Abstract
Purpose
Recent data have not demonstrated improved outcomes when guideline-concordant (GC) antibiotics are given to patients with healthcare-associated pneumonia (HCAP). This study was designed to evaluate the relationship between health outcomes and GC therapy in patients admitted to an ICU with HCAP.
Materials and Methods
We performed a population-based cohort study of patients admitted to >150 hospitals in the U.S. Veterans Health Administration system to compare baseline characteristics, bacterial pathogens, and health outcomes in ICU patients with HCAP receiving either GC-HCAP therapy, GC community-acquired pneumonia (GC-CAP) therapy, or non-GC therapy. The primary outcome was 30-day patient mortality. Risk factors for the primary outcome were assessed in a multivariable logistic regression model.
Results
A total of 3,593 patients met inclusion criteria and received GC-HCAP therapy (26%), GC-CAP therapy (23%), or non-GC therapy (51%). GC-HCAP patients had higher 30-day patient mortality compared to GC-CAP patients (34% vs. 22%, p<0.0001). After controlling for confounders, risk factors for 30-day patient mortality were vasopressor use (OR, 95% CI; 1.67, 1.30–2.13), recent hospital admission (1.53, 1.15–2.02), and receipt of GC-HCAP therapy (1.51, 1.20–1.90).
Conclusions
Our data do not demonstrate improved outcomes among ICU patients with HCAP who received GC-HCAP therapy.
Keywords: pneumonia, critical care, guideline-concordant therapy, health outcomes, antibiotic therapy
INTRODUCTION
The concept of healthcare-associated pneumonia (HCAP) has been surrounded by controversy since its introduction in 2005 [1]. The growing body of HCAP literature has demonstrated that community-dwelling patients admitted to the hospital with pneumonia and HCAP risk factors have more comorbidities, are more severely-ill, and experience higher rates of mortality than similar patients without HCAP risk factors [2–11]. These studies also indicate a higher incidence of multi-drug resistant (MDR) pathogens (e.g., Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus [MRSA]) in patients with HCAP, although some data from specific geographic regions reveal a pathogen distribution more similar to community-acquired pneumonia (CAP), with low absolute rates of MDR pathogens [4,6,7,9].
Multiple studies have correlated guideline-concordant (GC) CAP therapy with improved outcomes in patients with CAP [12]; however, this does not seem to be the case for GC-HCAP therapy. Several studies of hospitalized patients with HCAP, admitted mostly to medical wards, demonstrated either no effect, or increased mortality, with GC-HCAP therapy [5,13–16], while one found decreased mortality [17]. A study of intensive care unit (ICU) patients (including patients with HCAP, hospital-acquired pneumonia, and ventilator-associated pneumonia) at risk for MDR infections also found increased mortality with GC therapy [18].
There are few data to describe the effect of guideline-concordant antibiotic therapy in a pure cohort of ICU patients with HCAP. In the present study, we examined a cohort of ICU patients with HCAP to compare effects of GC-HCAP therapy and GC-CAP therapy on patient mortality and hospital length-of-stay (LOS).
MATERIALS AND METHODS
This study was performed using administrative data from the U.S. Veterans Health Administration (VHA) to examine pneumonia care and mortality among ICU patients with HCAP. The VHA databases are repositories of clinical data from more than 150 VHA hospitals and 850 VHA clinics. The Institutional Review Boards of The University of Texas Health Science Center at San Antonio and the VA North Texas Health Care System have approved this study.
Patient Eligibility
Similar methods are described in a previous study from our research group [13]. All patients were required to have a discharge diagnosis of pneumonia: either a primary diagnosis of pneumonia/influenza (International Classification of Disease-ninth edition [ICD-9] codes 480.0–483.99 or 485–487) or a secondary discharge diagnosis of pneumonia/influenza with a primary diagnosis of respiratory failure (ICD-9 code 518.81) or sepsis (ICD-9 code 038.xx), in fiscal years 2002 to 2007, and at least one documented HCAP risk factor. HCAP risk factors were defined as hospital admission in the previous 90 days, residence in a nursing home in the previous 90 days, receipt of outpatient intravenous antibiotics in the previous 90 days, and hemodialysis. Patients were also required to be admitted to the ICU and to have received antibiotics within 48 hours of hospital admission. Excluding patients who did not receive antibiotics within 48 hours minimizes the potential inclusion of cases of nosocomial pneumonia.
Baseline Characteristics
ICD-9 codes from outpatient and inpatient care at the time of admission were used to determine baseline characteristics in accordance with the Charlson comorbidity scoring system [19]. Patient race was recorded for white and black patients, and ethnicity was reported for patients identifying themselves as Hispanic. Native Americans, Hawaiians, and patient records missing race information were reported as “other.” Tobacco use was defined as patients with a diagnosis of nicotine dependence, a recorded visit to a VHA tobacco cessation clinic, a current procedural terminology (CPT) treatment code for smoking (99406 or 99407), or an outpatient prescription for a smoking cessation product (Zyban®, varenicline, Nicotrol®, or nicotine replacement). Alcohol abuse/dependence and organ failure were identified through ICD-9 codes, and medication use in the 90 days prior to admission was documented by medication classes, as previously described [13].
Antibiotic Therapy and Bacterial Pathogens
Current consensus guidelines were reviewed to evaluate antibiotic therapy received within the first 48 hours of hospital admission (Table 1) [1,12]. Patients receiving additional antibiotics beyond the minimum required to satisfy GC-HCAP or GC-CAP therapy remained in their respective treatment groups. The subset of patients who received both GC-HCAP and GC-CAP therapy was considered to have received GC-HCAP therapy. Patients receiving antibiotics that were not concordant with either CAP or HCAP guidelines were considered to have received non-GC therapy.
Table 1.
Definitions of CAP and HCAP Guideline-Concordant Therapy (ICU Patients)
| Guideline-Concordant CAP Therapy | Guideline-Concordant HCAP Therapy |
|---|---|
| Beta-lactam1†plus azithromycin Beta-lactam1†plus respiratory fluoroquinolone2 |
Antipseudomonal beta-lactam3†plus antipseudomonal fluoroquinolone4plus vancomycin or linezolid Antipseudomonal beta-lactam3†plus aminoglycoside5plus vancomycin or linezolid |
CAP: community-acquired pneumonia; HCAP: healthcare-associated pneumonia; ICU: intensive care unit
Aztreonam may be substituted for beta-lactam in penicillin-allergic patients
Beta-lactam includes cefotaxime, ceftriaxone, or ampicillin-sulbactam
Respiratory fluoroquinolone includes moxifloxacin, levofloxacin, or gatifloxacin
Antipseudomonal beta-lactam includes cefepime, ceftazidime, imipenem-cilastatin, meropenem, piperacillin-tazobactam, or ticarcillin-clavulanate
Antipseudomonal fluoroquinolone includes ciprofloxacin or levofloxacin
Aminoglycoside includes gentamicin, tobramycin, or amikacin
Pneumonia pathogens were identified using ICD-9 discharge diagnosis codes. Codes used during the study period do not differentiate between methicillin-sensitive S. aureus and MRSA; however, HCAP data in the U.S. suggest that methicillin-resistance is present in more than half of all S. aureus isolates [2,3,14].
Patient Mortality and Hospital Length-of-Stay
Our primary outcome was 30-day patient mortality. Admission and discharge dates were extracted for each hospital stay and length of stay (LOS) was defined as the date of hospital discharge minus the date of hospital admission plus one day. Mortality was determined using date of death provided by the VHA vital status file.
Statistical Analysis
For bivariable comparisons, a two-tailed alpha ≤0.05 was used for statistical significance. In comparisons among the three treatment groups, GC-HCAP was used as the reference group and was compared with both the GC-CAP and non-GC groups. In our multivariable logistic regression model, a two-tailed alpha ≤0.05 indicated statistical significance.
Patient demographics, baseline characteristics, comorbid conditions, bacterial pathogens, and health outcomes (hospital LOS and mortality) were compared between groups. Dichotomous variables were compared using Chi-square or Fisher’s Exact tests. The Wilcoxon Rank Sum test was used to compare continuous variables after all were tested for normality and were found to have non-normal distributions. A multivariable logistic regression model was used to examine the association between the receipt of GC antibiotics and 30-day patient mortality. Patients who received non-GC therapy were excluded from the regression analysis to isolate the effects of GC therapy (GC-HCAP vs. GC-CAP). We included variables that the investigative team believed were clinically important. Then, those variables were simultaneously entered into the regression model. The dependent variable was 30-day patient mortality and covariates included individual HCAP risk factors, comorbid conditions, mechanical ventilation, vasopressor use, and guideline-concordant antibiotic therapy.
All statistical analyses were conducted using JMP 8.0® (SAS Corp., Cary, NC, USA) and SPSS (SPSS, Inc., Chicago, IL, USA).
RESULTS
Baseline Characteristics
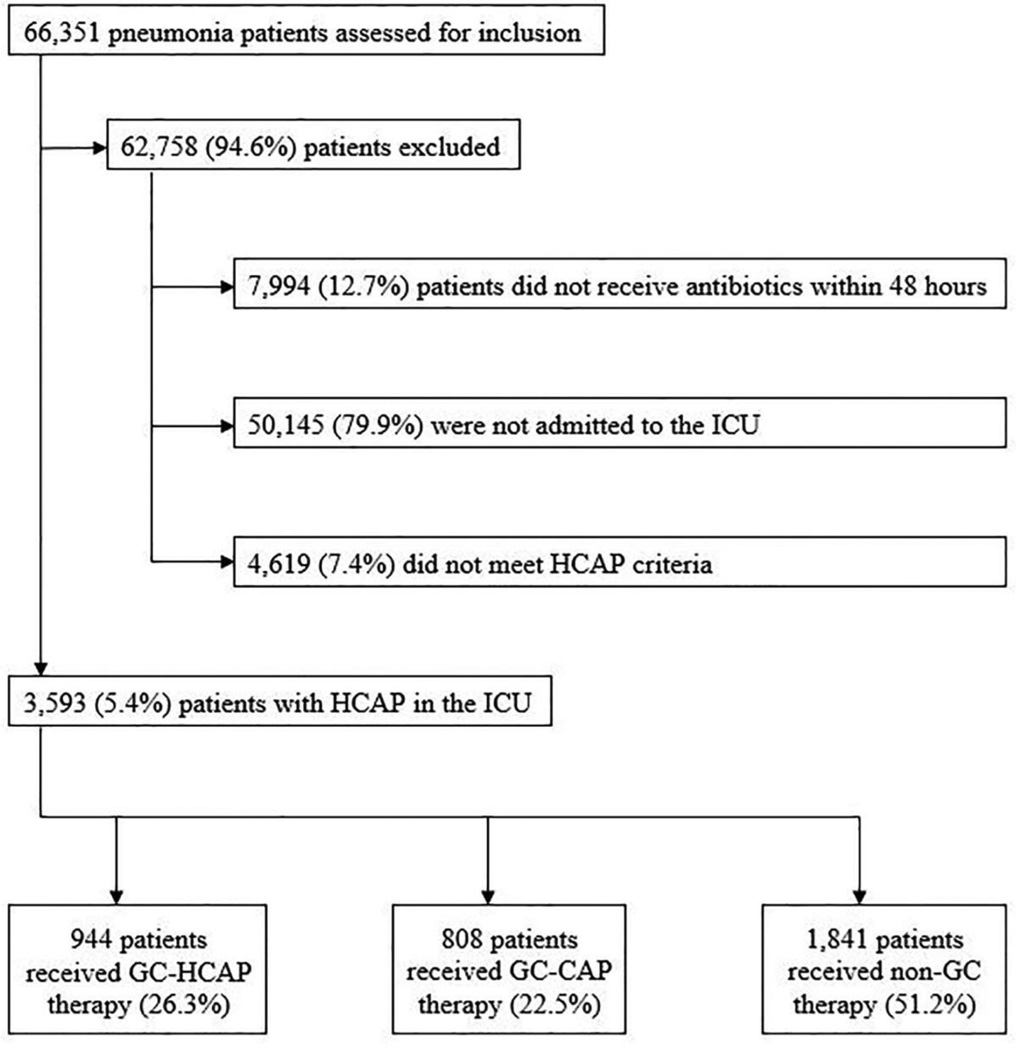
A total of 3,593 patients met inclusion criteria for our study (Figure 1). The most common HCAP risk factor was recent hospitalization in the past 90 days (72%), 27% had more than one HCAP risk factor, and invasive or non-invasive mechanical ventilation was necessary in 42% and 16%, respectively (Table 2).
Figure 1.
Patient inclusion and exclusion flow diagram
Table 2.
Baseline Characteristics
| Patient Characteristics |
Overall (n=3,593) |
GC- HCAP (n=944) |
GC- CAP (n=808) |
Non-GC (n=1,841) |
GC- HCAP versus GC-CAP (p-value) |
GC- HCAP versus Non-GC (p-value) |
|---|---|---|---|---|---|---|
| Age in years; median, IQR |
77.1, 71.8–81.8 |
76.8, 71.8– 81.4 |
77.1, 71.8– 81.7 |
77.3, 71.8–82.0 |
0.40 | 0.58 |
| Male, % | 98.4 | 99.3 | 98.6 | 97.9 | 0.20 | 0.009 |
| Race, % | 0.05 | 0.27 | ||||
| White | 80.7 | 78.7 | 84.0 | 80.3 | ||
| Black | 14.4 | 15.1 | 12.3 | 14.9 | ||
| Other | 4.9 | 6.1 | 3.7 | 4.7 | ||
| Hispanic ethnicity, % | 5.4 | 8.8 | 3.6 | 4.4 | <0.0001 | <0.0001 |
| HCAP Risk Factors, % |
||||||
| Recent hospitalization, 90d |
71.6 | 74.9 | 59.4 | 75.2 | <0.0001 | 0.87 |
| Nursing home resident, 90d |
2.1 | 2.8 | 2.6 | 1.6 | 0.84 | 0.03 |
| Hemodialysis | 45.5 | 46.1 | 50.7 | 42.9 | 0.05 | 0.10 |
| Outpatient IV antibiotic therapy, 90d |
10.9 | 10.9 | 11.3 | 10.8 | 0.82 | 0.90 |
| ≥2 HCAP Risk Factors, % |
27.2 | 30.7 | 21.6 | 27.9 | <0.0001 | 0.12 |
| Charlson Index score; median, IQR |
3, 2–5 | 3, 2–5 | 4, 2–6 | 3, 2–5 | 0.10 | 0.83 |
| Comorbid conditions, % |
||||||
| Myocardial infarction |
12.8 | 12.3 | 14.1 | 12.4 | 0.26 | 0.91 |
| Heart failure | 40.1 | 36.7 | 46.3 | 39.1 | <0.0001 | 0.21 |
| Cerebrovascular disease |
21.7 | 25.1 | 21.3 | 20.1 | 0.06 | 0.002 |
| COPD | 56.2 | 52.6 | 61.5 | 55.7 | 0.0002 | 0.13 |
| Liver disease | 1.3 | 1.5 | 1.0 | 1.3 | 0.36 | 0.70 |
| CKD | 33.5 | 32.6 | 39.7 | 31.2 | 0.002 | 0.45 |
| Diabetes | 40.9 | 41.5 | 45.4 | 38.6 | 0.10 | 0.14 |
| Neoplastic disease | 27.7 | 30.1 | 22.6 | 28.7 | 0.0004 | 0.44 |
| HIV/AIDS | 0.2 | 0.1 | 0.1 | 0.3 | 0.91 | 0.37 |
| Substance abuse or dependence |
||||||
| Tobacco use | 35.8 | 35.8 | 35.8 | 35.9 | 0.99 | 0.98 |
| Alcohol abuse or dependence |
5.1 | 4.7 | 5.4 | 5.2 | 0.45 | 0.57 |
| Outpatient medication use, 90d, % |
||||||
| Cardiovascular medications |
72.8 | 71.0 | 81.9 | 69.6 | <0.0001 | 0.47 |
| Antidiabetic medications |
27.5 | 27.3 | 33.0 | 25.2 | 0.009 | 0.23 |
| Inhaled corticosteroids |
21.8 | 20.8 | 23.9 | 21.5 | 0.12 | 0.65 |
| Systemic corticosteroids |
28.0 | 29.1 | 28.1 | 27.3 | 0.63 | 0.31 |
| Pulmonary medications |
39.4 | 36.7 | 44.1 | 38.8 | 0.002 | 0.26 |
| Vasopressors, % | 28.8 | 39.5 | 14.9 | 29.4 | <0.0001 | <0.0001 |
| Invasive mechanical ventilation, % |
41.6 | 53.7 | 28.5 | 41.2 | <0.0001 | <0.0001 |
| Non-invasive mechanical ventilation, % |
16.3 | 18.2 | 17.1 | 15.0 | 0.53 | 0.03 |
| Organ failure, % | ||||||
| Any organ failure | 68.9 | 76.6 | 64.4 | 67.0 | <0.0001 | <0.0001 |
| Respiratory | 46.0 | 55.5 | 36.5 | 45.2 | <0.0001 | <0.0001 |
| Cardiovascular | 18.3 | 18.5 | 12.1 | 20.9 | 0.0002 | 0.14 |
| Neurological | 4.5 | 5.6 | 5.0 | 3.6 | 0.54 | 0.02 |
| Renal | 36.9 | 40.8 | 34.2 | 36.2 | 0.004 | 0.02 |
| Hematologic | 6.8 | 9.4 | 5.2 | 6.2 | 0.0008 | 0.002 |
| Hepatic | 0.8 | 1.1 | 0.6 | 0.7 | 0.32 | 0.33 |
GC-HCAP: guideline-concordant healthcare-associated pneumonia
GC-CAP: guideline-concordant community-acquired pneumonia
GC: guideline-concordant
IQR: interquartile range
HCAP: healthcare-associated pneumonia
IV: intravenous
COPD: chronic obstructive pulmonary disease
CKD: chronic kidney disease
HIV/AIDS: human immunodeficiency virus/acquired immunodeficiency syndrome
Of the 3,593 ICU patients with HCAP, 944 (26%) received GC-HCAP therapy and 808 (23%) received GC-CAP therapy. Compared to patients who received GC-CAP therapy, those receiving GC-HCAP therapy were more likely to have been hospitalized in the past 90 days (75% vs. 59%, p<0.0001) and have more than one HCAP risk factor (31% vs. 22%, p<0.0001). Intensity of care was higher in GC-HCAP vs. GC-CAP, with patients receiving GC-HCAP therapy being more likely to receive vasopressors (40% vs. 15%, p<0.0001) and invasive mechanical ventilation (54% vs. 29%, p<0.0001). Patients receiving GC-HCAP therapy were also more likely than patients receiving GC-CAP therapy to experience organ failure (77% vs. 64%, p<0.0001), and when divided by type, had a statistically-significant higher rate of respiratory, cardiovascular, renal, and hematologic failure.
Patients receiving non-GC therapy had baseline characteristics, pathogens, and outcomes that were more similar to patients receiving GC-HCAP therapy vs. GC-CAP therapy. Of 1,841 patients in the non-GC therapy group, 33.4% receiving anti-MRSA therapy, 51.7% received antipseudomonal therapy, and 31.7% received both. Antibiotics with activity against atypical pathogens was prescribed in 40.4% of patients who received non-GC therapy. Macrolides were prescribed in 10.9% of patients who received non-GC therapy.
Bacterial Pathogens
Bacterial pathogens were identified in 26% of the cohort (Table 3). Among these patients, 86% had a single pathogen identified. The three most common pathogens were S. aureus (38%), Pseudomonas spp. (17%), and S. pneumoniae (16%). Patients receiving GC-HCAP therapy were more likely to be culture-positive than patients receiving GC-CAP therapy (36% vs. 19%, p<0.0001) or non-GC therapy (36% vs. 23%, p<0.0001). Compared to patients receiving GC-CAP therapy, those receiving GC-HCAP therapy were more likely to have pneumonia due to potentially drug-resistant pathogens including S. aureus (43% vs. 27%, p=0.0005) and Pseudomonas spp. (22% vs. 7%, p<0.0001) and less likely to have pneumonia due to Streptococcus pneumoniae (12% vs. 33%, p<0.0001) and H. influenzae (1% vs. 11%, p<0.0001).
Table 3.
Bacterial Pathogen Distribution for GC-HCAP, GC-CAP, and Non-GC Patients
| Patient Characteristics |
Overall | GC- HCAP |
GC- CAP |
Non- GC |
GC-HCAP versus GC-CAP (p-value) |
GC-HCAP versus Non- GC (p-value) |
|---|---|---|---|---|---|---|
| All patients, n | 3,593 | 944 | 808 | 1,841 | ||
| Organism identified | 25.6 | 36.4 | 18.9 | 22.9 | <0.0001 | <0.0001 |
| Single organism identified |
21.9 | 30.1 | 16.8 | 19.9 | <0.0001 | <0.0001 |
| Multiple organisms identified |
3.7 | 6.4 | 2.1 | 3.0 | <0.0001 | <0.0001 |
| Culture-positive patients, n |
919 | 344 | 153 | 422 | ||
| Gram-positive pathogens |
||||||
| S. pneumoniae | 16.4 | 12.2 | 33.3 | 13.7 | <0.0001 | 0.53 |
| Streptococcus other |
3.6 | 2.3 | 6.5 | 3.6 | 0.02 | 0.32 |
| Staphylococcus aureus |
37.5 | 43.3 | 26.8 | 36.7 | 0.0005 | 0.06 |
| Gram-negative pathogens |
||||||
| K. pneumoniae | 8.7 | 10.2 | 3.3 | 9.5 | 0.009 | 0.75 |
| Pseudomonas | 17.1 | 21.8 | 7.2 | 16.8 | <0.0001 | 0.08 |
| H. influenzae | 4.1 | 1.2 | 10.5 | 4.3 | <0.0001 | 0.01 |
| E. coli | 3.6 | 5.2 | 1.3 | 3.1 | 0.04 | 0.13 |
| Other gram- negatives |
4.6 | 2.9 | 5.9 | 5.5 | 0.11 | 0.08 |
| Atypical pathogens | ||||||
| Legionella | 1.4 | 1.2 | 2.6 | 1.2 | 0.24 | 0.98 |
| Mycoplasma | 0 | 0 | 0 | 0 | -- | -- |
| Chlamydia | 0 | 0 | 0 | 0 | -- | -- |
| Anaerobes | 0.4 | 0.6 | 0.7 | 0.2 | 0.92 | 0.45 |
GC-HCAP: guideline-concordant healthcare-associated pneumonia
GC-CAP: guideline-concordant community-acquired pneumonia
GC: guideline-concordant
Culture-positive patients with only one HCAP risk factor were more likely to have HCAP secondary to S. pneumoniae (19.1%) vs. those with two (9.6%) or three or more HCAP risk factors (10%) [p=0.002]. The opposite was true with pneumonia secondary to Pseudomonas spp., although this was not statistically-significant (15.3%, 21.7%, and 20% for one, two, and three or more risk factors, respectively, p=0.08). Rates of S. aureus pneumonias were similar regardless of the cumulative number of HCAP risk factors.
Outcomes
The overall 30-day patient mortality rate was 37.6%. Thirty-day patient mortality was significantly higher in patients receiving GC-HCAP vs. GC-CAP therapy (34% vs. 22%, p<0.0001). Patients receiving non-GC therapy experienced a higher rate of 30-day mortality (46%) than either of the other groups (p<0.0001 for both comparisons). The median hospital LOS was 11 days (IQR 6–20). Patients receiving GC-HCAP therapy had a nearly-double hospital LOS compared to patients in the GC-CAP group (median, IQR; 18, 11–34 vs. 10, 6.25–17, p<0.0001) or non-GC group (18, 11–34 vs. 9, 4–16, p<0.0001). Patients who received macrolide therapy (31% of total cohort) had lower 30-day mortality than those patients who did not receive this therapy (24.2% vs. 43.6%, p<0.0001). Additionally, macrolide therapy was a part of most GC-CAP therapy (78.1%), nearly one-third of GC-HCAP therapy (29.9%), and only 10.9% of non-GC therapy.
We compared patients receiving GC-HCAP and GC-CAP therapy in a multivariable logistic regression model, with 30-day patient mortality as the dependent variable (Table 4). After controlling for possible confounders, several characteristics maintained significant associations with 30-day patient mortality, including vasopressor use (odds ratio [OR], 95% confidence interval [CI]; 1.67, 1.30–2.13), recent hospital admission in the past 90 days (1.53, 1.15–2.02), and the receipt of GC-HCAP therapy (1.51, 1.20–1.90).
Table 4.
Risk Factors for 30-Day Mortality in GC-HCAP and GC-CAP Patients (n= 1,752)
| Risk Factors | Odds Ratio | 95% Confidence Interval |
p-value |
|---|---|---|---|
| HCAP risk factors | |||
| Recent hospital admission, 90d | 1.53 | 1.15 – 2.02 | 0.003 |
| Nursing home admission, 90d | 0.53 | 0.22 – 1.10 | 0.11 |
| Hemodialysis | 1.09 | 0.84 – 1.42 | 0.51 |
| Outpatient IV antibiotics, 90d | 1.17 | 0.83 – 1.62 | 0.37 |
| Charlson Index score ≤4 | 0.91 | 0.72 – 1.14 | 0.41 |
| Invasive mechanical ventilation | 1.1 | 0.87 – 1.39 | 0.42 |
| Non-invasive mechanical ventilation | 1.2 | 0.91 – 1.58 | 0.18 |
| Vasopressor use | 1.67 | 1.30 – 2.13 | <0.0001 |
| GC-HCAP versus GC-CAP antibiotics | 1.51 | 1.20 – 1.90 | 0.0004 |
DISCUSSION
This study examined the effects of guideline-concordant HCAP therapy in ICU patients with HCAP. Our results demonstrated that guideline-concordant HCAP therapy, compared to guideline-concordant CAP therapy, is not associated with improved outcomes and, after controlling for possible confounders, remains a significant risk factor for 30-day patient mortality.
The reason that GC-HCAP therapy did not improve outcomes in this cohort of ICU patients with HCAP is unclear. There are data to indicate that patients with HCAP are more likely to have restrictions on care (e.g., “do not resuscitate” or “not for ICU” orders) that may negatively affect survival [7]. By limiting our cohort to ICU patients, we expected that restrictions on care would have a smaller role, and that GC-HCAP antibiotics might be more likely to be beneficial. Despite this approach, the effects of GC-HCAP therapy remained consistent with prior studies, where GC-HCAP therapy given to patients in the medical ward or mixed ward/ICU patients either increased mortality or had no effect on mortality [5,13–15]. Data using a less strict combination of guideline-similar antibiotics (e.g., one anti-MRSA antibiotic and only one antipseudomonal antibiotic) in mixed ward/ICU cohorts have also demonstrated similar results to our study [15,16].
A recent meta-analysis by Troitino et al had similar findings, with GC-HCAP therapy being associated with increased mortality and no effect on hospital length of stay or time to clinical stability [20]. We do not believe the antibiotic regimens alone are fully responsible for these findings since GC-HCAP antibiotics have a high likelihood of being active against the isolated pathogens; however, we hypothesize that increases in adverse events with GC-HCAP therapy and fewer options for oral transition therapy (and thus increased intravenous [IV] therapy duration and increased hospital LOS) may have contributed to the association between GC-HCAP therapy and poor outcomes. Duration of IV therapy is responsible for a significant amount of variation among LOS in CAP patients [21], and shorter LOS minimizes exposure to hospital environments that may increase the risk of additional complications, including Clostridium difficile infection [22].
A lack of activity against atypical pneumonia pathogens with some GC-HCAP regimens and/or the absence of macrolides (and their potential immunomodulatory effects) may have had a role in poorer outcomes in patients receiving GC-HCAP therapy. While there are data to highlight potential risks of macrolide therapy [23], our finding that patients who received macrolides had lower 30-day mortality compared to those who did not is consistent with other published data [24,25]. Similarly, it is possible that a lower rate of atypical coverage (whether by macrolide or another antibiotic) in conjunction with inconsistent coverage of MRSA and Pseudomonas spp. may have been partially responsible for the poor outcomes of the non-GC therapy group.
After controlling for confounders, several other baseline characteristics were significantly-associated with 30-day mortality in our study. Vasopressor use increased mortality, but mechanical ventilation (invasive or non-invasive) did not. Among HCAP risk factors, recent hospital admission was the only factor associated with mortality. In most HCAP data, including the current data, it is the most common reason for classification as HCAP [26]. Several other studies, although not all [6,27], analyzing individual risk factors have indicated that recent hospitalization is an important factor in mortality [9,13,17,28] and in the recovery of resistant pathogens [10,29,30]. Recent hospitalization may be a particularly important HCAP risk factor, and it is prudent for clinicians to be aware of this history, especially in those receiving recent antibiotic therapy [7,9,10,27,28,31,32]. Further studies that review characteristics of recent hospitalizations (e.g., principal hospital diagnosis, receipt of antibiotic therapy) and/or measure changes in functional status as a measure of physiologic reserve may provide further insights into this risk factor.
Many findings, and a recent meta-analysis, support the idea that differences in baseline characteristics (comorbidities, severity of illness, and/or functional status) between patients with CAP and HCAP are a major driving factor behind HCAP mortality [7–9,11,17,27,28,33,34]. A meta-analysis from Chalmers et al, which included 24 eligible studies comparing patients with CAP and HCAP, found significantly higher mortality rates among those with HCAP. However, after including the only four studies that adjusted for age and comorbidities, there was no mortality difference between the two groups [34]. Our cohort of ICU patients with HCAP had increased mortality with GC-HCAP therapy, even after adjusting for comorbidities, which may suggest additional unmeasured confounders.
Our data indicate a high prevalence of S. aureus and Pseudomonas spp. among patients with HCAP, a finding consistent with the majority of HCAP literature [2,3,13,14,17,31,33,34]. There are data postulating that inappropriate initial antibiotic therapy, partially because of an increased prevalence of S. aureus and Pseudomonas spp., might have been responsible for higher rates of mortality in HCAP patients [2]; however, there are other data that do not support this notion [7,8,11]. Even among studies in which patients had very low rates of resistant pathogens and thus, were more likely to receive appropriate initial therapy, the population with HCAP (vs. CAP) had lower survival [4,6,7,9].
If GC-HCAP therapy does not improve outcomes among patients with HCAP, what strategy should be used to initiate empiric therapy in these patients? To date, there is no clear answer to this question. Research of risk factors for potentially drug-resistant pathogens has highlighted the suboptimal predictive ability of the HCAP criteria [31,32,34,35]. Several risk scores have been developed that perform better than the HCAP criteria in selecting resistant pathogens [8,10,35,36], and risk score strategies that separate out MRSA from other MDR organisms might prove to be useful [10,29,30]. Some have demonstrated that severity of illness might be used to help predict pneumonia pathogens [7,8,33]; however, this is not supported by all published studies [10]. In our study, patients with HCAP who received GC-HCAP therapy and GC-CAP therapy had no difference in Charlson Comorbidity Index scores, yet the pneumonia pathogens differed significantly between the two groups.
Quick de-escalation, improved diagnostics, and a restructuring of HCAP criteria may also be beneficial. At least for now, a quick de-escalation strategy, particularly in culture-negative patients, may be useful [37]. Improved and continued evaluation of diagnostics might improve our ability for either early de-escalation, or broadening of empiric therapy, when necessary. Based on the entirety of the HCAP data, calls to restructure criteria and separate recently-hospitalized patients and immunosuppressed patients from HCAP are logical [11,38].
Our study has several limitations that should be addressed. First, while the large, national sample is a strength of our study, the retrospective cohort study design is subject to limitations inherent to this type of research. Patients in the treatment groups had several significant differences in baseline characteristics (as outlined in Table 2). We performed multivariable logistic regression with a number of covariates to minimize confounding; however, this method will not account for unmeasured variables and is not as robust as a randomized, controlled trial. Prospective studies to assess outcomes for pneumonia patients with HCAP and other risk factors for multi-drug resistant pathogens may help validate our results. We also included Charlson Index scores as a measure of comorbidity burden, but we did not have sufficient data to classify patients with conventional pneumonia severity-of-illness scoring systems (e.g., Pneumonia Severity Index, CURB-65) [39,40]. Second, the use of ICD-9 codes to identify pneumonia patients and pathogens enabled us to analyze a large sample size but limited our ability to provide specific microbiologic information that would be of interest, including antibiotic susceptibilities, and may be responsible for a relatively low rate of culture positivity. We acknowledge that our method for identifying pathogens is suboptimal and may have missed some patients with known bacterial etiology because we did not have complete microbiologic information. ICD-9 codes did not allow us to differentiate between methicillin-sensitive and methicillin-resistant Staphylococcus aureus, and we were unable to return to individual patient records to review for any serologic tests that may have identified additional pathogens. For these reasons, we did not include the bacterial pathogens in the multivariable regression model. Third, our definition of guideline-concordant therapy was a strict, guideline-based definition. Less strict definitions, to include patients receiving only one antipseudomonal agent, patients receiving empiric antipseudomonal therapy and not anti-MRSA therapy, or patients receiving anti-MRSA therapy and not antipseudomonal therapy, may provide different results and should continue to be investigated in future studies. Additionally, while we were able to account for some potential confounders, we did not have data available on functional status. A positive relationship between poor outcomes and poor functional status has been demonstrated in some recent HCAP studies [9,28], and this may have had an effect on our finding of increased mortality among patients receiving GC-HCAP therapy.
CONCLUSION
GC-HCAP therapy, compared to GC-CAP therapy, was not associated with improved outcomes in ICU patients with HCAP.
Acknowledgments
This project was supported by a grant from the U.S. National Institute of Nursing Research (R01NR010828). The content is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. National Institute of Nursing Research or the National Institutes of Health.
Abbreviations
- GC
guideline-concordant
- HCAP
healthcare-associated pneumonia
- MDR
multi-drug resistant
- LOS
length-of-stay
- VHA
Veterans Health Administration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statements:
Russell Attridge: none
Christopher Frei: Dr. Frei has received grants outside the submitted work from Bristol Myers Squibb, Pfizer, and Ortho-McNeill Janssen.
Mary Jo Pugh: none
Kenneth Lawson: none
Laurajo Ryan: none
Antonio Anzueto: none
Mark Metersky: Dr. Metersky has served as a speaker for Pfizer (relationship ended in 2011).
Marcos Restrepo: Dr. Restrepo’s time is partially protected by Award Number K23HL096054 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute, the National Institutes of Health or the Department of Veterans Affairs.
Eric Mortensen: Dr. Mortensen reports grants from NIH received during the conduct of the study.
Contributor Information
Russell T. Attridge, Email: attridge@uiwtx.edu.
Christopher R. Frei, Email: freic@uthscsa.edu.
Mary Jo V. Pugh, Email: pughm@uthscsa.edu.
Kenneth A. Lawson, Email: ken.lawson@austin.utexas.edu.
Laurajo Ryan, Email: ryanl@uthscsa.edu.
Antonio Anzueto, Email: anzueto@uthscsa.edu.
Mark L. Metersky, Email: Metersky@nso.uchc.edu.
Marcos I. Restrepo, Email: restrepom@uthscsa.edu.
Eric M. Mortensen, Email: eric.mortensen@utsouthwestern.edu.
REFERENCES
- 1.American Thoracic Society, Infectious Diseases Society of America: Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 2.Kollef MH, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest. 2005;128:3854–3862. doi: 10.1378/chest.128.6.3854. [DOI] [PubMed] [Google Scholar]
- 3.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51:3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carratala J, Mykietiuk A, Fernandez-Sabe N, Suarez C, Dorca J, Verdaguer R, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med. 2007;167:1393–1399. doi: 10.1001/archinte.167.13.1393. [DOI] [PubMed] [Google Scholar]
- 5.Grenier C, Pepin J, Nault V, Howson J, Fournier X, Poirier M, et al. Impact of guideline-consistent therapy on outcome of patients with healthcare-associated and community-acquired pneumonia. J Antimicrob Chemother. 2011;66:1617–1624. doi: 10.1093/jac/dkr176. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Vidal C, Viasus D, Roset A, Adamuz J, Verdaguer R, Dorca J, et al. Low incidence of multidrug-resistant organisms in patients with healthcare-associated pneumonia requiring hospitalization. Clin Microbiol Infect. 2011;17:1659–1665. doi: 10.1111/j.1469-0691.2011.03484.x. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers JD, Taylor JK, Singanayagam A, Fleming GB, Akram AR, Mandal P, et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis. 2011;53:107–113. doi: 10.1093/cid/cir274. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama T, Fujisawa T, Okuno M, Toyoshima H, Tsutsui K, Maeda H, et al. A new strategy for healthcare-associated pneumonia: a 2-year prospective multicenter cohort study using risk factors for multidrug-resistant pathogens to select initial empiric therapy. Clin Infect Dis. 2013;57:1373–1383. doi: 10.1093/cid/cit571. [DOI] [PubMed] [Google Scholar]
- 9.Polverino E, Torres A, Menendez R, Cilloniz C, Valles JM, Capelastegui A, et al. Microbial aetiology of healthcare associated pneumonia in Spain: a prospective, multicentre, case-control study. Thorax. 2013;68:1007–1014. doi: 10.1136/thoraxjnl-2013-203828. [DOI] [PubMed] [Google Scholar]
- 10.Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188:985–995. doi: 10.1164/rccm.201301-0079OC. [DOI] [PubMed] [Google Scholar]
- 11.Valles J, Martin-Loeches I, Torres A, Diaz E, Seijas I, Lopez MJ, et al. Epidemiology, antibiotic therapy and clinical outcomes of healthcare-associated pneumonia in critically ill patients: a Spanish cohort study. Intensive Care Med. 2014;40:572–581. doi: 10.1007/s00134-014-3239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;(44 Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attridge RT, Frei CR, Restrepo MI, Lawson KA, Ryan L, Pugh MJV, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38:878–887. doi: 10.1183/09031936.00141110. [DOI] [PubMed] [Google Scholar]
- 14.Webb BJ, Dangerfield BS, Pasha JS, Agrwal N, Vikram HR. Guideline-concordant antibiotic therapy and clinical outcomes in healthcare-associated pneumonia. Respir Med. 2012;106:1606–1612. doi: 10.1016/j.rmed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Madaras-Kelly KJ, Remington RE, Sloan KL, Fan VS. Guideline-based antibiotics and mortality in healthcare-associated pneumonia. J Gen Intern Med. 2012;27:845–852. doi: 10.1007/s11606-012-2011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothberg MB, Zilberberg MD, Pekow PS, Priya A, Haessler S, Belforti R, et al. Association of guideline-based antimicrobial therapy and outcomes in healthcare-associated pneumonia. J Antimicrob Chemother. 2015:701573–701579. doi: 10.1093/jac/dku533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falcone M, Corrao S, Licata G, Serra P, Venditti M. Clinical impact of broad-spectrum empirical antibiotic therapy in patients with healthcare-associated pneumonia: a multicenter interventional study. Intern Emerg Med. 2012;7:523–531. doi: 10.1007/s11739-012-0795-8. [DOI] [PubMed] [Google Scholar]
- 18.Kett DH, Cano E, Quartin AA, Mangino JE, Zervos MJ, Peyrani P, et al. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11:181–189. doi: 10.1016/S1473-3099(10)70314-5. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Troitino AX, Porhomayon J, El-Solh AA. Guideline-concordant antimicrobial therapy for healthcare-associated pneumonia: a systematic review and meta-analysis. Lung. 2013;191:229–237. doi: 10.1007/s00408-013-9462-8. [DOI] [PubMed] [Google Scholar]
- 21.Laing R, Coles C, Chambers S, Frampton C, Jennings L, Karalus N, et al. Community-acquired pneumonia: influence of management practices on length of hospital stay. Intern Med J. 2004;34:91–97. doi: 10.1111/j.1444-0903.2004.00544.x. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers JD, Al-Khairalla M, Short PM, Fardon TC, Winter JH. Proposed changes to management of lower respiratory tract infections in response to the Clostridium difficile epidemic. J Antimicrob Chemother. 2010;65:608–618. doi: 10.1093/jac/dkq038. [DOI] [PubMed] [Google Scholar]
- 23.Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881–1890. doi: 10.1056/NEJMoa1003833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asadi L, Sligl WI, Eurich DT, Colmers IN, Tjosvold L, Marrie TJ, et al. Macrolide-based regimens and mortality in hospitalized patients with community-acquired pneumonia: a systematic review and meta-analysis. Clin Infect Dis. 2012;55:371–380. doi: 10.1093/cid/cis414. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen EM, Halm EA, Pugh MJ, Copeland LA, Metersky M, Fine MJ, et al. Association of azithromycin with mortality and cardiovascular events among older patients hospitalized with pneumonia. JAMA. 2014;311:2199–2208. doi: 10.1001/jama.2014.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attridge RT, Frei CR. Health care-associated pneumonia: an evidence-based review. Am J Med. 2011;124:689–697. doi: 10.1016/j.amjmed.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 27.Depuydt P, Putman B, Benoit D, Buylaert W, De Paepe P. Nursing home residence is the main risk factor for increased mortality in healthcare-associated pneumonia. J Hosp Infect. 2011;77:138–142. doi: 10.1016/j.jhin.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 28.Ding YY, Abisheganaden J, Chong WF, Heng BH, Lim TK. Short-term mortality among older persons hospitalized for pneumonia: influence of baseline patient characteristics beyond severity of illness. J Hosp Med. 2012;7:211–217. doi: 10.1002/jhm.985. [DOI] [PubMed] [Google Scholar]
- 29.Shorr AF, Zilberberg MD, Micek ST, Kollef MH. Prediction of infection due to antibiotic-resistant bacteria by select risk factors for health care-associated pneumonia. Arch Intern Med. 2008;168:2205–2210. doi: 10.1001/archinte.168.20.2205. [DOI] [PubMed] [Google Scholar]
- 30.Shorr AF, Myers DE, Huang DB, Nathanson BH, Emons MF, Kollef MH. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infect Dis. 2013;13:268. doi: 10.1186/1471-2334-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schreiber MP, Chan CM, Shorr AF. Resistant pathogens in nonnosocomial pneumonia and respiratory failure: is it time to refine the definition of health-care-associated pneumonia? Chest. 2010;137:1283–1288. doi: 10.1378/chest.09-2434. [DOI] [PubMed] [Google Scholar]
- 32.Madaras-Kelly KJ, Remington RE, Fan VS, et al. Predicting antibiotic resistance to community-acquired pneumonia antibiotics in culture-positive patients with healthcare-associated pneumonia. J Hosp Med. 2012;7:195–202. doi: 10.1002/jhm.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannella M, Pinilla B, Capdevila JA, Martinez Alarcon J, Munoz P, Lopez Alvarez J. Pneumonia treated in the internal medicine department: focus on healthcare-associated pneumonia. Clin Microbiol Infect. 2012;18:786–794. doi: 10.1111/j.1469-0691.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers JD, Rother C, Salih W, Ewig S. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58:330–339. doi: 10.1093/cid/cit734. [DOI] [PubMed] [Google Scholar]
- 35.Aliberti S, Cilloniz C, Chalmers JD, Zanaboni AM, Cosentini R, Tarsia T, et al. Multidrug-resistant pathogens in hospitalised patients coming from the community with pneumonia: a European perspective. Thorax. 2013;68:997–999. doi: 10.1136/thoraxjnl-2013-203384. [DOI] [PubMed] [Google Scholar]
- 36.Webb BJ, Dascomb K, Stenehjem E, Vikram HR, Agrwal N, Sakata K, et al. Derivation and multicenter validation of the Drug Resistance in Pneumonia clinical prediction score. Antimicrob Agents Chemother. 2016;60:2652–2663. doi: 10.1128/AAC.03071-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlueter M, James C, Dominguez A, Tsu L, Seymann G. Practice patterns for antibiotic de-escalation in culture-negative healthcare-associated pneumonia. Infection. 2010;8:357–362. doi: 10.1007/s15010-010-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ewig S, Welte T, Chastre J, Torres A. Rethinking the concepts of community-acquired and health-care-associated pneumonia. Lancet Infect Dis. 2010;10:279–287. doi: 10.1016/S1473-3099(10)70032-3. [DOI] [PubMed] [Google Scholar]
- 39.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 40.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]