the unstimulated VST product.
Supplemental Figure 5: Surface phenotyping of 6V-VSTs day 10 (n=9, error bars: standard deviation) showed a majority of CD4+ T-cells (mean: 63.8 ± 18.6%). CD3+ T cells were predominantly effector memory as shown by IFNγ capture (Figure 6E and Supplemental Figure 4) but also included smaller populations of central memory (CD3+CD45RA-/CCR7+/CD62L+, mean 4.12 ± 1.52%) and naïve (CD3+CD45RA+/CCR7+, mean 9.24 ± 1.52%) T cells.
Supplemental Figure 6: The sequence homology between the Wash/47885/57 strain and other sequenced HPIV3 strains is relatively high for the 4 tested viral proteins, with higher degree of conservation in the Matrix and Fusion proteins (Uniprot database, www.uniprot.org).