Abstract
Purpose
Sound levels in the intensive care unit (ICU) are universally elevated and are believed to contribute to sleep and circadian disruption. The purpose of this study is to compare overnight ICU sound levels and peak occurrence on A- versus C-weighted scales.
Materials and Methods
This was a prospective observational study of overnight sound levels in 59 medical ICU patient rooms. Sound level was recorded every 10 seconds on A- and C-weighted decibel scales. Equivalent sound level (Leq) and sound peaks were reported for full and partial night periods.
Results
The overnight A-weighted Leq of 53.6 dBA was well above World Health Organization (WHO) recommendations; overnight C-weighted Leq was 63.1 dBC (no WHO recommendations). Peak sound occurrence ranged from 1.8 to 23.3 times per hour. Illness severity, mechanical ventilation and delirium were not associated with Leq or peak occurrence. Leq and peak measures for A- and C-weighted decibel scales were significantly different from each other.
Conclusions
Sound levels in the medical ICU are high throughout the night. Patient factors were not associated with Leq or peak occurrence. Significant discordance between A- and C-weighted values suggests that low frequency sound is a meaningful factor in the medical ICU environment.
Keywords: Sound, Noise, A-weighted, C-weighted, low frequency, critical care
Introduction
Critically ill patients experience sleep loss, poor sleep quality and circadian misalignment. Causality is multifactorial and may include physiologic, psychological, and environmental factors [1-3]. Studies using 24hour polysomnography in medical intensive care unit (MICU, ICU) patients demonstrate severely reduced overall sleep time, decreased slow wave sleep, limited rapid eye movement sleep, frequent arousals, and increased sleep during daytime hours [3-7]. Similar findings have been observed in healthy subjects exposed to recordings of ICU sounds [8].
Health care providers struggle to balance the urgent needs of critically ill patients with the empiric observation that ICUs are loud and disruptive. Concerns regarding the impact of the ICU environment on the cognitive and psychiatric outcomes of patients were raised early in the inception of ICUs [8-13]. However, critical care units are still characterized by high sound levels that, in turn, may contribute to sleep deprivation, circadian disruption, and delirium. There have been improvements in treatment and prevention of delirium and promotion of sleep [14, 15], but understanding of how characteristics of the ICU environment, such as sound, impact patient sleep, circadian orientation, and delirium continues to be incomplete.
Sound has characteristics of amplitude or sound pressure (perceived as loudness, measured in decibels), frequency (perceived as pitch, measured in Hertz), and time pattern [16]. Decibel (dB) levels are reported on a logarithmic scale that accommodates the large range of sound intensity in our environment. The threshold for human hearing is set at 0 dB; painful sound is 140 dB. A change in 3-5 dB is perceptible to the human ear and a change of 10 dB represents approximate doubling of sound amplitude [16, 17]. Noise is unwanted or undesirable sound and is subjectively identified by the listener.
The sound level equivalent (Leq) reflects the average amplitude or sound pressure over an indicated interval. Leq is most commonly reported on either an A-weighted (unit dBA) or C-weighted (unit dBC) scale. Weighted scales integrate sound levels across varying frequencies and give higher or lower weights to particular frequencies. For example, reporting of sound in dBA units gives more weight to the higher frequency tones most readily heard by the human ear. The C-weighted scale incorporates a wider range of sound frequency and is weighted equally across low and high frequencies. Discordance between A- and C-weighted measures indicates the presence of a high proportion of low frequency sound [18]; low frequency sound is associated with building machine noise such as air handlers and small machine noise such as the “hum” from computers or televisions [19].
Studies of patients interviewed post ICU discharge identified lack of sleep and “noise” as significant stressors and linked “noise” to sleep loss [17, 20-23]. Average ICU sound levels are typically between 55 to 65 dBA [24-29] despite World Health Organization (WHO) recommendations for hospital sound averages and maxima of 30 and 40 dBA, respectively [30]. Maximum sound levels exceed 80 dBA in most studies of acute or critical care environments [24, 25, 31, 32]. Studies of critically ill patients demonstrate correlations between sound peaks greater than 75 dBA and polysomnographic arousals from sleep with approximately 17-18% of nighttime arousals occurring at the time of sound peaks [5, 33].
The purpose of this paper is to report overnight sound levels (amplitude and peak occurrence) in MICU patient rooms and to examine associations between sound levels and patient characteristics. Peak occurrence was measured in absolute and relative terms, and concurrent measures on A- and C-weighted scales were compared for differences. Patient characteristics include severity of illness, use of mechanical ventilation, use of vasopressors, need for contact precautions and presence of delirium. We hypothesized that these factors were either associated with sound producing machines or may require a higher degree of bedside presence that would increase sound levels.
Methods
Study Design and Setting
This was a prospective observational study investigating sound levels in the rooms of critically ill patients. The study was conducted in the MICU of a 1,000 bed tertiary hospital. The MICU was built in 2009 and is rectangular in shape with rooms on all four sides around a central core of workstations, supply closets, and conference rooms (Figure 1). Every patient has a private room with 3 solid walls and a hallway wall with a curtain and sliding clear glass door. There is no central nursing station. There is a main entrance desk that is continuously staffed by administrative personnel who answer phones and open the locked unit door. The majority (>70%) of nurses work 12-hour shifts from 07:00 to 19:00 and 19:00 to 07:00. Daytime physician staffing utilizes a traditional academic pyramid of one attending physician supported by fellows, residents, interns, advanced practice nurses, and physician assistants who conduct interdisciplinary rounds during the morning and perform work during the afternoon. Overnight physician staffing includes three intensivists and the on-call resident and intern who work from 19:00 to 07:00. Visitors are allowed in the unit 24 hours a day. It is hospital policy that all patients receive a “quiet kit” that includes earplugs, eye masks, and suggestions for being quiet after 21:00.
Figure 1.
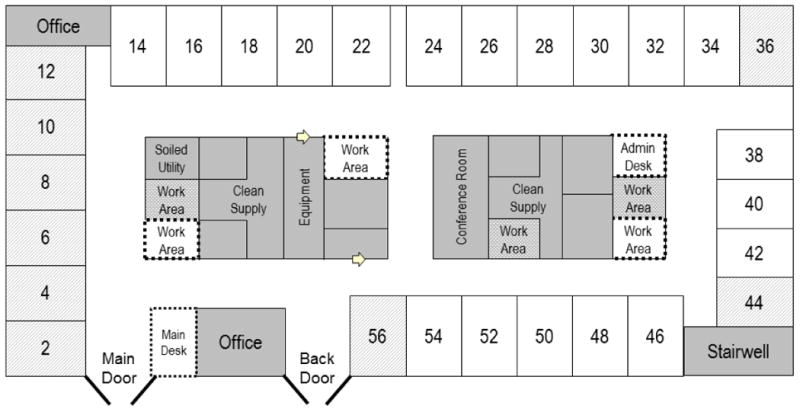
Medical intensive care unit floor plan. Patient rooms are indicated by sequential even numbers; grey-lined rooms were excluded from the study. Staff work areas were either enclosed (grey) or open (white with dashed borders).
Study Patients and Ethical Considerations
MICU study rooms were selected based on presumed average sound qualities with the goal of excluding rooms that may be extremely noisy or quiet. Therefore, rooms by the main MICU entrance which were subject to high staff and equipment traffic and corner rooms with lower traffic were excluded (Figure 1). The sample included patients over the age of 18 years who were admitted to MICU study rooms. Patients were eligible for screening if they had been admitted within the 48 hours prior to the next sound recording period. Patients were excluded if they were non-English speaking, expected to die in the next 24 hours, receiving comfort care only, undergoing therapeutic hypothermia, or expected to be transferred from the MICU before completing the overnight sound recording period. All study procedures were approved by the Institutional Review Board / Human Investigations Committee. Consent was obtained according to standard procedures.
Clinical Data Collection and Sound Recordings
Patient data was abstracted from the electronic medical record and included need for contact precautions, mechanical ventilation, vasopressor use, and severity of illness. Delirium was determined during daily interview via the Confusion Assessment for the ICU (CAM-ICU) scale [34]. Contact precaution status was considered “yes” if visitors to a patient's room were required by the hospital to don a protective gown before entering. Mechanical ventilation was considered positive if the patient was ventilated during the night of observation. Vasopressor use was positive if norepinephrine, epinephrine, neosynephrine, dopamine, or vasopressin was used for one hour or more during the 24 hours surrounding the overnight sound monitoring period. Severity of illness was defined by the APACHE II score calculated according to the published algorithm [35].
Sound data were obtained with two sound meters per room (HD600, Extech Instruments, New Hampshire, USA) set to read sound levels every 10 seconds on either an A- or C-weighted scale. We piloted sampling frequencies of every 2, 5 and 10 seconds. Sampling frequency did not change Leq; there was a nonsignificant trend suggesting a small decrease in peaks as the sampling frequency decreased. We selected a 10 second sampling frequency to allow full overnight monitoring and data storage on our portable devices. The Extech HD600 Sound Level Meter meets American National Standards Institute and IEC61672-1 Type 2 standards and was calibrated per National Institute of Standards and Technology (NIST) standards. Sound measurement was continuous and re-calibration occurred between patients using the Extech NIST Sound Calibrator. The decibel range was set at 30 to 130 decibels and the detector response setting was “Fast” (125 ms) as suggested by the manufacturer to capture sound peaks. Sound meters were placed at a standard central location in the patient's room adjacent to the foot of the patient's bed; telemetry monitors and ventilators were also placed at standard locations adjacent to the head of the patient's bed. Our sound meter position was optimal in terms of patient safety and for achieving a standard distance between the sound meters and patient equipment such as the mechanical ventilator, intravenous medication pumps and telemetry monitor.
Data analysis
Data analyses were performed with SAS software V9.3 (SAS Institute, North Carolina, USA). Continuous clinical variables were described by mean and standard deviation (SD) or by median and interquartile range (IQR). For each set of sound measurements (A- and C-weighted), mean Leq was calculated for the entire overnight period (20:00 to 08:00) as well as beginning (20:00 to 23:59), middle (00:00 to 03:59) and end of the night (04:00 to 08:00) periods. Average sound extremes were calculated by taking the highest/lowest sound level during the entire period under evaluation and calculating the mean across all patients. “LAF90” is the sound level exceeded for 90% of the measurement period indicated. By definition, LAF90 is calculated only in the A-weighted scale. Sound peaks were identified via the following criteria: 1) absolute A-weighted sound level greater than 60 dBA or absolute C-weighted sound level greater than 70 dBC and 2) relative sound level greater than 10 decibels above local mean sound level (A- or C-weighted) as determined for the 5 minutes during which the peak occurred. Frequencies and percentages were used to describe the categorical data. Differences in frequencies or percentages between groups were tested with a chi-square statistic; differences in means were tested with an unpaired Student's t-test or one-way ANOVA; and differences in medians were tested with the Wilcoxon Two Sample statistic. For significant differences detected with ANOVA, post-hoc comparisons were made with the Tukey-Kramer test that simultaneously tests all pairs of means while adjusting for multiple comparisons. Statistical significance was defined as a p-value ≤ 0.05.
Results
Patient characteristics
Of 266 screened patients, 134 were eligible, and 59 were enrolled (Figure 2). Baseline demographic, medical, and severity of illness characteristics of the enrolled participants are described in Table 1. Patients' mean age was 63 years (SD) and they had an average length of MICU stay of 5.0 days. Reasons for MICU admission were diverse, but the most frequent included sepsis (n=18/59) or respiratory failure (n=17/59). The mean APACHE II score was 19.4; 21/59 (35.6%) of patients requiring mechanical ventilation. Delirium was present in 23/59 (39.0%) of patients during the 24-hour period during which patients were monitored.
Figure 2.
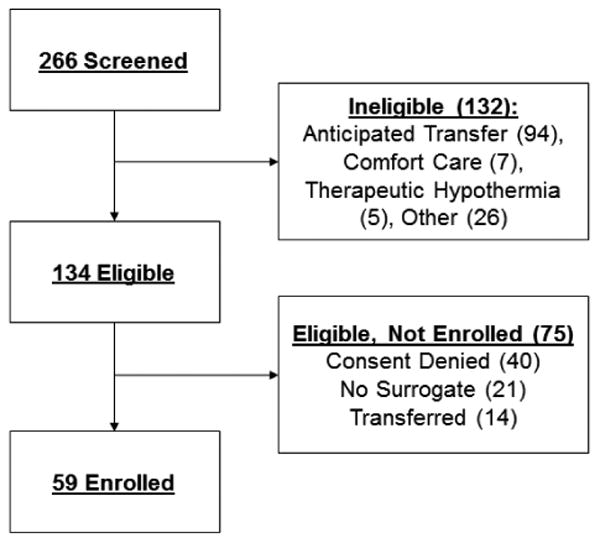
Study consort diagram.
Table 1. Clinical and demographic characteristics of enrolled study participants (N=59).
| Baseline Demographics | |
|---|---|
| Age in years: mean (SD) | 63.1 (16.8) |
| Male gender: n (%) | 31 (52.5%) |
| Race, non-white: n (%) | 13 (22.0%) |
| MICU length of stay in days: mean (SD) | 5.0 (2.8) |
| Hours from admission to study enrollment: mean (SD) | 31:45 (7:03) |
|
| |
| Reason for MICU Admission: n (%) | |
|
| |
| Infection, sepsis: n (%) | 18 (30.5%) |
| Respiratory failure: n (%) | 17 (28.8%) |
| Gastrointestinal bleed: n (%) | 6 (10.2%) |
| Liver disease: n (%) | 2 (3.4%) |
| Pulmonary embolism: n (%) | 2 (3.4%) |
| Heart failure: n (%) | 1 (1.7%) |
| Other disease: n (%) | 13 (22.0%) |
|
| |
| Severity of Illness Parameters | |
|
| |
| APACHE II score: mean (SD) | 19.4 (5.9) |
| Mechanical ventilation: n (%) | 21 (35.6%) |
| Delirium, n (%) | 23 (39.0%) |
Abbreviation: SD, standard deviation
Sound level equivalents and sound extremes in MICU patient rooms
The mean Leq (sound level equivalent) from 20:00 to 08:00 was 53.5 dBA and 63.1 dBC. The means of sound maxima from all patients in the overnight period (20:00 to 08:00) were 80.0 dBA and 84.9 dBC. The means for the sound minima were 46.5 dBA and 57.5 dBC. Within each set of A- and C-weighted measures, there were no statistically significant differences between mean equivalent sound levels, mean of sound maxima, or mean of sound minima. This was true for the full overnight period and each of the three designated four-hour blocks representing the beginning (20:00 to 23:59), middle (00:00 to 03:59) and end of night (04:00 to 08:00) periods. In contrast, when comparing A-weighted versus C-weighted measures, mean Leq and mean of sound minima on the C-weighted scale was approximately 10 decibels (a sound doubling) higher during all periods of comparison; these differences are statistically significant (Table 2). LAF90, the sound level exceeded for 90% of the measurement period, was 49.5 dBA for the overnight period (Table 2).
Table 2. Overnight MICU A-weighted and C-weighted Sound Levels.
| Mean Leq (SD) | Mean (SD) of Maxima | Mean (SD) of Minima | LAF90 | ||||
|---|---|---|---|---|---|---|---|
| Time Period | dBA | dBC | dBA | dBC | dBA | dBC | dBA |
|
Overnight: 20:00 – 08:00 |
53.5 (4.1) | 63.1 (3.8) | 80.0 (4.2) | 84.9 (5.5) | 46.5 (4.9) | 57.5 (3.6) | 49.5 (4.7) |
|
Beginning of Night: 20:00 – 23:59 |
54.6 (3.9) | 63.7 (3.6) | 77.6 (4.2) | 82.3 (5.8) | 47.9 (4.5) | 58.9 (3.3) | 50.5 (4.6) |
|
Middle of Night: 24:00 – 3:59 |
52.7 (4.5) | 62.6 (4.0) | 74.1 (4.5) | 79.0 (5.5) | 47.2 (5.0) | 57.9 (3.9) | 49.5 (4.9) |
|
End of Night: 04:00 – 08:00 |
53.2 (4.5) | 63.1 (4.0) | 76.1 (5.5) | 81.6 (5.8) | 47.9 (4.9) | 58.6 (3.3) | 49.8 (5.1) |
dBA n=53
dBC n=46
Difference of mean Leq for (dBA) and for (dBC) had p-value < 0.0001 (t-test) for all pairs.
Difference of means of minimal for (dBA) and for (dBC) had p-value < 0.0001 (t-test) for all pairs.
Abbreviations: dBA, A-weighted decibel unit; dBC, C-weighted decibel unit; LAF90, sound level exceeded for 90% of the measurement period; Leq, Sound Level Equivalent; SD, standard deviation
Sound peaks occur frequently across all portions of the overnight period
We analyzed the occurrence of the sound peaks in absolute and relative terms. For A-weighted sound measures, during the entire overnight period there was a median of 23.3 absolute peaks per hour and 5.6 relative peaks per hour. Neither absolute nor relative peak frequency varied significantly between the entire overnight period versus beginning, middle and end of night periods; however, the median values were lower and the interquartile range was narrowed during the middle of the night period (Table 3).
Table 3. Occurrence of Overnight MICU Sound Peaks.
| Time Period | Median Peaks per Hour [Q1, Q3] | |||
|---|---|---|---|---|
|
| ||||
| Absolute Peaks | Relative Peaks | |||
| dBA | dBC | dBA | dBC | |
|
Overnight: 20:00 – 08:00 |
23.3 [12.1, 47.7] | 7.9 [2.3, 16.0] | 5.6 [2.9,10.1]a | 1.8 [1.0, 2.9]a |
|
|
||||
|
Beginning of night: 20:00 – 23:59 |
32.0 [15.3, 68.3] | 9.8 [3.3, 23.8] | 6.3 [3.8, 10.0] | 1.6 [0.8, 3.8] |
|
|
||||
|
Middle of night: 24:00 – 3:59 |
12.8 [6.8, 33.8] | 4.8 [1.3, 15.8] | 4.0 [2.0, 8.8] | 1.3 [0.5, 3.0] |
|
|
||||
|
End of night: 04:00 – 08:00 |
21.3 [10.0, 43.3] | 6.0 [2.5, 19.0] | 5.3 [3.0, 9.3] | 1.5 [0.8, 3.3] |
dBA n=53
dBC n=46
p < 0.0001
Abbreviations: dBA, A-weighted decibel unit; dBC, C-weighted decibel unit; Q1 and Q3 indicate first (25%) and third (75%) quartiles
For C-weighted sound measures, peaks were defined similarly. For the entire overnight period there was a median of 7.9 absolute peaks per hour and 1.8 relative peaks per hour. Peak occurrence did not vary significantly between the entire overnight period versus beginning, middle and end of night periods. Similar to the dBA peak measurements, there were trends that suggested a decreased number of absolute peaks during the middle of the night (Table 3). Comparison of frequency of A- versus C-weighted peaks reveals significant differences between the two measures. The A- and C-weighted relative peak measures were defined identically and dBA measurements revealed significantly more frequent dBA peaks when compared to relative C-weighted peaks (Table 3).
Equivalent sound levels do not adequately reflect peak burden
Evaluation of raw A-weighted sound plots for overall sound patterns demonstrates that Leq does not adequately reflect variations in sound level that occur over time. Figure 3 displays four representative overnight sound plots; brief clinical summaries are included in the figure legend. Plot A shows the sound profile for a patient with overall low Leq (48.3 dBA) versus the total patient sample; however, the sound profile has a high relative peak burden with a mean of 8.3 relative peaks per hour across the overnight period. Plots B and C show sound profiles for two patients with virtually equivalent (54.5 and 54.3 dBA) Leq levels which are similar to the mean Leq for the overall patient sample. Despite having a similar Leq, the frequency of peak occurrence in plot B is very low at 4.1 relative peaks per hour, and the frequency of peak occurrence in plot C is much higher at 10.1 relative peaks per hour. Finally, D shows a subject with a high mean Leq (59.8) versus the overall patient sample; this patient's sound profile has a very low peak burden of 0.8 relative peaks per hour. Analysis over the whole sample demonstrates that Leq and the mean number of relative peaks were poorly correlated (correlation coefficient of -0.186 and R2 of 0.0345).
Figure 3.
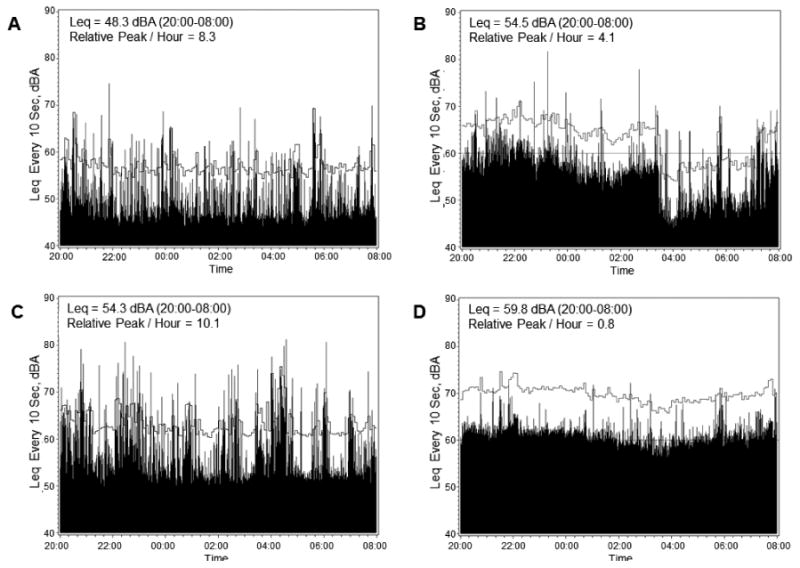
Single patient sound plots across the 20:00 to 08:00 overnight period. Leq and relative peaks are indicated in the upper left of each panel. Absolute dBA value every 10 seconds is indicated by black bars. Clock time is on the x-axis and decibels on the y-axis. Local mean is indicated by the horizontal grey line. Relative peaks are any values (black bars) that cross the local mean (horizontal grey line). Clinical vignettes with age rounded to the nearest decade are as follows: A. 80 year old with a history of stroke, atrial fibrillation and colon cancer who was admitted to the MICU for urosepsis. B. 50 year old with a history of end stage renal disease and peripheral vascular disease who was admitted to the MICU for sepsis related to a skin wound. C. 50 year old with end stage liver disease from hepatitis C virus who was admitted to the MICU for poor mental status in the setting of hepatic encephalopathy. D. 70 year old with chronic obstructive pulmonary disease and acute myeloid leukemia who was admitted to the MICU for hypoxemia in the setting of blast crisis.
Sound level and sound peak occurrence are not associated with patient characteristics
We evaluated the associations between patient clinical characteristics (severity of illness, delirium, need for contact precautions, vasopressor use, and mechanical ventilation) and equivalent sound levels and sound peak frequency. There were no associations between overnight Leq (A- or C-weighted measures) and any of the patient characteristics listed (Supplementary Material, Table S1, S2). The frequency of absolute and relative peaks was also not significantly associated with any of the patient characteristics.
Discussion
Our study is the first to describe the discrepancy between A- and C-weighted mean Leq measures in the ICU. In addition, the C-weighted mean of sound minima is approximately 10 decibels (a sound doubling) above that of A-weighted mean of sound minima during all measurement periods. These findings are important because they indicate that there is a high level of constant low frequency sound present in the ICU; this may be important in understanding the impact of sound levels on ICU patient outcomes. In contrast, A-weighted levels can be quite low, as indicated by the mean sound minima on the A-weighted scale. C-weighted sound peaks occur at a significantly lower frequency than A-weighted peaks. This suggests less C-weighted variability. Consistent with prior studies, mean A-weighted Leq over the full night period and in four-hour intervals during the night were elevated over the levels recommended by the WHO [24-29].
ICU-based sources of low frequency sound includes air handling systems, climate control mechanisms, mechanical ventilation machinery and small machine “hum” such as that produced by mobile computer workstations, medical beds or televisions. The implications of the difference between A-weighted and C-weighted findings are not clear. From a clinical perspective, it is not known whether prolonged elevated levels or sporadic peak elevations of sound are more important to sleep disruption. Polysomnography studies attribute about one-fifth of electrophysiological arousals from sleep to sound peaks in the ICU [5, 33]. Studies of the effects of low frequency sounds and perceived noise from wind turbines demonstrated negative effects on sleep, although the mechanism through which low frequency sound affects human health and sleep are not understood [36].
Precise comparison of our findings with previous studies is limited by the variations in methods used to record and report sound characteristics. For example, there is no established definition of peak sounds, and there is considerable uncertainty regarding the characteristics of sound that are most relevant to health-related outcomes. Leq is the predominant metric reported. However, Leq obscures important considerations such as variations in sound over time, the distribution of peaks, the relevance of a particular peak versus the underlying background sound, and how sound relates to the type of care and the severity of illness of the patient. In addition, the use of only an A-weighted scale has ignored the possible contribution of low frequency sound to patient well-being.
For both A-weighted and C-weighted scales, the mean Leq did not vary significantly across the entire night or over the beginning, middle and end of night periods. LAF90, an alternative measure of background sound, also demonstrates consistently high sound levels. However, the raw A-weighted sound level plots during single patient overnight observation periods demonstrate significant local variation in sound levels that is not reflected in Leq or LAF90 measures. Both low and high average Leq's can been associated with varying relative peak burdens. We therefore conclude that the Leq value can obscure significant sound characteristics such as peak occurrence, and local variations in sound may have a significant influence on sleep and circadian rhythms.
The ICU sound literature has inconsistently defined peaks while consistently reporting maxima greater than 80 dBA [24, 25, 31, 32]. Recently, Nannapaneni et al. [28] defined the concept of sound “defects” (aka peaks) as sound events greater than 60 dBA. Their sound reduction intervention did not change Leq, but decreased the number of defects / peaks [28]. In order to better understand sound variation, we examined the occurrence of sound peaks in detail. We included both relative and absolute peak definitions in order to capture both variation of sound levels relative to the local temporal sound background as well as capture all significantly loud sounds. In our study, absolute peak definitions of 60 dBA and 70 dBC directly contribute to and correlate well with the overall Leq. However, relative peak frequency did not correlate with overall A-weighted Leq. These findings underscore our conclusion that Leq may have limited utility as an ICU sound measure and should be interpreted with caution.
We did not demonstrate an association between patient characteristics such as severity of illness, mechanical ventilation, vasopressor use, delirium, contact precaution status and sound amplitude or hourly sound peaks. While this challenges our hypothesis that high levels of ICU sound are directly related to the need to provide more intensive in-room care to critically ill patients, given the small size of the sample, we are unable to draw firm conclusions. Future research on the associations between noise, intensity of patient care and clinical outcomes is needed. Our findings regarding the high levels of low frequency sound associated with machinery suggest that certain aspects of elevated sound in the ICU may not be modifiable except with structural unit redesign. Other ICU sound investigators have suggested that WHO recommendations for sound levels can only be achieved in side rooms with equipment turned off [32].
Study Limitations
Limitations of this study include the small sample size with sound data collected in a single intensive care unit. We did not have adequate power to detect differences in sound levels by patient clinical characteristics. We also did not perform spectral analysis of the ICU sounds, which would have more precisely defined the range and amplitude of individual sound frequencies. Inclusion of spectral analysis will be important to include in future work on sound in the ICU. It is possible that 10 second sampling rate and the location of the meters at the foot rather than the head of the bed may have resulted in a subtle loss of information on sound peaks. Loud sounds occurring for shorter than 10 seconds or sounds at the absolute peak threshold may not have been detected. However, pilot studies conducted to optimize sound meter settings suggest that these differences are non-significant. Finally, we did not measure sleep in our study and cannot therefore make statements regarding associations between sound levels in this study and patient sleep.
Conclusions
This work demonstrates significant discordances between A- and C-weighted sound measures. These results suggest that high levels of continuous low frequency sound may be a significant and disruptive source of ICU sound. Moreover, Leq and LAF90 measures obscure important sound level variability and should be interpreted with caution. Sound level variability can be described with frequency of absolute and relative peaks, which may improve our understanding of ICU sound patterns and allow future research examining the impact of sound on health outcomes in critically ill patients. An important direction for future research is the clarification of the relative harm of high levels of continuous low frequency sound (C-weighted sounds) versus highly variable sounds with frequent peaks (A-weighted sounds) as they relate to disruption of patient sleep.
Supplementary Material
Highlights.
Sound levels in the intensive care unit (ICU) are severely elevated and are believed to contribute to sleep and circadian disruption.
In this work we compare average sound levels and peak occurrence of overnight ICU sound on A- versus C-weighted scales.
The overnight A-weighted Leq of 53.6 dBA was well above World Health Organization recommendations, and overnight C-weighted Leq was 63.1 dBC.
Discordance between A- and C-weighted values suggests that low frequency sound is a significant factor contributing to overall ICU sound.
Acknowledgments
This study was funded by P20NR014126. Dr. Knauert is supported by career development funds from the Yale Claude D. Pepper Older Americans Independence Center P30AG021342 and a CTSA grant number KL2 TR000140 from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). The contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. Dr. Pisani is supported through a grant from the Donoghue Foundation.
Abbreviations
- dB
Decibel
- dBA
Equivalent Sound Level, A-weighted Scale
- dBC
Equivalent Sound Level, C-weighted Scale
- ICU
Intensive Care Unit
- IR
Interquartile Range
- LAF90
Sound level exceeded for 90% of the measurement period
- Leq
Equivalent Sound Level
- MICU
Medical Intensive Care Unit
- NIST
National Institute of Standards and Technology
- SD
Standard Deviation
- WHO
World Health Organization
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa Knauert, Email: melissa.knauert@yale.edu.
Sangchoon Jeon, Email: sangchoon.jeon@yale.edu.
Terrence E. Murphy, Email: terrence.murphy@yale.edu.
H. Klar Yaggi, Email: henry.yaggi@yale.edu.
Margaret A. Pisani, Email: margaret.pisani@yale.edu.
Nancy S. Redeker, Email: nancy.redeker@yale.edu.
References
- 1.Knauert MP, Yaggi HK, Redeker NS, Murphy TE, Araujo KL, Pisani MA. Feasibility study of unattended polysomnography in medical intensive care unit patients. Heart Lung. 2014;43(5):445–452. doi: 10.1016/j.hrtlng.2014.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knauert MP, Haspel JA, Pisani MA. Sleep Loss and Circadian Rhythm Disruption in the Intensive Care Unit. Clin Chest Med. 2015;36(3):419–429. doi: 10.1016/j.ccm.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Knauert MP, Malik V, Kamdar BB. Sleep and sleep disordered breathing in hospitalized patients. Semin Respir Crit Care Med. 2014;35(5):582–592. doi: 10.1055/s-0034-1390080. [DOI] [PubMed] [Google Scholar]
- 4.Cooper AB, Thornley KS, Young GB, Slutsky AS, Stewart TE, Hanly PJ. Sleep in critically ill patients requiring mechanical ventilation. Chest. 2000;117(3):809–818. doi: 10.1378/chest.117.3.809. [DOI] [PubMed] [Google Scholar]
- 5.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163(2):451–457. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 6.Cabello B, Thille AW, Drouot X, Galia F, Mancebo J, d'Ortho MP, Brochard L. Sleep quality in mechanically ventilated patients: comparison of three ventilatory modes. Crit Care Med. 2008;36(6):1749–1755. doi: 10.1097/CCM.0b013e3181743f41. [DOI] [PubMed] [Google Scholar]
- 7.Elliott R, McKinley S, Cistulli P, Fien M. Characterisation of sleep in intensive care using 24-hour polysomnography: an observational study. Crit Care. 2013;17(2):R46. doi: 10.1186/cc12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Topf M, Davis JE. Critical care unit noise and rapid eye movement (REM) sleep. Heart Lung. 1993;22(3):252–258. [PubMed] [Google Scholar]
- 9.Kornfeld DS. Psychiatric view of the intensive care unit. Br Med J. 1969;1(5636):108–110. doi: 10.1136/bmj.1.5636.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McFadden EH, Giblin EC. Sleep deprivation in patients having open-heart surgery. Nurs Res. 1971;20(3):249–254. [PubMed] [Google Scholar]
- 11.Falk SA, Woods NF. Hospital noise--levels and potential health hazards. N Engl J Med. 1973;289(15):774–781. doi: 10.1056/NEJM197310112891504. [DOI] [PubMed] [Google Scholar]
- 12.Redeker NS. Sleep in acute care settings: an integrative review. J Nurs Scholarsh. 2000;32(1):31–38. doi: 10.1111/j.1547-5069.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 13.Topf M, Bookman M, Arand D. Effects of critical care unit noise on the subjective quality of sleep. J Adv Nurs. 1996;24(3):545–551. doi: 10.1046/j.1365-2648.1996.22315.x. [DOI] [PubMed] [Google Scholar]
- 14.Kamdar BB, King LM, Collop NA, Sakamuri S, Colantuoni E, Neufeld KJ, Bienvenu OJ, Rowden AM, Touradji P, Brower RG, et al. The Effect of a Quality Improvement Intervention on Perceived Sleep Quality and Cognition in a Medical ICU. Crit Care Med. doi: 10.1097/CCM.0b013e3182746442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones SF, Pisani MA. ICU delirium: an update. Curr Opin Crit Care. 18(2):146–151. doi: 10.1097/MCC.0b013e32835132b9. [DOI] [PubMed] [Google Scholar]
- 16.Protective Noise Levels: Condensed Version of EPA Levels Document. Edited by Office of Noise Abatement and Control EPA; Washington, D.C: 1978. [Google Scholar]
- 17.Stafford A, Haverland A, Bridges E. Noise in the ICU. Am J Nurs. 2014;114(5):57–63. doi: 10.1097/01.NAJ.0000446780.99522.90. [DOI] [PubMed] [Google Scholar]
- 18.Ziaran S. The assessment and evaluation of low-frequency noise near the region of infrasound. Noise & Health. 2014;16(68):10–17. doi: 10.4103/1463-1741.127848. [DOI] [PubMed] [Google Scholar]
- 19.Ziaran S. Potential health effects of standing waves generated by low frequency noise. Noise & Health. 2013;15(65):237–245. doi: 10.4103/1463-1741.113518. [DOI] [PubMed] [Google Scholar]
- 20.Hweidi IM. Jordanian patients' perception of stressors in critical care units: a questionnaire survey. Int J Nurs Stud. 2007;44(2):227–235. doi: 10.1016/j.ijnurstu.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Ugras GA, Oztekin SD. Patient perception of environmental and nursing factors contributing to sleep disturbances in a neurosurgical intensive care unit. Tohoku J Exp Med. 2007;212(3):299–308. doi: 10.1620/tjem.212.299. [DOI] [PubMed] [Google Scholar]
- 22.Ma PL, Wang Y, Xi XM, Lin HY, Xu Y, Du B, Zhao HL, Zhang XY, Zeng L. [Epidemiology of unpleasant experiences in conscious critically ill patients during intensive care unit stay] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20(9):553–557. [PubMed] [Google Scholar]
- 23.Little A, Ethier C, Ayas N, Thanachayanont T, Jiang D, Mehta S. A patient survey of sleep quality in the Intensive Care Unit. Minerva Anestesiol. 78(4):406–414. [PubMed] [Google Scholar]
- 24.Balogh D, Kittinger E, Benzer A, Hackl JM. Noise in the ICU. Intensive Care Med. 1993;19(6):343–346. doi: 10.1007/BF01694709. [DOI] [PubMed] [Google Scholar]
- 25.Akansel N, Kaymakci S. Effects of intensive care unit noise on patients: a study on coronary artery bypass graft surgery patients. J Clin Nurs. 2008;17(12):1581–1590. doi: 10.1111/j.1365-2702.2007.02144.x. [DOI] [PubMed] [Google Scholar]
- 26.Cordova AC, Logishetty K, Fauerbach J, Price LA, Gibson BR, Milner SM. Noise levels in a burn intensive care unit. Burns. doi: 10.1016/j.burns.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 27.Park M, Vos P, Vlaskamp BN, Kohlrausch A, Oldenbeuving AW. The influence of APACHE II score on the average noise level in an intensive care unit: an observational study. BMC anesthesiology. 2015;15:42. doi: 10.1186/s12871-015-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nannapaneni S, Lee SJ, Kashiouris M, Elmer JL, Thakur LK, Nelson SB, Bowron CT, Danielson RD, Surani S, Ramar K. Preliminary noise reduction efforts in a medical intensive care unit. Hospital practice. 2015;43(2):94–100. doi: 10.1080/21548331.2015.1015389. [DOI] [PubMed] [Google Scholar]
- 29.Czaplik M, Rossaint R, Kaliciak J, Follmann A, Kirfel S, Scharrer R, Guski M, Vorlander M, Marx G, Coburn M. Psychoacoustic analysis of noise and the application of earplugs in an ICU: A randomised controlled clinical trial. Eur J Anaesthesiol. 2015 doi: 10.1097/EJA.0000000000000313. [DOI] [PubMed] [Google Scholar]
- 30.B B, T L, DH S. Guidelines for Community Noise. Geneva: World Health Organization; 1999. http://whqlibdoc.who.int/hq/1999/a68672.pdf. [Google Scholar]
- 31.Lawson N, Thompson K, Saunders G, Saiz J, Richardson J, Brown D, Ince N, Caldwell M, Pope D. Sound intensity and noise evaluation in a critical care unit. Am J Crit Care. 2010;19(6):e88–98. doi: 10.4037/ajcc2010180. quiz e99. [DOI] [PubMed] [Google Scholar]
- 32.Darbyshire JL, Young JD. An investigation of sound levels on intensive care units with reference to the WHO guidelines. Crit Care. 2013;17(5):R187. doi: 10.1186/cc12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabor JY, Cooper AB, Crombach SA, Lee B, Kadikar N, Bettger HE, Hanly PJ. Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects. Am J Respir Crit Care Med. 2003;167(5):708–715. doi: 10.1164/rccm.2201090. [DOI] [PubMed] [Google Scholar]
- 34.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 35.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 36.Onakpoya IJ, O'Sullivan J, Thompson MJ, Heneghan CJ. The effect of wind turbine noise on sleep and quality of life: A systematic review and meta-analysis of observational studies. Environ Int. 2015;82:1–9. doi: 10.1016/j.envint.2015.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


