Abstract
Objective
Compare five comorbidity indices to predict community discharge and functional status following post-acute rehabilitation.
Design
Retrospective study of Medicare beneficiaries with stroke, lower extremity fracture and joint replacement discharged from inpatient rehabilitation in 2011 (N=105,275). Community discharge and self-care, mobility and cognitive function were compared using the Charlson, Elixhauser, Tier, Functional Comorbidity and Hierarchical Condition Category comorbidity indices.
Results
Patients were 64.4% female and 84.6% non-Hispanic white. Mean age was 79.3 years (SD=7.6). Base regression models including sociodemographic and clinical variables explained 56.6%, 42.2% and 23.0% of the variance (R2) for discharge self-care; 47.4%, 30.9% and 18.6% for mobility; and, 62.0%, 55.3% and 37.3% for cognition across the three impairment groups. R2 values for self-care, mobility and cognition increased by 0.2% to 3.3% when the comorbidity indices were added to the models. The base model C-statistics for community discharge were 0.58 (stroke), 0.61 (fracture) and 0.62 (joint replacement). The C-statistics increased over 25% with the addition of discharge functional status to the base model. Adding the comorbidity indices individually to the base model resulted in C-statistic increases of 1% to 2%.
Conclusion
Comorbidity indices were poor predictors of community discharge and functional status in Medicare beneficiaries receiving inpatient rehabilitation.
Keywords: Comorbidity, Medicare, Inpatient Rehabilitation, Functional Status, Community Discharge
INTRODUCTION
Discharge to community and gain in functional status are important patient-centered outcomes for persons receiving inpatient rehabilitation. Under the Affordable Care Act, the Centers for Medicare and Medicaid Services (CMS) have identified functional status as a future quality measure for inpatient rehabilitation facilities (IRFs).1 The Improving Medicare Post-Acute Care Transformation (IMPACT) Act recently mandated uniform reporting of patient functional assessments across post-acute care settings (IRFs, skilled nursing homes, and home health agencies) to improve coordination of care and outcomes for Medicare beneficiaries.2
The majority of patients receiving post-acute inpatient rehabilitation are 65 years or older with multiple comorbidities.3 Comorbidities increase the risk of developing medical complications and negatively impact discharge functional status, length of stay, discharge destination, 30-day hospital readmission, and mortality.4–6 Several standardized comorbidity indices have been developed to predict mortality and other health outcomes.7–10 Tier comorbidities were developed and validated by CMS for IRF prospective payment.1, 3 The Charlson and Elixhauser comorbidity indices were developed and validated to estimate mortality risk in hospitalized patients.7 Their associations with post-acute rehabilitation outcomes among older adults are largely unknown.
The Functional Comorbidity Index (FCI) was developed and validated to predict physical function using clinical records.10 The FCI has not been tested in IRF settings to examine its relationship with post-acute outcomes using administrative (claims) data. CMS recently developed the Hierarchical Condition Category (HCC) for risk adjustment in capitated payments for Medicare Advantage plans.11 The HCC has also not been tested for its ability to predict post-acute rehabilitation outcomes.
The purpose of this study is to assess the contribution of the five comorbidity indices listed above in predicting: 1) discharge to the community, and 2) self-care, mobility and cognitive functional status at discharge in persons with stroke, lower extremity fracture, and lower extremity joint replacements. We selected persons in these three rehabilitation impairment categories because they represent 45 to 50% of Medicare beneficiaries receiving inpatient rehabilitation.3 We hypothesized that the Functional Comorbidity Index would be the strongest predictor of rehabilitation outcomes because the FCI was developed to assess physical function in persons with chronic disease and disability using clinical records.10
METHODS
Data Source and Description
Secondary analyses of Medicare data were conducted using the Beneficiary Summary File, the Medicare Provider Analysis and Review File (MedPAR) and the Inpatient Rehabilitation Facility-Patient Assessment Instrument (IRF-PAI) file for the calendar year 2011.12 The beneficiary summary file contains Medicare enrollment indicators and patient sociodemographic characteristics.
The MedPAR file contains claims for all inpatient stays, including acute hospitals and IRFs, as well as information about diagnostic conditions and surgical procedures. The IRF-PAI file includes data on the patient’s functional status at the time of admission and discharge. Data from the IRF-PAI file were also used to derive the patient’s Tier category. A Data Use Agreement was established with the CMS. The study was reviewed by the University’s Institutional Review Board.
Sample
The eligible sample included 181,443 Medicare fee-for-service beneficiaries 66 years or older and discharged from an inpatient rehabilitation facility in 2011. The sample included the following rehabilitation impairment categories: stroke (n=82,615), lower extremity fracture (n=57,092) and lower extremity joint replacement (n=41,736). The rehabilitation impairment categories were developed by the CMS based on the primary etiologic diagnosis for which the patient was admitted to inpatient rehabilitation.3 We excluded patients who were younger than 66 years of age (n=19,115), living in non-community settings before IRF admissions (n=1,801) or died during their IRF stay (n=222). The final study sample included 105,275 fee-for-service beneficiaries. The study cohort selection process is presented in Figure 1.
Figure 1.
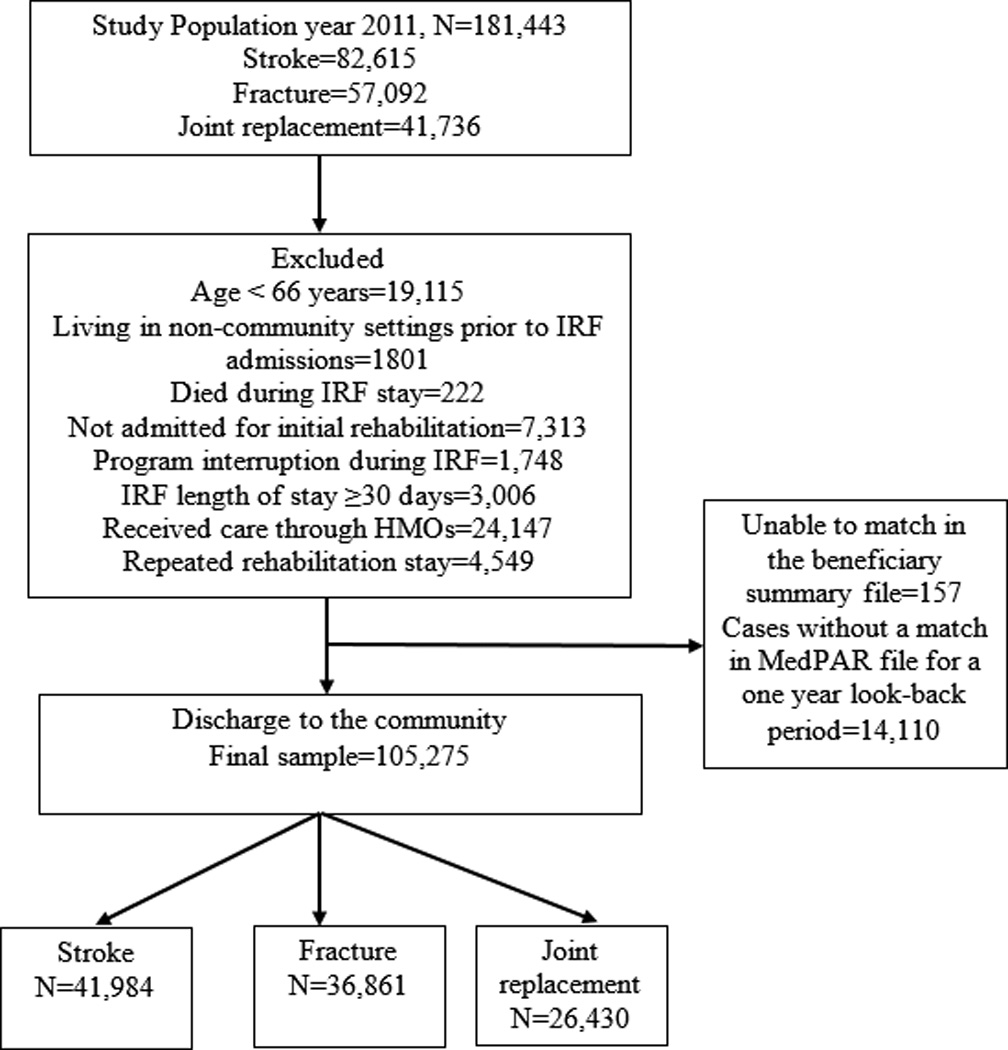
Flow chart of the study sample discharged from the Inpatient Rehabilitation Facilities.
Variables
The primary outcomes were discharge functional status (self-care, mobility and cognition) and discharge setting. Discharge functional status was measured using items from the Functional Independence Measure (FIM instrument) included in the IRF-PAI assessment.12 The IRF-PAI is administered within three days of admission and three days before discharge. The IRF-PAI includes 18 items that provide a total functional status rating. The items can also be used to create three subscales: self-care, mobility and cognition. The self-care subscale includes eight items: eating, grooming, bathing, upper body dressing, lower body dressing, toileting, bladder and bowel management. The mobility subscale includes five items: bed to chair transfers, toilet transfers, shower transfers, walking and climbing stairs. The cognition subscale includes five items: comprehension, expression, social interaction, problem-solving and memory. Each item is rated on a 7-point scale, ranging from complete dependence (level 1) to complete independence (level 7). Codes of “0,” indicating an activity did not occur at the time of admission. These were re-coded as a “1” for this study to allow comparisons with previous research.1, 12 The self-care rating ranged from 8 to 56; mobility and cognition ratings ranged from 5 to 35.
We categorized community discharge setting using destinations noted in the IRF-PAI.12, 13 The community discharge category included patients discharged to private home/apartment, board/care, assisted living, group home and transitional living settings. Discharge to an institutional setting included skilled nursing facility, intermediate care, acute hospital, sub-acute setting, long-term care hospital and rehabilitation facility.
Demographic information
Patient demographic variables included age, gender, race/ethnicity, Medicare qualifying disability, dual eligibility and length of stay. Age was used as a continuous variable in the descriptive analysis. In the regression models, age was entered as a categorical variable with three levels: 66–75, 76–85 and >85 years. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic and other races. Medicare qualifying disability was dichotomized (yes/no). Medicare qualifying disability referred to beneficiaries who qualified for Medicare Disability benefits. Medicaid dual eligibility was dichotomized (yes/no). Dual eligible beneficiaries included patients who received benefits from both Medicaid and Medicare.
Comorbidity Indices
The comorbid conditions for each index were derived using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes listed in the Medicare claims data. Dichotomous indicators (yes/no) were used for each condition included in the Charlson, Elixhauser, FCI and HCC indices. The Tier comorbidity index (see below) was used as a single 4-level variable. We did not use a summary score indicating the number of comorbid conditions because we believed that doing so would dilute the distinct effect of the specific comorbidities included in the indices. Brief descriptions of the five comorbidity indices are presented below.
Tier Comorbidity
As noted above, the Tier comorbidity system was developed by CMS and classifies medical conditions into one of four categories (no tiered comorbidity, Tier 1, Tier 2 and Tier 3) based on costs during an inpatient rehabilitation stay.1 Tier 1 represents the highest cost category and includes 8 diagnostic codes. Tier 3 includes 932 diagnostic codes and is the lowest cost tier category. The IRF-PAI file includes information on the Tier categories for each patient.1 The Tier was used as a single 4-level variable in the regression models.
Charlson Comorbidity Index
The Charlson Comorbidity Index consists of 18 medical conditions developed to predict one-year all-cause mortality in patients with breast cancer using hospital medical records.7 Deyo adapted the original Charlson index for use with administrative data using the ICD-9-CM codes, creating 17 comorbid categories for predicting health services utilization.14 The Deyo version of the Charlson Comorbidity Index was used in this study. We used ICD-9-CM codes for each of the 17 conditions as documented in the literature.14 Dichotomous indicators for each of the Charlson’s 17 comorbidity conditions for each patient were included in the regression models.
Elixhauser Comorbidity Index
The Elixhauser Comorbidity Index consists of 30 medical conditions developed to predict in-hospital mortality, length of stay and hospital charge.9 The Elixhauser method was based on ICD-9-CM codes from administrative data and has been widely used in health services research.15 We used the ICD-9-CM codes for the 30 conditions as described in the original study.15 Dichotomous indicators for each of the Elixhauser’s 30 comorbidity conditions were included in the regression models.
Functional Comorbidity Index
The FCI consists of 18 medical conditions and was developed to predict physical function.10 In comparison to the Charlson Comorbidity Index, the FCI captures more chronic conditions (e.g., arthritis, hearing impairment and degenerative disk disease) that are associated with physical functioning.10 The Charlson and FCI demonstrated similar associations with function in an acute hospital setting in a small prospective cohort from Canada.16 Dichotomous indicators for each of the 18 comorbidity conditions in the FCI were included in the regression models.
Hierarchical Condition Category
The HCC was developed by the CMS to estimate annual expenditures for beneficiaries enrolled in the Medicare Advantage program using demographic characteristics and medical conditions documented by inpatient claims from the previous year.11 The CMS identified 70 HCCs from 805 diagnostic groups and more than 14,000 ICD-9-CM diagnostic codes. We used the ICD-9-CM codes for HCC conditions as reported in the CMS manual.17 Dichotomous indicators for each of the 70 HCC comorbidity conditions were included in the regression models.
Statistical Analysis
Descriptive statistics for patient demographic and functional characteristics were tabulated for each of the three impairment categories. Separate linear regression models were computed to assess the impact of each comorbidity index on discharge functional status during inpatient rehabilitation. We developed six models for patients in each of the three impairment categories for each dependent variable. Dependent variables for the models included: discharge self-care, discharge mobility and discharge cognition ratings.
The base model included age, gender, race/ethnicity, Medicare qualifying disability, dual eligibility, length of stay and admission functional status. Five subsequent models were computed for each dependent variable with one of the comorbidity indices included in each model. Variance explained (R2) values were compared across the models for all functional outcomes.
Logistic regression models were computed to examine the associations between each comorbidity index and discharge setting. We developed seven models for patients in each of the three impairment categories for discharge setting. Seven receiver operating characteristics (ROC) curves were constructed to differentiate patients discharged to the community compared to institutional settings.
Two models were computed without comorbidity indices. The first model included age, gender, race/ethnicity, Medicare qualifying disability, dual eligibility and length of stay. The second model added total functional status at discharge to show the discriminatory ability explained by functional status related to discharge setting. In each of the five remaining models, one comorbidity index was added. The C-statistic was used to quantify the ability of the models to discriminate discharge to community across the three impairment categories.18 All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC).
RESULTS
The sample included 105,275 patients discharged from inpatient rehabilitation facilities in 2011. The characteristics of the sample are presented in Table 1, stratified by impairment category. The mean discharge functional score for the total sample was 39.5 (SD = 10.4) for self-care, 20.5 (SD = 6.6) for mobility, and 27.1 (SD = 6.8) for cognition. Approximately 73% of the sample was discharged to the community after inpatient rehabilitation. Table 2 shows the R2 estimates from the linear regression models predicting discharge self-care, mobility and cognitive ratings among patients with stroke, lower extremity fractures and joint replacements.
Table 1.
Descriptive Characteristics of the Sample by three Impairment Categories: Values are presented as N (%) or mean ±SD.
| Variable | Stroke | Fracture | Joint Replacement |
|---|---|---|---|
| Total number patients | 41,984 (40.1) | 36,861 (35.0) | 26,430 (24.7) |
| Age | 79.0 ± 7.5 | 81.8 ± 7.5 | 76.6 ± 6.7 |
| 66 – 75 | 35.1% | 22.3% | 46.4% |
| 76 – 85 | 42.5% | 42.6% | 41.3% |
| >85 | 22.3% | 35.0% | 12.2% |
| Female | 55.0% | 72.3% | 68.4% |
| Race/Ethnicity | |||
| White | 79.5% | 89.3% | 86.1% |
| Black | 12.1% | 3.5% | 7.2% |
| Hispanic | 5.1% | 4.9% | 4.5% |
| Other | 3.2% | 2.0% | 2.0% |
| Length of stay | 14.7 ± 6.6 | 13.4 ± 4.5 | 9.7 ± 3.6 |
| Dual eligibility | 17.6% | 13.7% | 10.1% |
| Medicare qualifying disability | 11.1% | 10.7% | 10.7% |
| Admission functional status | |||
| Self-care | 24.4 ±9.7 | 25.4 ±7.7 | 31.0 ±6.8 |
| Mobility | 11.0 ±4.3 | 9.4 ±3.0 | 11.7 ±3.4 |
| Cognition | 19.1 ±7.3 | 23.8 ±6.8 | 27.7 ±5.4 |
| Discharge functional status | |||
| Self-care | 36.1 ±11.8 | 39.2 ±7.7 | 45.3 ±6.3 |
| Mobility | 19.3 ±7.2 | 19.3 ±6.0 | 24.1 ±4.9 |
| Cognition | 23.8 ± 7.2 | 27.9 ±6.0 | 31.4 ± 3.9 |
| Community Discharge | 65.2% | 68.6% | 90.4% |
Table 2.
R2 Values for Predicting Discharge Self-Care, Mobility and Cognition in Six Models in three Impairment Categories.
| Model | Self-Care R2 | Mobility R2 | Cognition R2 |
|---|---|---|---|
| Stroke | |||
| Base Model | 0.566 (0.564 – 0.577) | 0.474 (0.468 – 0.481) | 0.620 (0.619 – 0.630) |
| Base + Charlson | 0.571 (0.564 – 0.577) | 0.476 (0.469 – 0.482) | 0.626 (0.620 – 0.631) |
| Base + TIER | 0.572 (0.565 – 0.578) | 0.478 (0.471 – 0.484) | 0.627 (0.621 – 0.632) |
| Base + FCI | 0.576 (0.569 – 0.582) | 0.479 (0.472 – 0.485) | 0.628 (0.622 – 0.633) |
| Base + Elixhauser | 0.575 (0.568 – 0.581) | 0.483 (0.476 – 0.489) | 0.629 (0.623 – 0.634) |
| Base + HCC | 0.584 (0.577 – 0.590) | 0.493 (0.486 – 0.499) | 0.632 (0.626 – 0.637) |
| Fracture | |||
| Base Model | 0.422 (0.414 – 0.429) | 0.309 (0.301 – 0.316) | 0.553 (0.546 – 0.559) |
| Base + Charlson | 0.425 (0.417 – 0.432) | 0.315 (0.307 – 0.322) | 0.554 (0.547 – 0.560) |
| Base + TIER | 0.430 (0.422 – 0.437) | 0.321 (0.313 – 0.328) | 0.555 (0.548 – 0.561) |
| Base + FCI | 0.429 (0.421 – 0.436) | 0.319 (0.311 – 0.326) | 0.556 (0.549 – 0.562) |
| Base + Elixhauser | 0.440 (0.432 – 0.447) | 0.332 (0.324 – 0.339) | 0.564 (0.557 – 0.576) |
| Base + HCC | 0.446 (0.438 – 0.453) | 0.337 (0.329 – 0.344) | 0.560 (0.553 – 0.566) |
| Joint Replacement | |||
| Base Model | 0.230 (0.221 – 0.238) | 0.186 (0.177 – 0.194) | 0.373 (0.363 – 0.382) |
| Base + Charlson | 0.233 (0.224 – 0.241) | 0.192 (0.183 – 0.200) | 0.375 (0.365 – 0.384) |
| Base + TIER | 0.231 (0.222 – 0.239) | 0.189 (0.180 – 0.197) | 0.374 (0.364 – 0.383) |
| Base + FCI | 0.242 (0.233 – 0.250) | 0.194 (0.185 – 0.202) | 0.379 (0.369 – 0.388) |
| Base + Elixhauser | 0.246 (0.236 – 0.255) | 0.208 (0.199 – 0.216) | 0.385 (0.375 – 0.394) |
| Base + HCC | 0.252 (0.242 – 0.261) | 0.219 (0.210 – 0.227) | 0.382 (0.372 – 0.391) |
Base model included age, gender, race/ethnicity, Medicare qualifying disability, dual eligibility, length of stay and admission functional status. Charlson: Charlson Comorbidity Index; Tier: CMS-Tier; FCI: Functional Comorbidity Index; Elixhauser: Elixhauser Comorbidity Index; HCC: CMS-Hierarchical Condition Category.
Stroke
The base model explained 56.6% of the variance in discharge self-care scores for patients with stroke. The amount of explained variance increased marginally when the individual comorbidity indices were added to the base model: Charlson (0.5%), Tier (0.6%), FCI (1.0%), Elixhauser (0.9%) and HCC (1.8%). The base model explained 47.4% of the variance in discharge mobility scores. The increases in variance explained with the addition of the comorbidity indices to the base model were: Charlson (0.2%), Tier (0.4%), FCI (0.5%), Elixhauser (0.9%) and HCC (1.9%). The base model explained 62.0% of the variance in predicting discharge cognition scores. The increases in variance explained with the addition of the comorbidity indices to the base model were: Charlson (0.6%), Tier (0.7%), FCI (0.8%), Elixhauser (0.9%) and HCC (1.2%).
Lower Extremity Fracture
The base model explained 42.2% of the variance in discharge self-care scores for patients with lower extremity fractures. The amount of explained variance increased slightly when the individual comorbidity indices were added to the base model: Charlson (0.3%), Tier (0.8%), FCI (0.7%), Elixhauser (1.8%) and HCC (2.4%). The base model explained 30.9% of the variance in discharge mobility scores. The increases in variance explained with the addition of the comorbidity indices to the base model were: Charlson (0.6%), Tier (1.2%), FCI (1.0%), Elixhauser (2.3%) and HCC (2.8%). The base model explained 55.3% of the variance in predicting discharge cognition scores. The increases in variance explained with the addition of the comorbidity indices to the base model were: Charlson (0.1%), Tier (0.2%), FCI (0.3%), Elixhauser (1.1%) and HCC (0.7%).
Lower Extremity Joint Replacement
The base model explained 23.0% of the variance in discharge self-care scores for patients with lower extremity joint replacements. The amount of explained variance increased little when the individual comorbidity indices were added to the base model: Charlson (0.3%), Tier (0.1%), FCI (1.2%), Elixhauser (1.6%) and HCC (2.2%). The base model explained 18.6% of the variance in discharge mobility scores. The increases in variance explained with the addition of the comorbidity indices to the base model were: Charlson (0.6%), Tier (0.3%), FCI (1.8%), Elixhauser (2.2%) and HCC (3.3%). The base model explained 37.3% of the variance in predicting discharge cognition scores. The increases in variance explained with the addition of the comorbidity indices to the base model were: Charlson (0.2%), Tier (0.1%), FCI (0.6%), Elixhauser (1.2%) and HCC (0.9%).
Community Discharge
Table 3 reports the C-statistics from the logistic regression models predicting discharge setting in each of the three impairment categories. In patients with stroke, the C-statistic for the base model was 0.58. The C-statistic increased to 0.86 after adding discharge functional status to the model. There was no increase in C-statistics with the addition of the comorbidity indices.
Table 3.
C-Statistics from Logistic Regression Models Predicting Community Discharge in three Impairment Categories.
| Model | Stroke C-statistic 95% CI |
Fracture C-statistic 95% CI |
Joint Replacement C-statistic 95% CI |
|---|---|---|---|
| Base Model | 0.589 (0.583 – 0.595) | 0.610 (0.604 – 0.616) | 0.629 (0.618 – 0.641) |
| Base Model + Discharge Function | 0.861 (0.860 – 0.867) | 0.831 (0.829 – 0.837) | 0.878 (0.871 – 0.884) |
| Base Model + Discharge Function + Charlson | 0.871 (0.868 – 0.877) | 0.835 (0.830 – 0.838) | 0.887 (0.880 – 0.891) |
| Base Model + Discharge Function + Tier | 0.870 (0.869 –0.867) | 0.833 (0.829 – 0.837) | 0.885 (0.881 – 0.893) |
| Base Model + Discharge Function + FCI | 0.873 (0.870 – 0.877) | 0.837 (0.834 – 0.841) | 0.888 (0.881 – 0.895) |
| Base Model + Discharge Function + Elixhauser | 0.874 (0.871 – 0.880) | 0.839 (0.832 – 0.842) | 0.889 (0.882 – 0.896) |
| Base Model + Discharge Function + HCC | 0.879 (0.873 – 0.885) | 0.841 (0.837 – 0.848) | 0.890 (0.884 – 0.897) |
Base model included age, gender, race, Medicare qualifying disability, dual eligibility and length of stay. Charlson: Charlson Comorbidity Index; Tier: CMS-Tier; FCI: Functional Comorbidity Index; Elixhauser: Elixhauser Comorbidity Index; HCC: CMS-Hierarchical Condition Category.
In patients with lower extremity fracture, the C-statistic for the base model was 0.61. The C-statistic increased to 0.83 after adding discharge functional status to the model. There was no meaningful change in the C-statistic values with the addition of the comorbidity indices.
In patients with lower extremity joint replacement, the C-statistic for the base model was 0.62. The C-statistic increased to 0.87 after adding functional status to the base model. The C-statistics show very small changes with the addition of the comorbidity indices (Table 3).
DISCUSSION
This study compared the performances of five comorbidity indices to determine if there were any statistically significant or clinically important differences in their ability to predict discharge functional status in self-care, mobility and cognition ratings, or to predict community discharge following inpatient rehabilitation. The study hypothesis that the Functional Comorbidity Index would outperform the other indices was not supported. Adding information from the CMS Tier, Charlson, FCI and Elixhauser indices added little to a base model including demographic and clinical factors in the ability to predict patients’ discharge functional status or discharge to the community.
The results indicate that none of the five comorbidity indices predicted discharge functional status in a statistically significant or clinically meaningful way in our sample of Medicare beneficiaries. The inclusion of the HCC to the base model explained an additional 1–3% of the variance in discharge functional status across the three rehabilitation impairment categories, but this difference was not statistically significant. The slightly better performance of HCC may have two possible explanations. First, the HCC index includes more medical conditions (70) than the other indices.9–11, 14 Second, the HCC has more ICD-9 codes per condition than the Elixhauser or Charlson. These factors may have made the HCC a slightly more sensitive index to clinical outcomes.
The results suggest that comorbidity information from the Tier, Charlson, FCI, Elixhauser and HCC does not improve the ability to discriminate discharge to the community for patients included in the rehabilitation impairment groups we examined. Adding discharge functional status, however, did significantly increase the discriminatory ability of the models in classifying community discharge (Table 3).
IRFs and other post-acute settings were exempt from the original prospective payment system implemented in acute care hospitals in the 1980s because Diagnosis Related Groups were not found to be associated with resource utilization in post-acute care environments.19 The IRF prospective payment system, implemented in 2002, is based on Function Related Groups developed by Stineman and colleagues20 and now referred to as Case-Mix Groups by CMS.1 Based on the different histories and objectives associated with acute versus post-acute settings, it is not surprising that comorbidity indices such as the Charlson and Elixhauser, derived from medical diagnoses and focused on mortality, are weakly associated with post-acute outcomes in Medicare patients.21
The Functional Comorbidity Index, however, was developed with the goal of creating an index sensitive to physical function. Groll and colleagues10 state that the underlying premise in developing the FCI was that “diagnoses associated with physical function” would be different than those associated with mortality, and that the FCI “would perform better than indices designed with mortality as the outcome of interest.”10, p. 599 Although the FCI was developed to predict physical function, our results suggest that the conditions included in the FCI are not strong predictors of functional status or community discharge for IRF patients. The lack of association with functional status as measured in our study may be related to the fact that the FCI was developed and validated using samples including community-based middle-aged and younger adults. While diagnostic conditions associated with rehabilitation such as arthritis and neurological diseases are included in the FCI, information regarding the severity of these conditions is not incorporated in the index.10 A goal of future research should be to expand the FCI to include conditions that would make it a more sensitive measure of functional outcomes associated with post-acute rehabilitation.
The weak association in our results between CMS Tier and post-acute outcomes is consistent with previous research.22–25 Schneider et al., reported poor performance for the CMS Tier, Charlson and Elixhauser indices in predicting functional gain and community discharge in patients with burn-related conditions.23 Horn and colleagues, also found that the Charlson and Tier indices had weak correlations with discharge motor function ratings and discharge to the community in patients with spinal cord injury.24 Our findings regarding functional status as a predictor of IRF outcomes, including readmission, are consistent with the results from recent studies.26, 27 Slocum et al. and Shih et al. reported an increase in C-statistics for acute hospital readmissions from IRF after adding functional status to the model.26, 27 Our study adds new information regarding the association of the HCC and FCI comorbidity with post-acute inpatient rehabilitation outcomes. The findings from our study and previous studies suggest a positive role for functional status in developing risk-prediction models for post-acute outcomes.
The relationship between post-acute outcomes and comorbidity indices in older adults is complex. The majority of research on comorbidity indices and health outcomes in older adults has occurred in acute care settings.25 Evidence exists indicating that the HCC is a reliable measure for estimating Medicare costs28 and the Elixhauser and Charlson indices are useful in predicting hospital-related outcomes such as mortality, hospital length of stay and hospital payment.9, 14, 15 None of these indices, however, were strongly associated with post-acute functional outcomes among the older adults in our study.
There is growing interest in the role of comorbidity, multi-morbidity, chronic conditions and functional status in post-acute care settings as a result of health care reform.2 The Institute of Medicine and Department of Health and Human Services recently issued a report describing the need for practice guidelines to address the impact of multiple comorbid conditions when making treatment decisions for older adults.29 A logical place to begin exploring the relationship between multimorbidity and functional status is to evaluate existing comorbidity indices. Our results suggest a weak relationship among the five comorbidity indices examined and self-care, mobility, cognitive functional status and discharge to the community. Additional research is needed to explore alternative approaches for operationalizing and assessing comorbidity in post-acute care environments.
In a recent study using large data visual analytics, Bhavnani and colleagues30 found that small clusters or pairs of comorbid conditions were associated with a higher risk of hospital readmission for patients with hip fracture who received post-acute rehabilitation. The comorbidity clusters were more useful predictors of readmission than a single comorbidity or larger pre-defined groups of comorbidities such as the Charlson or Elixhauser. New methods associate with “Big Data” and the emerging field of data science, such as those used by Bhavnani and colleagues27, may help identify small clusters or unique combinations of comorbidities from existing indexes such as the HCC, that are sensitive to positive (discharge to the community) or negative (readmissions) outcomes in patients from specific impairment groups.30
Limitations
This study has several limitations. First, we did not use a single score for the comorbidity indices or outcome-specific weights as none of the indices were developed to predict post-acute rehabilitation outcomes. Some indices do not apply weights, and those with weighting schemes were developed for specific outcomes. For example, Charlson weights were originally developed to predict one-year mortality in patients with cancer. Using weights for generating composite scores for comorbidity indices for rehabilitation outcomes may dilute the effect of severity. Therefore, our study used separate indicators for the comorbid conditions contained in each index and included these in the regression models. The study was also limited to patients in three rehabilitation impairment categories and, therefore, the findings are not generalizable to all patients receiving inpatient rehabilitation. Finally, our results are based on data contained in Medicare files. These data represent administrative information and are subject to reporting and coding errors.
CONCLUSION
Comorbid indices including the Charlson, Functional Comorbidity Index, Elixhauser, Hierarchical Condition Category, and the CMS Tier comorbidity classification system did not significantly predict discharge self-care, mobility, and cognitive function in patients receiving inpatient rehabilitation. The comorbidity indices did not improve the ability to discriminate between patients discharged to the community versus to institutional settings. In the current environment of health care reform emphasizing quality of care indicators and patient-centered outcomes, it is essential to develop robust approaches that will improve functional independence for patients and families receiving post-acute rehabilitation. The role of comorbid indices in improving outcomes for patients receiving post-acute rehabilitation is potentially important and requires additional research attention.
Acknowledgments
This study was supported by the National Institutes of Health (grants #: R24-HD065702 and R01-HD069443, PI- K. Ottenbacher) and the Administration for Community Living, National Institute on Disability, Independent Living and Rehabilitation Research (grant #: 90IF0071-02-00, PI- K. Ottenbacher). Sponsors had no role in the study design, analysis or interpretation of the data. Study sponsors did not have any role in the writing of the manuscript or the submission to a journal. The authors acknowledge the assistance of Sarah Toombs Smith, PhD, ELS, in manuscript preparation.
Footnotes
Disclosures: No authors have conflicts of interest or financial disclosures to report.
This work has not been previously published nor is it under review at another journal. An abstract was presented at the Academy Health Annual Meeting in June 2015.
REFERENCES
- 1.Department of Health and Human Services. Federal Registry: Proposed rule Medicare program; Inpatient Rehabilitation Facility prospective payment system for federal fiscal year 2015. [Accessed January 21, 2015];2014 79(88):26309–26353. Available at http://www.gpo.gov/fdsys/pkg/FR-2014-05-07/pdf/2014-10321.pdf. [Google Scholar]
- 2.United States Congress. The Improving Medicare Post-Acute Care Transformation Act of 2014, H.R. 4994 (113th): IMPACT Act of 2014. [Accessed March 25, 2015];2014 Available at https://www.govtrack.us/congress/bills/113/hr4994.
- 3.The Medicare Payment Advisory Commission (MedPAC) Report to the Congress: Medicare Payment Policy, March 2012: Chapter 9, Inpatient rehabilitation facilities. [Accessed April 3, 2015];:233–253. Available at http://www.medpac.gov/documents/reports/mar12_ch09.pdf?sfvrsn=0.
- 4.Graham JE, Ripsin CM, Deutsch A, et al. Relationship between diabetes codes that affect medicare reimbursement (Tier comorbidities) and outcomes in stroke rehabilitation. Arch Phys Med Rehabil. 2009;90:1110–1116. doi: 10.1016/j.apmr.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottenbacher KJ, Graham JE, Ottenbacher AJ, et al. Hospital readmission in persons with stroke following postacute inpatient rehabilitation. J Gerontol A Biol Sci Med Sci. 2012;67:875–881. doi: 10.1093/gerona/glr247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neuman MD, Silber JH, Magaziner JS, Passarella MA, Mehta S, Werner RM. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273–1280. doi: 10.1001/jamainternmed.2014.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, Mackenzie CR. A new method of classifying prognostic co-morbidity in longitudinal-studies - development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 8.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data - differing perspectives. J Clin Epidemiol. 1993;46:1075–1079. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Pope GC, Kautter J, Ingber MJ, Freeman S, Sekar R, Newhart C. Evaluation of the CMS-HCC Risk Adjustment Model. Research Triangle Park, NC: Research Triangle Institute International; 2011. [Google Scholar]
- 12.UB Foundation Activities Inc. Uniform Data System for Medical Rehabilitation: IRF-PAI Training Manual. [Accessed January 15, 2015];2004 Available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/InpatientRehabFacPPS/downloads/irfpaimanual040104.pdf.
- 13.Research Data Distribution Center. Medicare Provider Analysis And Review Record - Dictionary For SAS and CSV Datasets. [Accessed October 9, 2015];2014 Available at http://www.cms.gov/research-statistics-data-and-systems/files-for-order/identifiabledatafiles/downloads/sasidmedpar.pdf.
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Dominick KL, Dudley TK, Coffman CJ, Bosworth HB. Comparison of three comorbidity measures for predicting health service use in patients with osteoarthritis. Arthritis Rheum. 2005;53:666–672. doi: 10.1002/art.21440. [DOI] [PubMed] [Google Scholar]
- 16.Tessier A, Finch L, Daskalopoulou SS, Mayo NE. Validation of the Charlson Comorbidity Index for predicting functional outcome of stroke. Arch Phys Med Rehabil. 2008;89:1276–1283. doi: 10.1016/j.apmr.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 17.The Centers for Medicare & Medicaid Services. Risk Adjustment. 2006–2011 Model Software/ICD-9-CM Mappings. [Accessed October 9, 2015]; Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Risk-Adjustors.html.
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd. New York, NY: John Wiley & Sons; 2000. [Google Scholar]
- 19.Hosek Susan D, Kane Robert L, Carney Maureen F, et al. Charges and Outcomes for Rehabilitative Care: Implications for the Prospective Payment System. Santa Monica CA: RAND; 1986. [Google Scholar]
- 20.Stineman MG, Tassoni CJ, Escarce JJ, et al. Development of function-related groups version 2.0: a classification system for medical rehabilitation. Health Services Research. 1997;32:529–548. [PMC free article] [PubMed] [Google Scholar]
- 21.Zekry D, Loures Valle BH, Graf C, et al. Prospective comparison of 6 comorbidity indices as predictors of 1-year post-hospital discharge institutionalization, readmission, and mortality in elderly individuals. J Am Med Dir Assoc. 2012;13:272–278. doi: 10.1016/j.jamda.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Slocum CS, Goldstein R, DiVita MA, et al. Assessing the ability of comorbidity indexes to capture comorbid disease in the inpatient rehabilitation burn injury population. Am J Phys Med Rehabil. 2015;94:373–384. doi: 10.1097/PHM.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 23.Schneider JC, Gerrard P, Goldstein R, et al. The impact of comorbidities and complications on burn injury inpatient rehabilitation outcomes. PM R. 2013;5:114–121. doi: 10.1016/j.pmrj.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 24.Horn SD, Smout RJ, DeJong G, et al. Association of various comorbidity measures with spinal cord injury rehabilitation outcomes. Arch Phys Med Rehabil. 2013;94:036. doi: 10.1016/j.apmr.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Graham JE, Resnik L, et al. Examining the association between comorbidity indexes and functional status in hospitalized Medicare fee-for-service beneficiaries. Phys Ther. 2015;96:232–240. doi: 10.2522/ptj.20150039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shih SL, Gerrard P, Goldstein R, et al. Functional status outperforms comorbidities in predicting acute care readmissions in medically complex patients. J Gen Intern Med. 2015;30:1688–1695. doi: 10.1007/s11606-015-3350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slocum C, Gerrard P, Black-Schaffer R, et al. Functional status predicts acute care readmissions from inpatient rehabilitation in the stroke population. PLoS One. 2015;10:e0142180. doi: 10.1371/journal.pone.0142180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noyes K, Liu H, Temkin-Greener H. Medicare capitation model, functional status, and multiple comorbidities: model accuracy. Am J Manag Care. 2008;14:679–690. [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman RA, Boyd C, Tinetti ME, Von Kohorn I, Parekh AK, McGinnis JM. IOM and DHHS meeting on making clinical practice guidelines appropriate for patients with multiple chronic conditions. Ann Fam Med. 2014;12:256–259. doi: 10.1370/afm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhavnani SK, Dang B, Visweswaran S, Divekar R, Karmarkar A, Ottenbacher K. How comorbidities co-occur in readmitted hip fracture patients: from bipartite networks to insights for post-discharge planning. AMIA Joint Summits Translational Science Proceedings. 2015 Mar; [PMC free article] [PubMed] [Google Scholar]


