SUMMARY
Zika virus (ZIKV) related neuropathology is an important global health concern. Several studies have shown that ZIKV can infect neural stem cells in the developing brain, but infection in the adult brain has not been examined. Two areas in the adult mouse brain contain neural stem cells: the subventricular zone of the anterior forebrain and the subgranular zone of the hippocampus. Here using six week old mice triply deficient in interferon regulatory factor (IRF) as a model, we show that blood-borne ZIKV administration can lead to pronounced evidence of ZIKV infection in these adult neural stem cells, leading to cell death and reduced proliferation. Our data therefore suggest that adult as well as fetal neural stem cells are vulnerable to ZIKV neuropathology. Thus, although ZIKV is considered a transient infection in adult humans without marked long-term effects, there may in fact be consequences of exposure in the adult brain.
Keywords: Adult neurogenesis, Zika virus, Interferon, Neural progenitor cells
Recent world attention has been drawn to a global Zika virus (ZIKV) outbreak and its link with devastating cases of microcephaly and Guillain-Barre syndrome. ZIKV infection is spreading rapidly within the Americas after originating from an outbreak in Brazil (Campos et al., 2015). Mounting evidence suggests that ZIKV infection in pregnant women can cause congenital abnormalities as well as fetal demise (Calvet et al., 2016; Cugola et al., 2016; Miner et al., 2016; Wu et al., 2016). Initial case descriptions of microcephaly and spontaneous abortions have been supported by evidence of viral RNA and antigen in the brains of congenitally infected fetuses and newborns (Martines et al., 2016; Mlakar et al., 2016).
The radial glial-derived cortical neural stem cells (NSCs) in the fetal brain appear to be especially impacted by ZIKV infection, either through greater susceptibility to the viral infection or virus-induced cytotoxicity. This same population is affected by inherited forms of microcephaly, suggesting that loss of these cells are responsible for the microcephaly after ZIKV infection. Indeed recent work demonstrated that ZIKV can infect human cortical NSCs and attenuate their growth and survival, either when applied directly to monolayer culture (Tang et al., 2016), or to cerebral organoids or neurospheres (Dang et al., 2016; Garcez et al., 2016; Nowakowski et al., 2016; Qian et al., 2016). Vertical transmission from ZIKV infected murine dams to fetuses yielded virus in brain and histopathological evidence of cytotoxicity, supporting direct infection of NSCs. Effects of ZIKV on the placenta, and secondary effects on brain may have also contributed (Miner et al., 2016).
Many vector-borne flaviviruses have to overcome host type I IFN responses to replicate and cause disease in vertebrates. Wild-type mice are resistant to parenteral infection with DENV, and unlike in human cells where the virus is able to block type I and type II IFN receptor signaling, murine cells do not show the same block (Aguirre et al., 2012; Ashour et al., 2010; Yu et al., 2012). Therefore, similar to DENV mouse models, current ZIKV models utilize mice lacking the type I IFN signaling including the use of the IFN regulatory factor (IRF) transcription factors IRF-3, -5, and -7 strain (aka IRF-TKO) (Zellweger and Shresta, 2014). These mouse models appear to reproduce key features of human ZIKV infection, including viremia and neuronal tissue tropism, and are proving to be valuable for answering fundamental questions about ZIKV pathogenesis.
In the adult brain, neurogenesis contracts after birth to just the anterior subventricular zone (SVZ) of the forebrain and the subgranular zone (SGZ) of the hippocampal dentate gyrus. These restricted niches contain progenitor cells that divide to produce neurons or glia, depending upon intrinsic and environmental cues. Neurogenic niches are characterized by a comparatively high vascular density and proximity to cerebrospinal fluid (CSF) (Stolp and Molnar, 2015), allowing for not just communication through signaling molecules but also proximity to circulating viruses.
To identify direct target cells of ZIKV in adult central nervous system, we infected 5-6 week old Irf3−/− Irf5−/− Irf7−/− TKO mice with an Asian lineage ZIKV strain (FSS13025, 2010 Cambodian isolate) via retro-orbital injection (see Supplemental Experimental Procedures for section of strain rationale). Retro-orbital injection was selected as a method to introduce virus into the peripheral circulation, rather than direct introduction into the brain, to model the blood-borne route of Arboviruses transmission. Similar to a previous report, TKO mice were vulnerable to ZIKV infection (Lazear et al., 2016) and began to exhibit ruffled fur and lethargy as evidence for viral illness by 3-4 days post-infection (DPI) and developed evidence of hindlimb weakness by 6 DPI.
To examine the potential for virus infection in the brain, we screened serial coronal sections of whole brain from infected and mock-treated mice with the monoclonal 4G2 antibody that reacts with the Flavivirus-specific family envelope protein (see Methods). We observed dramatic immunoreactivity in proximity to the SVZ of the anterior forebrain, as well as the SGZ of the hippocampus (Figure 1A-C), the two regions in mouse that maintain stem cell populations throughout adulthood, in infected but not mock-infected mice. In contrast there was less immunoreactivity in other regions of brain under these conditions (Figure S1A-C), suggesting a particular tropism of the virus for proliferative regions of the brain. Quantification across major brain regions showed statistically significant selective vulnerability to these proliferative zones (Figure S1D).
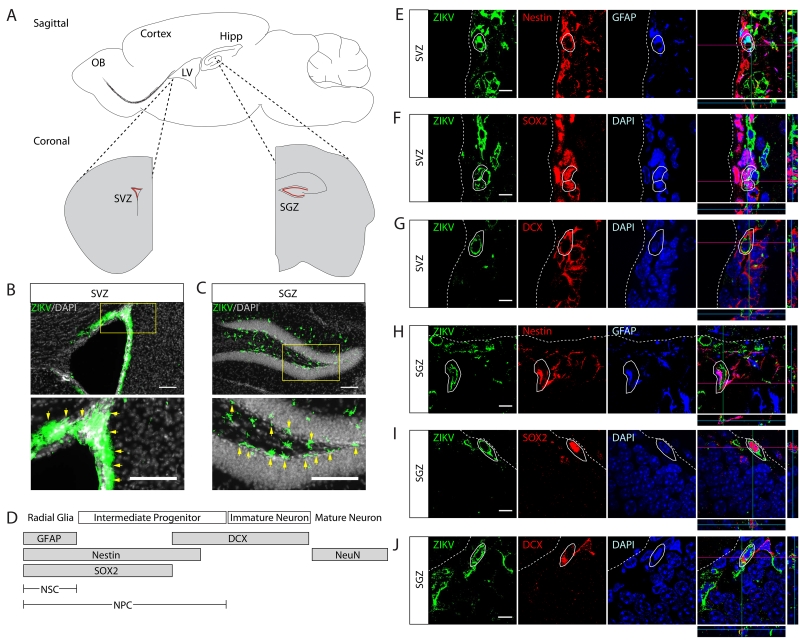
Figure 1. Systemic ZIKV can infect neural progenitor cells in the adult brain.
(A) Schematic of stem cell niches in adult mouse brain. Neural progenitor cells (NPCs) located in the subventricular zone (SVZ) in the anterior forebrain (red) adjacent to the cerebral ventricles contribute new neurons to the olfactory bulb. NPCs in the subgranular zone (SGZ) in the dorsal forebrain (red) contribute new neurons to the hippocampal dentate gyrus. LV: Lateral ventricle. OB: Olfactory bulb. Hipp: Hippocampus.
(B-C) Adult TKO mice were infected with ZIKV then sacrificed, brains serially sectioned, and immunostained for ZIKV envelope protein (green). Evidence of ZIKV was found in the SVZ and SGZ, with less expression elsewhere in adult brain. High power inset below with arrows (yellow) highlighting immunoreactive cells. LV: Lateral ventricle. Scale bar, 100μm.
(D) Marker expression during neural stem cell (NSC) differentiation from radial glia to mature neuron.
(E-J) Confocal images and orthogonal projection of SVZ and SGZ regions co-stained for GFAP, Nestin, SOX2, and DCX with ZIKV envelop protein, evidencing ZIKV in NPCs and immature neuron populations. White circle outlines: infected cells. LV: Lateral ventricle. SVZ: subventricular zone. DG: dentate gyrus. Scale bar, 10μm.
In adult SVZ and SGZ, radial glia-like NSCs give rise to intermediate progenitor cells (IPCs), which then migrate to final destinations, where they express developmental-dependent markers and integrate into neuronal circuitry (Figure 1D, see Supplement). For the remainder of this paper, neural progenitor cell (NPC) is used to refer to both NSC and IPC. To identify which cells were positive for 4G2, we co-stained with different cell type markers. We detected the presence of ZIKV in GFAP and Nestin expressing NSCs, SOX2 expressing IPCs, as well as DCX expressing immature neurons (Figure 1E-J). 4G2 reactivity in sagittal sections confirmed ZIKV infected DCX+ cells along the rostral migratory stream (Figure S1E). Consistent with previous reports where ZIKV was introduced directly into newborn and juvenile brain (Bell et al., 1971), we detected 4G2 reactivity in NeuN expressing neurons and S100β expressing astrocytes (Figure S2A-D), but much less than SOX2+ or DCX+ cells (Figure S2F,G). We rarely observed 4G2 reactivity in NG2 expressing oligodendrocytes (Figure S2E). We conclude that ZIKV has tropism for proliferative NPCs and immature neurons over terminal-differentiated cell population in the adult brain.
ZIKV infection can lead to caspase-3 activation in both NPCs differentiated from human ES/IPS cells and in embryonic mouse brain (Cugola et al., 2016; Miner et al., 2016). In order to determine whether systemic ZIKV infection can induce cell death in adult NPC populations we stained for cleaved (i.e. activated) caspase-3 (CASP3) in NPC niches. Mock infected TKO mice showed scant evidence of CASP3+ cells. In contrast abundant CASP3+ cells were detected in ZIKV infected brains within these neurogenic niches (Figure 2A-D), and were also usually positive for Nestin. Similarly, the ZIKV staining colocalized with CASP3 in NPCs in the SVZ and SGZ (Figure S2I, J), suggesting that ZIKV infection can induce apoptotic cell death in adult NPCs in these regions.
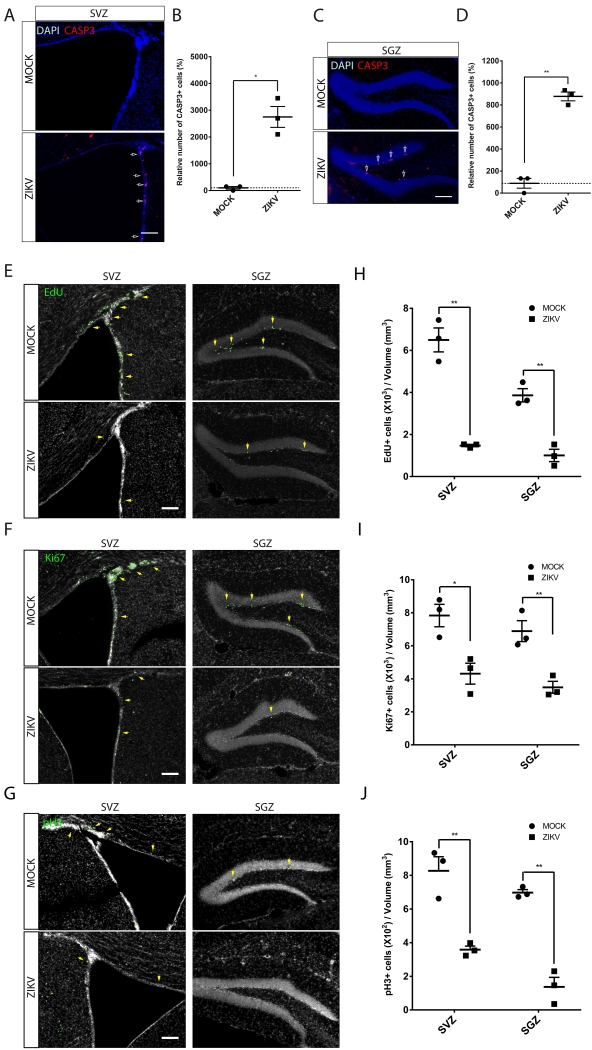
Figure 2. ZIKV-infected adult NPCs undergo cell death and show reduced proliferation.
(A, C) Representative images of SVZ (A) or SGZ (C) region from ZIKV infected showing more CASP3+ cells (arrows) compared regions with mock infected. Scale bar, 100μm.
(B, D) Quantification of the number of CASP3+ cells relative to MOCK infected brains. All data represent means ± SEM, n=3 animals for each group. Student’s t test, *P < 0.05, ** P < 0.01.
(E) Adult TKO mice were infected with ZIKV or mock, injected with EdU after 6 days, then sacrificed after 2 hr. ZIKV infected animals show reduced incorporation of EdU in both SVZ and SGZ, indicating reduced entry into S-phase. Scale bar, 100μm.
(F) Reduced Ki67 staining in ZIKV-infected mice in both SVZ and SGZ, indicating reduced cell proliferation. Scale bar, 100μm.
(G) Reduced phospho-histone H3 (pH3) staining in ZIKV-infected mice in both SVZ and SGZ, indicating reduced mitotic cells. Scale bar, 100μm.
(H-J)Stereological quantification of the number of EdU+, Ki67+ and pH3+ cells in each mouse were performed in every 6 section of the entire brains from 3 pairs of ZIKV and MOCK infected animals. All data represent means ± SEM, n=3 animals for each group. Student’s t test, * P < 0.05, ** P < 0.01.
We assessed the impact of systemic ZIKV infection on cell proliferation in niches in adult brain using the thymidine analog EdU and a series of cell cycle markers. We performed EdU pulse-labeling in TKO mice 6-days after ZIKV or MOCK infection. Quantitative analysis at 2 hours after EdU injection showed that, in both neurogenic regions, although the brain size and volume of the SGZ and SVZ was not notably different (Figure S2H), ZIKV infected mice had approximately a 4-5 fold reduction in the number of EdU+ cells per section, compared with MOCK (Figure 2E, H).
Consistent with the EdU-labeling results, there were many fewer cells positive for the proliferation marker Ki67 in the SVZ and SGZ, which was reduced by approximately 2-3-fold (Figure 2F, I). Similarly, there were many fewer cells positive for the mitotic marker phospho-Histone H3 in the SVZ and SGZ, reduced by approximately 2-4 fold (Figure 2G, J). Results were statistically significant and consistent across the three infected and three mock infected animals. These results indicate that ZIKV infection leads to decreased NPC proliferation in the adult SGZ and SVZ.
Here we demonstrate that ZIKV exposure in adult mice shows infection of brain with a predilection for neurogenic niches, associated with cellular apoptosis and reduction of cellular proliferation. Based upon the presence of the ZIKV antigen following exposure, the virus was able to infect SVZ and SGZ niche cells to a much greater degree than non-neurogenic regions. This infection correlated with evidence of apoptosis and reduced numbers of cells evidencing DNA synthesis or proliferation. However, the relative contribution of these features, as well as the long-term effects, on the NPC niches remains unknown. Our results suggest that ZIKV infection can enter the adult brain and lead to neuropathology in mammals.
The degree to which IFN deficient mice model the extent and severity of flavivirus infection in humans is unknown. We recognize that healthy humans may be able to mount an effective antiviral response and prevent entry into the CNS, but it remains a possibility that some immunocompromised humans and even some apparently healthy humans may be susceptible in ways modeled by the TKO mice. It will be important to determine the extent of involvement of stem cell niches with less immunocompromised strains of mice. Brain inflammation in general, including IFN-α induction, can lead to reduction in adult neurogenesis (Kaneko et al., 2006; Kohman and Rhodes, 2013), and therefore the interaction between ZIKV infection and IFN signaling pathway and its impact on adult neurogenesis merit further investigation.
There are several caveats to our study, representing a single viral strain, single mouse strain and single time point analysis. First ZIKV infection can downregulate expression of neural progenitor markers like Nestin and SOX2 (Tang et al., 2016) and thus our analysis may underestimate the proportion of infected cells co-expressing these markers. Second, infected NPCs could have divided or differentiated between infection and harvest, and therefore future time course studies could be important. Third, we noticed that some ZIKV-infected cells in non-neurogenic regions of the brain, such as the hilus of the hippocampus (Figure S1B2), were not positive for NPC markers. Further examination will help identify whether these cells represent microglia or infiltrating neutrophils (Aliota et al., 2016) or some other cell type.
Central nervous system (CNS) involvement associated with other flavivirus infections in adults is receiving increasing attention. Although dengue infection in humans usually produces a self-limited illness, CNS involvement is now considered criteria for severe dengue in the World Health Organization (WHO) classification (http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf), including but not limited to cerebrospinal fluid viremia, encephalitis or meningoencephalitis, and was detected in nearly half of fatal dengue cases (Araujo et al., 2012). Approximately 1:150 cases of West Nile infection shows neuroinvasive disease or encephalitis, with potential manifestations of flaccid paralysis, extrapyramidal findings, coarse tremor, myoclonus, and substantial cognitive difficulties. ZIKV can produce neurological findings to include demyelinating polyneuropathy and brachial plexopathy and recently acute meningoencephalitis (Carteaux et al., 2016). It will be important to evaluate the areas damaged following ZIKV CNS infection in humans, especially in neurogenic regions, and consider potential consequences on neurocognition.
Neurotropic viruses can gain entry into the CNS and cause disease through different means (Luethy et al., 2016), but how ZIKV gains entry into the brain remains unknown. Current models include entry of the virus directly across the blood brain barrier (BBB), across synapses from peripheral nerve, or through entry of infected microglia. Once across the BBB, the means of entry into NPCs may be through specific transmembrane receptors, such as the candidate AXL receptor (Miner and Diamond, 2016; Nowakowski et al., 2016). The means by which infection leads to NPC death is also under active study. We hypothesize that the particular cell death in NPCs may be p53 mediated, in keeping with current models of human microcephaly (Pilaz et al., 2016; Tang et al., 2016).
Infection of NPCs in stem cell niches may relate to the emergent cases of ZIKA-linked GBS, an acute postinfectious immune-mediated polyradiculoneuropathy. The dramatic increased GBS-incidence in ZIKV endemic regions (Watrin et al., 2016) and evidence of acute ZIKV infection in GBS patients from these regions (Beckham et al., 2016) suggests a causal relationship. A broad range of antibodies directed to neuronal glycolipids, possibly emerging from cross-reaction to viral proteins, or release of neural antigens from damaged cells, are observed in some GBS cases. Although identified in many non-ZIKV-related GBS cases, less than 50% of sera at admission from ZIKV-related GBS showed significant auto-antibodies to glycolipids, and moreover complementary analysis did not show any competition between the glycolipid GA1 and ZIKV proteins, suggesting absence of antigenic mimicry (Beckham et al., 2016). Our data suggests ZIKV infected NPCs could release neural antigens as a possible source of auto-antibodies, although we cannot rule out that other neural cells are the target relevant to GBS.
A recent study using radioactive carbon dating reported a striking annual turnover rate of 1.75% in the human dentate gyrus, where approximately 700 new neurons are added every day from the SGZ to the pool of excitatory granule neuron population to support plasticity (Spalding et al., 2013). Furthermore, hippocampal neurogenesis deficits have been linked to cognitive deficits characteristic of depression (Patricio et al., 2013) and Alzheimer’s disease (Demars et al., 2010; Rodriguez et al., 2011). SGZ neurogenesis is critical in rodents for hippocampal-dependent contextual conditioning learning (Saxe et al., 2006; Winocur et al., 2006), longer-term spatial memory (Deng et al., 2009) and pattern separation (Clelland et al., 2009; Sahay et al., 2011). Whether there are long-term effects of ZIKV on adult neurogenesis or cognition in rodents or humans will be an important question for the future.
Supplementary Material
HIGHLIGHTS.
Zika virus (ZIKV) can infect neural progenitor in the adult mouse brain.
ZIKV-infected adult NPCs show evidence of cell death
Cell proliferation is also impacted in ZIKV-infected adult NPC populations.
ACKNOWLEDGMENTS
Supported by the NIH R01NS041537, R01NS048453, R01NS052455, P01HD070494, P30NS047101, the Simons Foundation Autism Research Initiative (SFARI), the Howard Hughes Medical Institute, California Institute of Regenerative Medicine (J.G.G.) and NIH R01 AI116813 (S.S.) and Druckenmiller Fellowship from New York Stem Cell Foundation (H.L). We thank Alysson Muotri and Charles Rice for discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTION
H.L. and L.SC. performed histological assessment and statistics, J.A.RN, N.S. and W.T. performed mouse breeding and infections, G.C. and WT performed animal surgery and perfusions. H.L., L.SC., S.S., and J.G.G designed the experiments and wrote the paper. A.V.T assisted with experimental design and edited the manuscript. S.S. and J.G.G supervised the project.
DISCLOSURE
Authors report no disclosures.
REFERENCES
- Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, et al. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8:e1002934. doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Negl Trop Dis. 2016;10:e0004682. doi: 10.1371/journal.pntd.0004682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo FM, Araujo MS, Nogueira RM, Brilhante RS, Oliveira DN, Rocha MF, Cordeiro RA, Araujo RM, Sidrim JJ. Central nervous system involvement in dengue: a study in fatal cases from a dengue endemic area. Neurology. 2012;78:736–742. doi: 10.1212/WNL.0b013e31824b94e9. [DOI] [PubMed] [Google Scholar]
- Ashour J, Morrison J, Laurent-Rolle M, Belicha-Villanueva A, Plumlee CR, Bernal-Rubio D, Williams KL, Harris E, Fernandez-Sesma A, Schindler C, et al. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe. 2010;8:410–421. doi: 10.1016/j.chom.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckham JD, Pastula DM, Massey A, Tyler KL. Zika Virus as an Emerging Global Pathogen: Neurological Complications of Zika Virus. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch. 1971;35:183–193. doi: 10.1007/BF01249709. [DOI] [PubMed] [Google Scholar]
- Calvet G, Aguiar RS, Melo AS, Sampaio SA, de Filippis I, Fabri A, Araujo ES, de Sequeira PC, de Mendonca MC, de Oliveira L, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- Campos GS, Bandeira AC, Sardi SI. Zika Virus Outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, et al. Zika Virus Associated with Meningoencephalitis. N Engl J Med. 2016;374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr., Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, Rana TM. Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids through Activation of the Innate Immune Receptor TLR3. Cell Stem Cell. 2016 doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu YS, Gadadhar A, Lazarov O. Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res. 2010;88:2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK. Zika virus impairs growth in human neurospheres and brain organoids. Science. 2016;352:816–818. doi: 10.1126/science.aaf6116. [DOI] [PubMed] [Google Scholar]
- Kaneko N, Kudo K, Mabuchi T, Takemoto K, Fujimaki K, Wati H, Iguchi H, Tezuka H, Kanba S. Suppression of cell proliferation by interferon-alpha through interleukin-1 production in adult rat dentate gyrus. Neuropsychopharmacology. 2006;31:2619–2626. doi: 10.1038/sj.npp.1301137. [DOI] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS. Neurogenesis, inflammation and behavior. Brain Behav Immun. 2013;27:22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luethy LN, Erickson AK, Jesudhasan PR, Ikizler M, Dermody TS, Pfeiffer JK. Comparison of three neurotropic viruses reveals differences in viral dissemination to the central nervous system. Virology. 2016;487:1–10. doi: 10.1016/j.virol.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, Goldsmith C, Hale G, Ritter J, Rollin D, et al. Notes from the Field: Evidence of Zika Virus Infection in Brain and Placental Tissues from Two Congenitally Infected Newborns and Two Fetal Losses - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–160. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika Virus Infection during Pregnancy in Mice Causes Placental Damage and Fetal Demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JJ, Diamond MS. Understanding How Zika Virus Enters and Infects Neural Target Cells. Cell Stem Cell. 2016;18:559–560. doi: 10.1016/j.stem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Nowakowski TJ, Pollen AA, Di Lullo E, Sandoval-Espinosa C, Bershteyn M, Kriegstein AR. Expression Analysis Highlights AXL as a Candidate Zika Virus Entry Receptor in Neural Stem Cells. Cell Stem Cell. 2016;18:591–596. doi: 10.1016/j.stem.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patricio P, Mateus-Pinheiro A, Sousa N, Pinto L. Re-cycling paradigms: cell cycle regulation in adult hippocampal neurogenesis and implications for depression. Mol Neurobiol. 2013;48:84–96. doi: 10.1007/s12035-013-8422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilaz LJ, McMahon JJ, Miller EE, Lennox AL, Suzuki A, Salmon E, Silver DL. Prolonged Mitosis of Neural Progenitors Alters Cell Fate in the Developing Brain. Neuron. 2016;89:83–99. doi: 10.1016/j.neuron.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Noristani HN, Olabarria M, Fletcher J, Somerville TD, Yeh CY, Verkhratsky A. Voluntary running and environmental enrichment restores impaired hippocampal neurogenesis in a triple transgenic mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2011;8:707–717. doi: 10.2174/156720511797633214. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding KL, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner HB, Bostrom E, Westerlund I, Vial C, Buchholz BA, et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013;153:1219–1227. doi: 10.1016/j.cell.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Molnar Z. Neurogenic niches in the brain: help and hindrance of the barrier systems. Front Neurosci. 2015;9:20. doi: 10.3389/fnins.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, et al. Zika Virus Infects Human Cortical Neural Progenitors and Attenuates Their Growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watrin L, Ghawche F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barre Syndrome (42 Cases) Occurring During a Zika Virus Outbreak in French Polynesia. Medicine (Baltimore) 2016;95:e3257. doi: 10.1097/MD.0000000000003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wu KY, Zuo GL, Li XF, Ye Q, Deng YQ, Huang XY, Cao WC, Qin CF, Luo ZG. Vertical transmission of Zika virus targeting the radial glial cells affects cortex development of offspring mice. Cell research. 2016;26:645–654. doi: 10.1038/cr.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CY, Chang TH, Liang JJ, Chiang RL, Lee YL, Liao CL, Lin YL. Dengue virus targets the adaptor protein MITA to subvert host innate immunity. PLoS Pathog. 2012;8:e1002780. doi: 10.1371/journal.ppat.1002780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellweger RM, Shresta S. Mouse models to study dengue virus immunology and pathogenesis. Front Immunol. 2014;5:151. doi: 10.3389/fimmu.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.