Abstract
Purpose
Neuron specific enolase (NSE) concentrations are prognostic following traumatic and anoxic brain injury, and may provide a method to quantify neuronal injury in other populations. We determined the association of admission plasma NSE concentrations with mortality and delirium in critically ill septic patients.
Methods
Retrospective analysis of 124 patients from a larger sepsis cohort. Plasma NSE was measured in the earliest blood draw at intensive care unit (ICU) admission. Primary outcomes were 30-day mortality and ICU delirium determined by chart review.
Results
Sixty-one patients (49.2%) died within 30 days and delirium developed in 34 (31.5%) of the 108 patients who survived at least 24 hours and were not persistently comatose. Each doubling of the NSE concentration was associated with a 7.3% (95% CI 2.5-12.0, p=0.003) increased risk of 30-day mortality and a 5.2% (95% CI 3.2-7.2, p<0.001) increased risk of delirium. An NSE concentration > 12.5ug/L was independently associated with a 23.3% (95% CI 6.7-39.9, p=0.006) increased risk of 30-day mortality and a 29.3% (95% CI 8.8-49.8, p=0.005) increased risk of delirium.
Conclusions
Higher plasma NSE concentrations were associated with mortality and delirium in critically ill septic patients, suggesting NSE may have utility as a marker of neuronal injury in sepsis.
Keywords: sepsis, critical care, brain injury, delirium, neuron specific enolase
Introduction
Sepsis is one of the most common reasons for hospitalization in the United States and often leads to organ failure and death [1-3]. Acute brain dysfunction, manifesting as coma and/or delirium, is one of the most common organ failures in sepsis and is associated with increased mortality [4-8] as well as long-term cognitive impairment in survivors [7, 9].
Although the pathogenic mechanisms of acute brain dysfunction and subsequent long-term cognitive impairment are poorly understood, imaging studies comparing intensive care unit (ICU) survivors to matched controls reveal volume loss in the superior frontal lobes and hippocampus [10, 11]. The degree of volume loss is linked to the duration of acute brain dysfunction and severity of post-ICU cognitive impairment [10, 11]. In combination with animal studies showing neuronal degeneration and apoptosis during sepsis [12], these data suggest neuron cell death plays an important role in the pathophysiology of acute brain dysfunction and how it may lead to long-term cognitive impairment.
Early recognition and intervention aimed at limiting organ injury is a major priority for patients and clinicians; however, prompt detection of brain injury in critically ill patients with sepsis remains challenging. The frequent need for deep sedation during early critical illness limits the neurologic exam and may delay delirium recognition [5, 6]. Neuroimaging during early critical illness is impractical because patients often require respiratory and cardiovascular support making safe transport difficult [13]. Thus, novel approaches to identify brain injury during early critical illness are needed. Peripheral blood is easily accessible and measurement of organ-specific proteins allows for timely recognition of organ injury, such as measurement of troponin for cardiac injury or transaminases and bilirubin for hepatic injury [14, 15]. Following brain injury, neuron cell membrane integrity is compromised allowing brain-specific proteins to leak into the interstitial space, from which they enter peripheral blood via the brain's glymphatic system [16] or diffusion across a disrupted blood brain barrier [17].
Neuron specific enolase (NSE) is one of the more promising peripheral blood markers of neuronal injury because it is a cytosolic enzyme nearly exclusive to neurons and neuroendocrine cells, and is expressed in high levels in the brain [18]. NSE has proven useful for determining brain injury severity and aiding early prognosis following traumatic brain injury [19, 20] and cardiac arrest [21-23]. A prior study demonstrated high serum NSE concentrations were associated with mortality in sepsis, but found no association with sepsis-associated encephalopathy [24]. We sought to further investigate NSE as a marker of neuronal injury in critical illness by determining the association of plasma NSE concentration at ICU admission with 30-day mortality and delirium in a cohort of critically ill patients with sepsis.
Methods
Study design
We performed a retrospective analysis of patients enrolled in the Molecular Epidemiology of Severe Sepsis in the ICU (MESSI) study between January and September 2011 [25, 26]. The MESSI study is an ongoing prospective cohort of patients admitted to the medical ICU at the Hospital of the University of Pennsylvania, an urban academic tertiary referral center, with severe sepsis as defined by the American College of Chest Physicians consensus criteria [2]. Patients were enrolled if they had ≥ 2 systemic inflammatory response syndrome criteria, a known or strongly suspected infection, and evidence of organ dysfunction or shock [2]. Exclusion criteria included a lack of commitment to life sustaining treatment at the time of admission, primary reason for admission unrelated to sepsis (i.e. cardiac arrest, head injury), and previous enrollment. We excluded transfers from outside hospital ICUs given the objective to evaluate plasma NSE concentrations at initial ICU presentation. We excluded one patient with neuroendocrine cancer because NSE is a neuroendocrine tumor marker [27].
This study was approved by the Institutional Review Board of the University of Pennsylvania with a waiver of timely informed consent. Informed consent was obtained from patients or their surrogates as soon as feasible, and patients or their surrogates could withdraw from the study at any time.
Data collection
Research personnel collected data using structured case report forms with standardized definitions. Demographics and medical history were collected at the time of enrollment. We collected continuous analgesic and sedative infusion dosages during the ICU stay through day 15. Opiate dosages were converted into equivalent doses of fentanyl and benzodiazepine dosages were converted into equivalent doses of lorazepam [7]. We reviewed nursing and physician documentation during the ICU stay through day 15 to determine delirium status. During the study period, nurses assessed level of consciousness as part of standard care at least once per shift using the Richmond Agitation Sedation Scale (RASS) [28]. Patients were considered persistently comatose if they had a RASS of ≤ −4 throughout the study period. Nurses assessed for delirium using the Confusion Assessment Method for the ICU (CAM-ICU) [29], but at the time of this study the CAM-ICU was not performed every shift as part of our standard care. We defined patients as having delirium if the patient's bedside nurse documented at least one positive CAM-ICU assessment or if the patient's attending physician documented a diagnosis of delirium in their daily progress note at least once during the study period.
Acute Physiology and Chronic Health Evaluation (APACHE) III scores were calculated based on data within the first 24 hours of ICU admission. Acute kidney injury (AKI) was defined by Acute Kidney Injury Network creatinine and renal replacement therapy criteria [30]. Acute respiratory distress syndrome (ARDS) was defined using the Berlin definition with the added requirement of invasive mechanical ventilation [31].
Plasma biomarker measurement
Residual citrated plasma was collected from the earliest blood draw at or just prior to ICU admission. This corresponds to the initial blood draw at presentation to the emergency department for patients directly admitted to the ICU, and to blood drawn during or just after decompensation for patients transferred to the ICU from the medical ward. Plasma was collected in citrated vacutainers, centrifuged within 30 minutes for clinical testing, and then kept at 4°C for 12-48 hours before storage at −80°C until analysis. NSE concentrations were measured using a commercially available enzyme linked immunosorbent assay (R&D Systems, Minneapolis MN) with an intra-assay coefficient of variation of 6.1%. The lower limit of detection for NSE was 0.038 ug/L. Samples with visible evidence of hemolysis were excluded [32].
Statistical analysis
Comparisons of baseline characteristics were made using Pearson's Chi-square for categorical data and the Wilcoxon rank-sum test for continuous data. We used multivariable logistic regression to test the association of the plasma NSE concentration, defined as both a continuous and a categorical variable, with 30-day mortality and ICU delirium. We calculated standardized risks and risk differences (RD) using regression risk analysis [33, 34]. We used locally weighted scatterplot smoothing curves to determine if continuous variables required transformation prior to inclusion in logistic regression models [35]. We log (base 2) transformed the NSE concentration and therefore report our results when using NSE as a continuous variable as the risk difference for each two-fold increase in the NSE concentration. In our analyses using NSE as a categorical variable we defined a high NSE concentration as a concentration > 12.5 ug/L, which represents the 95th percentile in healthy subjects [36] and has been used in several prior studies in critically ill patients [19-22, 24].
We adjusted for illness severity using the APACHE III score in multivariable mortality models, and adjusted for APACHE III score and treatment with sedative and analgesic medications as categorical exposures in multivariable delirium models. We also assessed potential confounding by sedative and analgesic medications in delirium models when defined as the cumulative and mean daily dose during the study period. Additional potential confounders were selected a priori based on existing literature and were retained in multivariable models if they resulted in a ≥ 10% change in the point estimate in bivariate analysis (see Tables S1-S4 in the data supplement) [37].
In secondary analyses, we performed sensitivity analyses to test whether the association of NSE with 30-day mortality was driven by early deaths or modified by assumptions about survival of patients who were lost to follow-up. We also performed sensitivity analyses to test whether the association of NSE with delirium was modified by early deaths. Given the possibility that delirium was underdiagnosed, we assessed potential misclassification of the delirium outcome using logistic regression with an expectation-maximization algorithm (Stata logitem command) [38]. This method accounts for potential outcome misclassification by incorporating the sensitivity and specificity of the outcome measure in the model [38]. We varied the sensitivity of our delirium classification from 0.1 – 1.0 and determined the sensitivity at which our results would become non-significant. We assumed delirious patients were correctly classified (specificity 1.0). We also tested the association of the plasma NSE concentration with coma using multivariable logistic regression and with the number of coma/delirium-free days using negative binomial regression.
All analyses were performed using Stata version 12.1 (College Station, TX). A two-sided p value < 0.05 was considered statistically significant.
Results
Patient characteristics
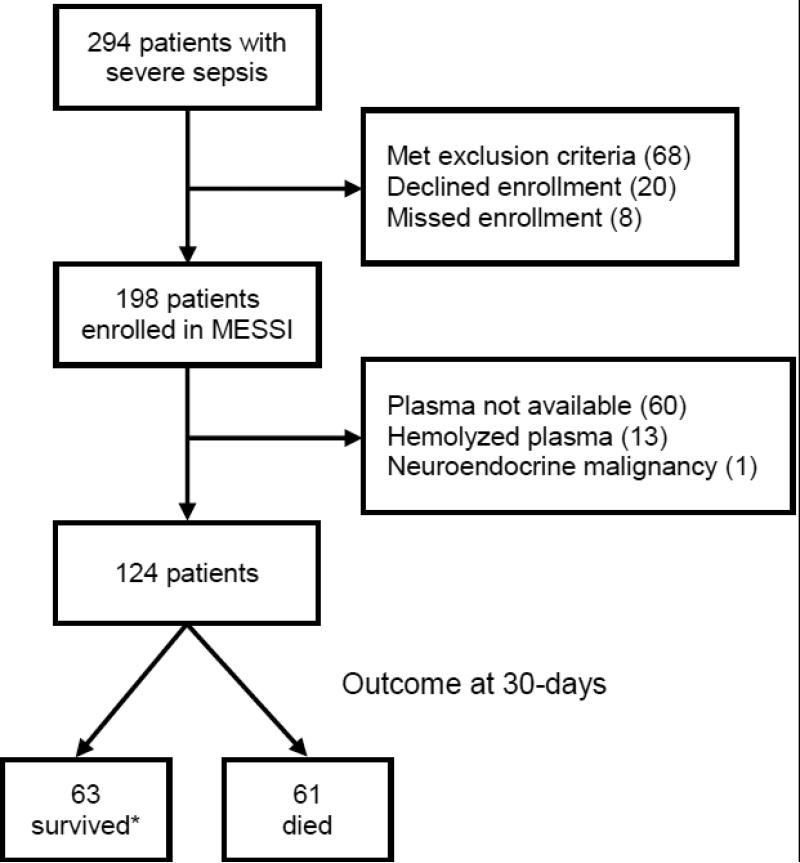
We screened 294 patients admitted to the ICU with sepsis and enrolled 198 into the MESSI study (Figure 1). One hundred twenty-four patients had available non-hemolyzed plasma and represent our study population; baseline characteristics are summarized in Table 1. Patients who underwent NSE measurement were slightly older but had no statistically significant differences in other baseline characteristics (see Table S5 in the data supplement). Septic shock occurred in 83 patients (66.9%) and 61 patients (49.2%) died within 30 days of ICU admission. All patients had detectable NSE at ICU admission and the median plasma NSE concentration was 6.6 ug/L (interquartile range 4.1-13.8). Thirty-five patients (28.2%) had high (>12.5 ug/L) plasma NSE concentrations indicative of neuronal injury at ICU admission. A history of alcohol abuse appeared to be associated with lower plasma NSE concentrations at ICU admission. The primary source of infection was not associated with NSE concentration, and we found no association of either AKI or ARDS with NSE concentration.
Figure 1.
Flowchart illustrating the enrollment and follow-up of the study population. *Three patients lost to follow-up after discharge to home were assumed to survive in primary analyses.
Table 1.
Characteristics of the study population categorized by high plasma neuron specific enolase (NSE) concentration at intensive care unit admission (n = 124)
| Variable | NSE ≤12.5ug/L (n = 89) | NSE > 12.5ug/L (n = 35) | p value |
|---|---|---|---|
| Age (yrs.) | 63 (55-71) | 61 (52-71) | 0.42 |
| Male gender | 54 (61%) | 20 (57%) | 0.72 |
| Race | |||
| White | 49 (55%) | 20 (57%) | 0.96 |
| Black or African American | 33 (37%) | 12 (34%) | |
| Other | 7 (8%) | 3 (9%) | |
| Comorbidities | |||
| Hypertension | 42 (47%) | 23 (66%) | 0.06 |
| Diabetes | 31 (35%) | 12 (34%) | 0.95 |
| Congestive heart failure | 13 (15%) | 5 (14%) | 0.96 |
| Chronic kidney disease | 9 (10%) | 6 (17%) | 0.28 |
| Cirrhosis | 11 (12%) | 4 (11%) | 0.89 |
| Malignancy | 34 (38%) | 15 (43%) | 0.63 |
| Current smoking | 11 (12%) | 4 (11%) | 0.33 |
| Alcohol abuse | 12 (13%) | 2 (6%) | 0.04 |
| Source of admission | |||
| Emergency Department | 36 (40%) | 19 (54%) | 0.16 |
| Medical Wards | 53 (60%) | 16 (46%) | |
| APACHE III score | 79 (66-97) | 89 (71-101) | 0.16 |
| Source of infection | |||
| Pulmonary | 31 (35%) | 13 (37%) | |
| Genitourinary | 14 (16%) | 6 (17%) | 0.79 |
| Gastrointestinal | 7 (8%) | 1 (3%) | |
| Other | 37 (41%) | 15 (43%) | |
| Septic shock | 56 (63%) | 27 (77%) | 0.13 |
| Mechanically ventilated | 54 (61%) | 24 (69%) | 0.41 |
| Sedative or analgesic infusion | |||
| Opiate | 39 (44%) | 17 (49%) | 0.63 |
| Benzodiazepine | 11 (12%) | 9 (26%) | 0.069 |
| Propofol | 10 (11%) | 3 (9%) | 0.66 |
| ARDS | 37 (42%) | 16 (46%) | 0.68 |
| AKI (N=121) | 47 (53%) | 20 (63%) | 0.34 |
| Coma | 28 (31%) | 18 (51%) | 0.038 |
| Persistent comaa | 4 (4%) | 4 (11%) | 0.16 |
| Delirium (n=108)b | 21 (24%) | 14 (40%) | 0.068 |
| 30-day mortality | 12 (19%) | 23 (38%) | 0.021 |
Data expressed as frequency (percent) or median (interquartile range)
Definition of abbreviations: APACHE = acute physiology and chronic health evaluation; ARDS = acute respiratory distress syndrome; AKI = acute kidney injury; NSE = neuron specific enolase
Persistent coma defined as a Richmond Agitation Sedation Score ≤ −4 throughout the study period
Delirium assessed in 108 patients who survived >24 hours and were not persistently comatose
Association of NSE with mortality
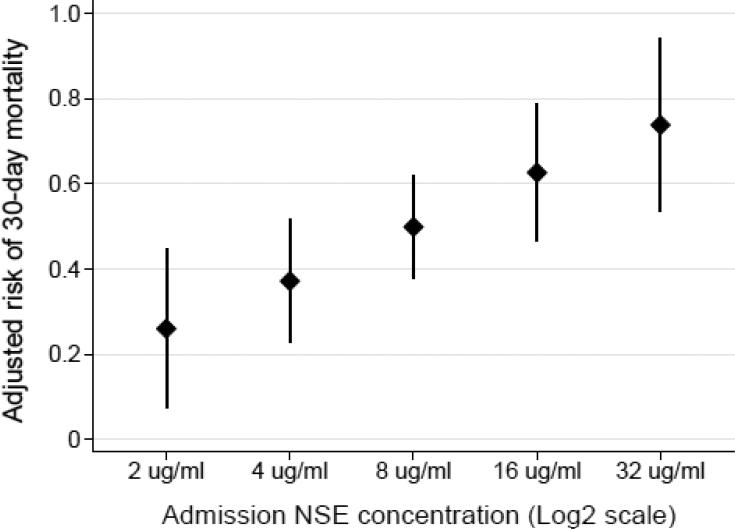
Higher plasma NSE concentrations at ICU admission were associated with increased risk of mortality (Table 2, Figure 2). Each two-fold increase in the plasma NSE concentration was associated with a 7.3% (95% CI 2.5-12.0, p=0.003) increased risk of 30-day mortality after adjusting for APACHE III score, admission location (medical ward versus emergency department), race and ARDS. When defined as a categorical variable, a high plasma NSE concentration at ICU admission was associated with a 23.3% (95% CI 6.7-39.9, p=0.006) increased risk of 30-day mortality after adjusting for APACHE III score and admission location.
Table 2.
Association of each two-fold increase in plasma neuron specific enolase concentration at intensive care unit admission with risk of mortality and delirium
| Risk Difference | 95% CI | p value | |
|---|---|---|---|
| 30-day Mortality | |||
| Unadjusted | 6.6% | 1.5-11.6 | 0.011 |
| Adjusteda | 7.3% | 2.5-12.0 | 0.003 |
| ICU Delirium | |||
| Unadjusted | 5.1% | 3.3-7.0 | <0.001 |
| Adjustedb | 5.2% | 3.2-7.2 | <0.001 |
Risk differences were calculated using regression risk analysis following logistic regression
Adjusted for Acute Physiology and Chronic Health Evaluation (APACHE) III, admission location (medical ward versus emergency room), race, and acute respiratory distress syndrome
Adjusted for APACHE III and receipt of sedative and analgesic infusions as categorical exposures
Figure 2.
Adjusted probability of 30-day mortality according to the plasma neuron specific enolase (NSE) concentration at intensive care unit admission. Points represent the adjusted mortality risk and vertical error bars represent 95% confidence intervals. The NSE concentration is plotted on the log base 2 scale. After adjustment for Acute Physiology and Chronic Health Evaluation III score, admission location, race, and acute respiratory distress syndrome each two-fold increase in the plasma NSE concentration was associated with a 7.3% increased risk of 30-day mortality (p=0.003).
To ensure the association of higher NSE concentrations with mortality was not driven by early deaths, we excluded 15 patients who died within the first 4 days and the association of each two-fold increase in NSE with 30-day mortality was similar (RD 6.8%, 95% CI 3.1-10.5, p<0.001). The association of each two-fold increase in NSE was also similar in a sensitivity analysis assuming the 3 patients who were lost to follow-up had died (adjusted RD 7.5%, 95% CI 2.2-12.8, p=0.005).
Association of NSE with delirium
One hundred eight patients who survived at least 24 hours after ICU admission and were not persistently comatose during the study period were included in our primary delirium analyses. Delirium developed in 34 (31.5%) of these 108 patients during the ICU stay.
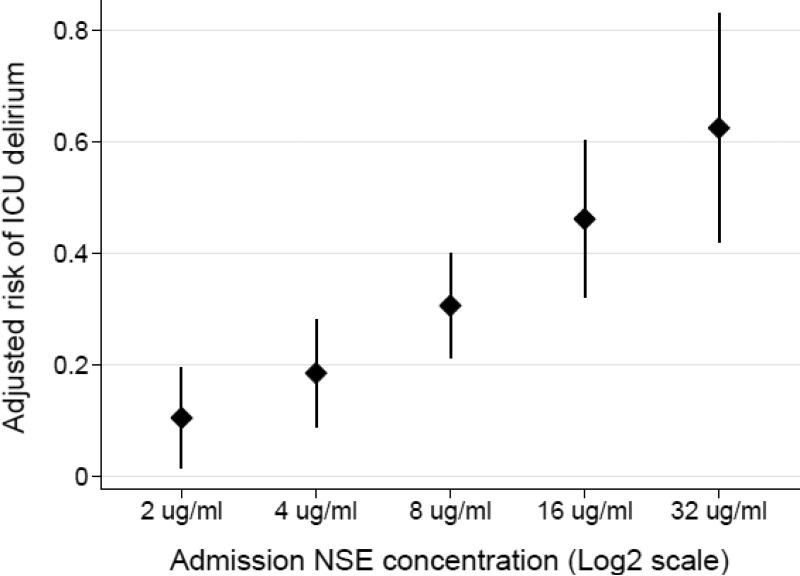
Higher plasma NSE concentrations at ICU admission were associated with increased risk of delirium (Table 2, Figure 3). Each two-fold increase in the plasma NSE concentration was associated with a 5.2% (95% CI 3.2-7.2, p<0.001) increased risk of delirium after adjusting for APACHE III score, and receipt of sedative and analgesic infusions. The results were similar when adjusting for the cumulative dose of sedative and analgesic infusions (adjusted RD 5.2%, 95% CI 3.4-6.9, p<0.001) and when adjusting for the mean daily dose of sedative and analgesic infusions (adjusted RD 5.1%, 95% CI 3.1-7.2, p<0.001). When defined as a categorical variable, a high plasma NSE concentration at ICU admission was associated with a 29.3% (95% CI 8.8-49.8, p=0.005) increased risk of delirium after adjustment for APACHE III score and receipt of sedative and analgesic infusions.
Figure 3.
Adjusted probability of delirium according to plasma neuron specific enolase (NSE) concentration at intensive care unit admission. Points represent the adjusted delirium risk and vertical error bars represent 95% confidence intervals. The NSE concentration is plotted on the log base 2 scale. After adjustment for Acute Physiology and Chronic Health Evaluation III score and receipt of sedative and analgesic infusions, each two-fold increase in the plasma NSE concentration was associated with a 5.2% increased risk of delirium (p<0.001).
The association of each two-fold increase in NSE with delirium was similar in sensitivity analyses assuming the eight patients who died within the first 24 hours were not delirious (adjusted RD 4.8%, 95% CI 3.0-6.6, p<0.001) and assuming they were delirious (adjusted RD 6.2%, 95% CI 4.4-8.0, p<0.001). To assess the robustness of our results to potential underdiagnosis of delirium, we performed sensitivity analysis varying the sensitivity of our delirium detection. Only at a sensitivity of ≤ 44%, corresponding to a false-negative rate of ≥ 56%, would the association of NSE with delirium have become non-significant. In secondary analyses, high NSE levels were associated with coma, but this association was attenuated after adjusting for APACHE III score, and receipt of sedative and analgesic infusions (see Table S6 in the data supplement). We also found that higher NSE concentrations at ICU admission were associated with significantly fewer coma/delirium free days (see Table S7 in the data supplement).
Discussion
Our study demonstrates that high plasma NSE concentrations at ICU admission for sepsis are independently associated with increased risk of mortality and delirium. These results support the potential role of neuronal injury in the pathophysiology of delirium in sepsis and support the need for further research of NSE as a novel early marker of neuronal injury in sepsis and other similar critical illnesses.
Our finding that high plasma NSE concentrations at ICU admission for sepsis were associated with 30-day mortality is consistent with the prior study by Nguyen and colleagues that demonstrated higher NSE concentrations at 24 and 48 hours after ICU admission for sepsis in patients who died within the first 4 days [24]. Our study extends these findings by demonstrating that high plasma NSE concentrations can be detected as early as ICU admission and that these early NSE concentrations are associated with both early and late mortality. Our study also demonstrates that the association of high NSE concentrations with mortality is independent of illness severity and other potential confounders at the time of presentation. The underlying nature of this association is unclear; however, it may be that neuronal injury impairs neurologic homeostatic functions resulting in endocrine, metabolic and autonomic disturbances that could contribute to increased mortality. Further research is needed to better understand the underlying mechanisms linking neuronal injury and increased mortality in patients with sepsis.
To our knowledge, our study is the first to demonstrate that high plasma NSE concentrations at ICU admission are independently associated with risk of delirium in critically ill patients with sepsis. One study of 60 general ICU patients demonstrated that delirious patients had higher serum NSE concentrations at the time of ICU admission [39], and a second study of 74 patients demonstrated higher postoperative serum NSE concentrations in patients who experienced delirium after cardiac surgery with cardiopulmonary bypass [40]. In their study of sepsis patients, Nguyen and colleagues reported that a high NSE concentration within the first 4 days of ICU admission was common in patients who developed encephalopathy, but did not report adjusted analyses and did not assess patients for delirium [24]. In contrast, no association of NSE with delirium was identified in a study of patients undergoing elective abdominal surgery or a study in elderly patients admitted with hip fracture [41, 42]. The varying results across these studies may be explained by different outcome definitions or differences in pathophysiologic mechanisms leading to delirium in different patient populations. Sepsis and cardiopulmonary bypass are both characterized by periods of hypoperfusion, hypoxemia and a robust systemic inflammatory response, which may lead to neuronal injury and play an important role in the development of delirium. It may be that these processes are less common following elective abdominal surgery or hip fractures, or other mechanisms may play more predominant roles in the development of delirium in such patients.
Our reported association of high plasma NSE concentrations at ICU admission with delirium in sepsis adds to a growing body of literature suggesting critical illness is associated with acute neuronal injury and that neuronal injury may be in the causal pathway of delirium. Future studies are needed to validate our findings and understand more fully the association of NSE with delirium across different patient populations. Studies should also investigate the association of NSE with post-ICU cognitive function given the strong association of delirium with subsequent long-term cognitive impairment [7, 9]. Further validation of NSE or similar markers could provide a novel method to quantify brain injury early in critical illness, which could be useful for studies investigating pathogenic mechanisms of delirium in critical illness. Although our study was not designed to investigate risk factors associated with NSE concentrations, our results suggest a history of alcohol abuse may be was associated with lower NSE levels at ICU admission for sepsis. These findings are hypothesis-generating but may be due to small sample size or potentially be random due to multiple comparisons. Future studies are needed to better understand risk factors associated with neuronal injury in sepsis.
Despite more than a dozen clinical trials over the past decade, there are few specific treatments to prevent or treat delirium in critically ill patients [43, 44]. Failed attempts at identifying effective therapies for delirium may be due to late intervention or due to its heterogeneous nature [45]. NSE or similar markers may have utility in defining subphenotypes of delirium, which could reduce this heterogeneity. The importance of reducing heterogeneity is highlighted by a recent study demonstrating different responses to treatment among subphenotypes of ARDS [46]. NSE or similar markers may also be useful in combination with clinical variables to improve patient selection for future clinical trials [47, 48].
Our study has several important limitations. The study was completed at a single center so generalizability may be limited. Three patients were lost to follow-up after discharge to home; however, sensitivity analyses demonstrated our results were robust to this potential misclassification of 30-day mortality. The incidence of delirium in our study was lower than other studies, possibly due to differences in patient populations. The Bringing to Light the Risk Factors and Incidence of Neuropyschological Dysfunction in ICU Survivors (BRAIN-ICU) study, the largest delirium study in critically ill patients, reported an incidence of 74% [7]. However, the BRAIN-ICU study had a higher rate of mechanical ventilation (91% versus 63%), as well as more frequent treatment with benzodiazepines (62% versus 16%), opiates (78% versus 45%) and propofol (52% versus 10%) compared to our study. Alternatively, a study by Ouimet and colleagues that similarly enrolled patients admitted to the ICU regardless of the need for mechanical ventilation or presence of shock reported a delirium incidence of 31.8%, which is consistent with our results [4]. The low incidence of delirium in our study could also be due to underdiagnosis because we determined delirium by chart review. To minimize underdiagnosis we reviewed attending physician notes in addition to routine nursing assessments, which have been shown to have varied sensitivity [49, 50]. We also performed sensitivity analyses and demonstrated our results were robust to underdiagnosis of delirium; only when the sensitivity of our delirium classification was ≤ 44%, corresponding to a false-negative rate of ≥ 56%, would the association of NSE with delirium have become non-significant. Misclassification could also have occurred among the few patients who died shortly after ICU admission prior to undergoing delirium assessment, but sensitivity analysis including these patients demonstrated similar results. Finally, although we considered multiple confounders in our adjusted analyses, unmeasured confounders could still have affected our results.
Conclusions
Our results demonstrate that higher plasma concentrations of NSE at the time of ICU admission were independently associated with an increased risk of mortality and delirium in patients with sepsis. These findings suggest acute neuronal injury is common in early sepsis and that neuronal injury may play a role in the pathophysiology of delirium. Future research is needed to validate our findings, and investigate whether NSE or similar plasma markers are associated with long-term cognitive outcomes, can help elucidate pathogenic mechanisms of neuronal injury in critical illness, and be used for early identification of patients to target for novel neuroprotective interventions.
Supplementary Material
Acknowledgements
Funding sources:
This study was supported in part by National Institutes of Health grants T32NS061779 (B.J.A.), F32GM116637 (B.J.A.), K23HL125723 (J.P.R.), K23DK097307 (M.G.S.S.), K23HL102254 (N.J.M.), K24HL115354 (J.D.C.), and an American Thoracic Society Foundation Award (N.J.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
N.J.M. and J.D.C. report research funding from GlaxoSmithKline unrelated to the present study. All remaining authors declare that they have no conflict of interest.
References
- 1.Pfuntner A, W. L.M., Stocks C. Most frequent conditions in U.S. hospitals, 2011. HCUP Statistical Brief #162. Agency for Healthcare Research and Quality. 2013 [PubMed] [Google Scholar]
- 2.Levy MM, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–6. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Xu JQ, et al. National vital statistics reports. 2. Vol. 64. National Center for Health Statistics; 2016. Deaths: Final data for 2013. [PubMed] [Google Scholar]
- 4.Ouimet S, et al. Incidence, risk factors and consequences of ICU delirium. Intensive Care Med. 2007;33(1):66–73. doi: 10.1007/s00134-006-0399-8. [DOI] [PubMed] [Google Scholar]
- 5.Ely EW, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 6.Shehabi Y, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med. 2012;186(8):724–31. doi: 10.1164/rccm.201203-0522OC. [DOI] [PubMed] [Google Scholar]
- 7.Pandharipande PP, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNicoll L, et al. Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc. 2003;51(5):591–8. doi: 10.1034/j.1600-0579.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- 9.Girard TD, et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–20. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunther ML, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–32. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semmler A, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84(1):62–9. doi: 10.1136/jnnp-2012-302883. [DOI] [PubMed] [Google Scholar]
- 12.Bozza FA, et al. Bioenergetics, mitochondrial dysfunction, and oxidative stress in the pathophysiology of septic encephalopathy. Shock. 2013;39(Suppl 1)(Suppl 1):10–6. doi: 10.1097/SHK.0b013e31828fade1. [DOI] [PubMed] [Google Scholar]
- 13.Schwebel C, et al. Safety of intrahospital transport in ventilated critically ill patients: a multicenter cohort study*. Crit Care Med. 2013;41(8):1919–28. doi: 10.1097/CCM.0b013e31828a3bbd. [DOI] [PubMed] [Google Scholar]
- 14.Mehta NJ, et al. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol. 2004;95(1):13–7. doi: 10.1016/j.ijcard.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Kramer L, et al. Incidence and prognosis of early hepatic dysfunction in critically ill patients--a prospective multicenter study. Crit Care Med. 2007;35(4):1099–104. doi: 10.1097/01.CCM.0000259462.97164.A0. [DOI] [PubMed] [Google Scholar]
- 16.Plog BA, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35(2):518–26. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonneville R, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care. 2013;3(1):15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor CB, et al. Diagnostic aspects of enolase isozymes. Isozymes Curr Top Biol Med Res. 1983;11:95–119. [PubMed] [Google Scholar]
- 19.Meric E, et al. The prognostic value of neuron-specific enolase in head trauma patients. J Emerg Med. 2010;38(3):297–301. doi: 10.1016/j.jemermed.2007.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Vos PE, et al. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology. 2004;62(8):1303–1310. doi: 10.1212/01.wnl.0000120550.00643.dc. [DOI] [PubMed] [Google Scholar]
- 21.Meynaar IA, et al. Serum neuron-specific enolase predicts outcome in post-anoxic coma: a prospective cohort study. Intensive Care Med. 2003;29(2):189–95. doi: 10.1007/s00134-002-1573-2. [DOI] [PubMed] [Google Scholar]
- 22.Martens P, Raabe A, Johnsson P. Serum S-100 and neuron-specific enolase for prediction of regaining consciousness after global cerebral ischemia. Stroke. 1998;29(11):2363–6. doi: 10.1161/01.str.29.11.2363. [DOI] [PubMed] [Google Scholar]
- 23.Stammet P, et al. Neuron-Specific Enolase as a Predictor of Death or Poor Neurological Outcome After Out-of-Hospital Cardiac Arrest and Targeted Temperature Management at 33 degrees C and 36 degrees C. J Am Coll Cardiol. 2015;65(19):2104–14. doi: 10.1016/j.jacc.2015.03.538. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen DN, et al. Elevated serum levels of S-100beta protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34(7):1967–74. doi: 10.1097/01.CCM.0000217218.51381.49. [DOI] [PubMed] [Google Scholar]
- 25.Reilly JP, et al. The ABO Histo-Blood Group and AKI in Critically Ill Patients with Trauma or Sepsis. Clin J Am Soc Nephrol. 2015;10(11):1911–20. doi: 10.2215/CJN.12201214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palakshappa JA, et al. Low Plasma Levels of Adiponectin Do Not Explain Acute Respiratory Distress Syndrome Risk: a Prospective Cohort Study of Patients with Severe Sepsis. Crit Care. 2016;20(1):71. doi: 10.1186/s13054-016-1244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barakat MT, Meeran K, Bloom SR. Neuroendocrine tumours. Endocr Relat Cancer. 2004;11(1):1–18. doi: 10.1677/erc.0.0110001. [DOI] [PubMed] [Google Scholar]
- 28.Ely EW, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS). JAMA. 2003;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 29.Ely EW, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAMICU). JAMA. 2001;286(21):2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 30.Mehta RL, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Force ADT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 32.Kato K, et al. Immunoassay of three enolase isozymes in human serum and in blood cells. Clin Chim Acta. 1983;127(3):353–63. doi: 10.1016/0009-8981(83)90162-6. [DOI] [PubMed] [Google Scholar]
- 33.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata Journal. 2013;13(3):492–509. [Google Scholar]
- 34.Kleinman LC, Norton EC. What's the Risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res. 2009;44(1):288–302. doi: 10.1111/j.1475-6773.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleveland WS. Robust Locally Weighted Regression and Smoothing Scatterplots. Journal of the American Statistical Association. 1979;74(368):829–836. [Google Scholar]
- 36.Fizazi K, et al. Normal serum neuron specific enolase (NSE) value after the first cycle of chemotherapy: an early predictor of complete response and survival in patients with small cell lung carcinoma. Cancer. 1998;82(6):1049–55. doi: 10.1002/(sici)1097-0142(19980315)82:6<1049::aid-cncr6>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 37.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 38.Magder LS, Hughes JP. Logistic regression when the outcome is measured with uncertainty. Am J Epidemiol. 1997;146(2):195–203. doi: 10.1093/oxfordjournals.aje.a009251. [DOI] [PubMed] [Google Scholar]
- 39.Grandi C, et al. Brain-derived neurotrophic factor and neuron-specific enolase, but not S100beta, levels are associated to the occurrence of delirium in intensive care unit patients. J Crit Care. 2011;26(2):133–7. doi: 10.1016/j.jcrc.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Herrmann M, et al. Neurobehavioral outcome prediction after cardiac surgery: role of neurobiochemical markers of damage to neuronal and glial brain tissue. Stroke. 2000;31(3):645–650. doi: 10.1161/01.str.31.3.645. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen LS, et al. Do blood concentrations of neurone specific enolase and S-100 beta protein reflect cognitive dysfunction after abdominal surgery?ISPOCD Group. Br J Anaesth. 2000;84(2):242–4. doi: 10.1093/oxfordjournals.bja.a013410. [DOI] [PubMed] [Google Scholar]
- 42.van Munster BC, et al. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. 2009;9:21. doi: 10.1186/1471-2377-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barr J, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 44.Al-Qadheeb NS, et al. Randomized ICU trials do not demonstrate an association between interventions that reduce delirium duration and short-term mortality: a systematic review and meta-analysis. Critical Care Medicine. 2014;42(6):1442–1454. doi: 10.1097/CCM.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel SB, et al. Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med. 2014;189(6):658–65. doi: 10.1164/rccm.201310-1815OC. [DOI] [PubMed] [Google Scholar]
- 46.Calfee CS, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–20. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matthay MA, Liu KD. New Strategies for Effective Therapeutics in Critically Ill Patients. JAMA. 2016;315(8):747–8. doi: 10.1001/jama.2016.0661. [DOI] [PubMed] [Google Scholar]
- 48.Salluh JIF, de Souza-Dantas VC, Gusmao-Flores D. Improved risk stratification for clinical trials of delirium. The Lancet Respiratory Medicine. 2016;4(5):e17. doi: 10.1016/S2213-2600(16)30020-0. [DOI] [PubMed] [Google Scholar]
- 49.Reade MC, et al. Routine use of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) by bedside nurses may underdiagnose delirium. Critical Care and Resuscitation. 2011;13(4):217–225. [PubMed] [Google Scholar]
- 50.Vasilevskis EE, et al. Delirium and sedation recognition using validated instruments: reliability of bedside intensive care unit nursing assessments from 2007 to 2010. J Am Geriatr Soc. 2011;59(Suppl 2)(Suppl 2):S249–55. doi: 10.1111/j.1532-5415.2011.03673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.