Abstract
BACKGROUND
Oral contraceptive (OC) use has been consistently linked to increased risk of inflammatory bowel disease (IBD). Nonetheless, a specific role of OC on the natural history of ulcerative colitis (UC) is unknown.
METHODS
We identified 6,104 incident female UC cases aged 16–51 years at diagnosis from the Swedish National Patient Register starting in January of 2003. Information on current OC use was obtained from the Prescribed Drug Register starting in July of 2005. We followed cases through December of 2014 for primary outcome defined as first UC-related surgery, and the secondary outcomes defined by recipient of the first prescription of oral steroids or anti-TNF use. We used Cox proportional hazard modeling with time-varying covariates to estimate multivariable-adjusted hazard ratio (aHR) and 95% confidence interval (CI).
RESULTS
Over 31,421 person-years of follow up, we observed 162 cases of UC-related surgery. Compared to non-users, current and past use of OC were not significantly associated with risk of UC-related surgery (aHR = 0.79, 95% CI, 0.52–1.18, and 0.74, 95% CI 0.46–1.18, respectively). The association did not appear to be modified by type of OC use (progestin-only vs. combination of progestin and estrogen), longer duration of use or higher number of dispensed prescriptions (All Ptrend > 0.28). Similarly, longer use or higher cumulative number of OC prescriptions were not associated with increased risk of receiving a steroid prescription (Ptrend = 0.68 and 0.63, respectively). In exploratory analyses restricted to Stockholm county, current OC use was not associated with increased risk of receiving anti-TNF therapy (aHR = 0.83, 95% CI, 0.59 – 1.18).
CONCLUSIONS
In a large nationwide registry of UC patients, we found no association between OC use and UC progression. Our data offer reassurance regarding the safety of OC assessed by its effect on risk of surgery and steroid or anti-TNF use in women with established UC.
Keywords: Inflammatory Bowel Disease, Ulcerative Colitis, Oral contraceptives, Surgery, Steroid use, Swedish National Patient Register
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disorder of the gastrointestinal tract with heterogeneous disease presentation. In nearly 20% of cases, the initial presentation is an acute severe colitis requiring hospitalization, while nearly 50% of patients may never require hospitalization related to their disease (1–3). Although the pathophysiology of the disease remains largely unknown, a number of novel environmental and genetic factors have recently been linked to the onset of UC further expanding our knowledge of potential key biologic pathways involved in development of the disease (4–7). Despite these advances in disease etiology, little is known about factors that are associated with disease progression.
Because a significant number of women in reproductive age with UC may consider use of oral contraceptives (OC), understanding the impact of these medications on progression of UC can help inform clinical recommendations. Previously we have shown an association between long-term use of OCs, particularly the combination type, and risk and progression of CD (8, 9). However, the data on the effect of OC on risk and progression of UC is sparse. Although some studies have shown an association between OC use and risk of UC, most recent meta-analysis as well as our recent cohort study did not support this association (9, 10). Nonetheless, a specific role for OC use on progression of UC is unclear.
We therefore sought to examine the association between OC use and UC progression defined by need for surgery or use of steroids or anti-TNF using a large population-based cohort in Sweden. To our knowledge, this is the first study examining the association between OC use and disease progression among women with established UC. Because of the availability of detailed and updated information on dispensed prescriptions, inpatient, and hospital-based ambulatory care visits, this cohort provided us with a unique opportunity to explore such associations.
METHODS
Study Population
The Swedish healthcare system is tax-funded and offers universal access including prescription coverage. The Swedish National Board of Health and Welfare has collected individual-level data on hospital discharges on a countywide level since 1964 (nationwide since 1987)(11). Each record, organized according to an individual's personal identity number, includes date of birth, sex, dates of hospital admission, hospital department, and discharge diagnoses (including surgical procedures), coded according to the International Classification of Diseases(12). Since 2001, this registry was expanded to include specialized outpatient care(11). Our study population included women between the ages of 16 (typical earliest age used in prior studies in Sweden (13, 14)) and 51 years (median age of menopause) (15) with at least two inpatient or outpatient encounters with a primary or secondary diagnosis of UC (ICD9: ‘556’ or ICD10: ‘K51’) after January 2003. We selected January 2003 as our study starting point to allow for a two-year lag between introduction of outpatient data in the National Patient Register and identification of prevalent cases of UC previously not captured through hospitalizations. Participants with any diagnoses codes for UC or Crohn’s disease (CD) at any time prior to 2003 (1964–2003) or those who had a change in their diagnosis from UC to CD or indeterminate colitis (ICD10: ‘K52.3’) at any time during follow up were excluded from the study. The accuracy of ICD coding for disease ascertainment for the inpatient component of the National Patient Register has been previously validated, with a positive predictive value of 85–95% (16). In addition, a recent study from this cohort has shown that this definition yields a stable and reliable prevalence for UC, comparable to those previously reported in Scandinavia and Canada(17). Although there has not been a formal validation study in Sweden about the accuracy of capturing incident cases of UC, preliminary results from our ongoing validation study showed a positive predictive value greater than 93% with ≥2 IBD encounters within the registry.
Primary Exposure and Other Covariates
Since July 1st 2005, information on all dispensed prescriptions for the entire Swedish population, including date of redemption, amount of drug, and dosage has been collected in the Swedish Prescribed Drug Register(18). Specifically sale information about each prescription for all individuals is directly transferred from the cashier to the registry. The registry is virtually complete for the entire population, with identity data missing for <0.3% of all outpatient prescribed medications (18). Information on the defined daily dose (DDD) is also available for each prescription. DDD is a statistical measure of drug consumption, defined by the World Health Organization to standardize the comparison of drug usage between different drugs or between different health care environments. Categories of DDD use were defined based on approximate annual or biannual use of medications. OC use was identified through Anatomical Therapeutic Chemical codes (ATC) of G03AA or G03AB (combined hormonal contraceptives) and G03AC (progestin-only). These ATC codes have previously been used to describe patterns of OC use in Sweden(13). Information on OC use was updated monthly and entered in our models as current, past, or non-users. In addition, we calculated and updated the cumulative duration of intake of OC and cumulative defined daily dose (DDD) of use from July of 2005 (earliest time prescription data was available) for each participant by adding the number of months and DDD use over follow up time, respectively. Information on UC-related medications including 5-aminosalicylic acid, steroids, thiopurines (azathioprine and 6-mercaptipurines) and methotrexate, and anti-TNF therapy (infliximab and adalimumab) were also obtained from the prescription registry (Supplementary Table 1). As infliximab is primarily infused in hospitals, data on infliximab use was not available nationwide. However, in the Stockholm county, which accounts for 20% of population in Sweden, starting in 2007 information on outpatient infusions of biologics including infliximab in the hospital was collected (European commission granted approval of infliximab for treatment of UC in March of 2006).
We obtained data on education level from the longitudinal integrated database for health insurance and labor market studies (LISA), which integrates annually updated administrative information from the labor market and educational and social sectors from 1990 onward on all individuals 16 years or older registered as residents in Sweden(19). Data from LISA are linked to patients with diagnosis of UC using Swedish resident’s unique personal identity number. Information on age, sex, dates of birth, death, or immigration were collected form the Swedish Total Population Register kept by Statistics Sweden (20). We also calculated and updated the number of UC-related encounters (both inpatient and outpatient) for each participant during the follow up. Finally, information on parity was collected from the Swedish Medical Birth Register, which includes information on more than 98% of all births in Sweden since 1973. Information is prospectively collected by midwives from standardized pre-natal, obstetrical, and neonatal records(21). We adjusted for parity in our sensitivity analyses because it may account for differences in disease severity as women with more severe disease are less likely to become pregnant.
Outcome Ascertainment
Information on UC-related surgery was obtained from surgical procedure codes available nationwide in the registry since 1987. The validity of surgical procedure coding in the National Patient Register has been reported to be high (positive predictive value > 85%) (22). For UC-related surgeries, we used ‘JFH’ subcodes referring to open or laparoscopic colectomy (subtotal or proctocolectomy) and ileostomy with or without creation of ileal pouch-anal anastomosis. We defined need for steroid as the first dispensed prescription for steroids (ATC codes = ‘H02AB06’, ‘H02AB07’ for oral prednisolone) at least 90 days after start of follow up. Similarly, first anti-TNF use was ascertained from prescription registry for adalimumab (ATC code = L04AB02) and Stockholm anti-TNF registry for infliximab as described above.
Statistical Analysis
Person-time for each participant was calculated from July 1, 2005 or second UC-related encounter, whichever came last, to the date of first UC-related surgery, death from any cause, date of emigration, or December 31, 2014, whichever came first. We used Cox proportional hazards modeling adjusting for covariates to calculate adjusted hazard ratios (aHR) and 95% confidence interval (CIs). As there could be differences in the duration of disease at the time of study entry in July of 2005, we adjusted for duration of disease, which was calculated from the time of first UC-related encounter (2003 – 2005) to the study entry, in our multivariable analyses. In addition, to account for secular trends in patterns of OC use over follow up time, we stratified all analyses by calendar year. Finally, we explored the possibility that the effect of OC use on risk of surgery or steroid prescription may be modified by age of diagnosis (e.g. defined as age at the time of second UC-related encounter) and parity by entering OC use and binary age of diagnosis (high vs. low based on median value) or parity (parous vs. nulliparious) in our models as a multiplicative interaction terms. We used SAS version 9.4 (Cary, NC) for these analyses. All P-values were 2-sided and < 0.05 was considered statistically significant. The study was approved by the regional ethics committee at Karolinska Institutet (Stockholm, Sweden).
Results
Through December of 2014, we identified 162 incident surgeries among 6,104 UC patients who contributed 31,421 person-years with a median follow up of 60 months. Compared to non-users, past and current users were younger and more likely to have had more than 12 years of education (Table 1). However, the county of residence, number of UC-related encounters over follow up, and patterns of UC-related medication use were similar across the groups (Table 1).
Table 1.
Characteristics of Participants at Mid-Point of the Study in 2010*
| Oral contraceptive use§ | ||||
|---|---|---|---|---|
| Never N = 1,812 |
Past N = 816 |
Current N = 572 |
||
| Age (yrs), mean (std) | 37.5 (8.8) | 30.1 (7.8) | 27.1 (7.7) | |
| Age at diagnosis (yrs), % | ||||
| 16–20 | 4.4 | 9.2 | 23.1 | |
| 21–30 | 17.8 | 47.3 | 44.9 | |
| 31–40 | 35.2 | 30.9 | 24.8 | |
| 41–51 | 42.6 | 12.6 | 7.2 | |
| Medications (ever use), % | ||||
| 5-Aminosalicylic acid (oral/topical) | 89.8 | 92.0 | 94.9 | |
| Steroids | 66.4 | 72.1 | 70.1 | |
| Immunomodulators¶ | 28.4 | 33.5 | 34.6 | |
| Anti-TNF† | 5.5 | 6.3 | 7.2 | |
| Health care region of residence, % | ||||
| Northern Sweden | 9.6 | 6.9 | 6.5 | |
| Stockholm-Gotland | 22.4 | 20.1 | 17.0 | |
| Southeastern Sweden | 9.9 | 14.1 | 12.4 | |
| Southern Sweden | 19.2 | 19.4 | 21.7 | |
| Uppsala-Örebro | 18.9 | 19.5 | 25.4 | |
| Western Sweden | 20.0 | 20.1 | 17.1 | |
| Education** (yrs), % | ||||
| ≤ 9 | 7.8 | 4.2 | 4.7 | |
| 10–12 | 43.3 | 37.9 | 40.0 | |
| ≥ 13 | 48.5 | 57.8 | 55.1 | |
| Number of encounters, median (range) | 8 (2–96) | 9 (2–112) | 9 (2–55) | |
Abbreviations: standard deviation (std) and years (yrs).
6,104 participants contributed person-years to this study with nearly 54% of women using OC through follow up.
Consists of methotrexate, azathioprine, and 6-mercaptopurine (6-MP).
refers to use of infliximab and adalimumab in Stockholm county only.
Percentages do not add up to 100 as there is <1% missing in each group of OC use.
Oral Contraceptives and Risk of Surgery
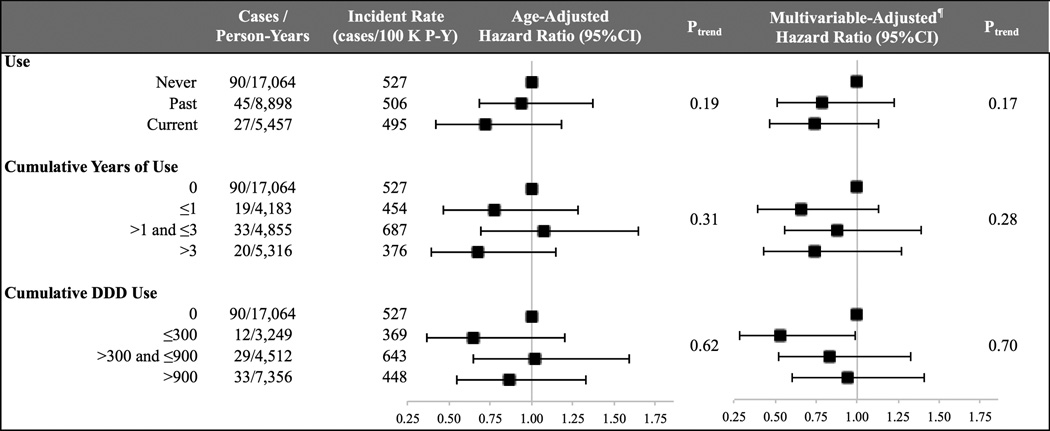
Compared to non-users, the age-adjusted HRs of surgery were 0.94 (95% CI, 0.64–1.40) for past users and 0.72 (95% CI, 0.46–1.15) for current users (Figure 1). These estimates were not materially altered after adjusting for other covariates (aHR = 0.79 (95% CI, 0.52–1.18) among past users and 0.74 (95% CI, 0.46–1.18) among current users, Ptrend = 0.17). In addition, the risk of surgery did not appear to increase with longer duration of use (Ptrend = 0.28) or higher number of DDD (Ptrend = 0.70) (Figure 1). Specifically, compared to non-users, the aHRs of surgery were 0.66 (95% CI, 0.39–1.13) with ≤ 1 year of use, 0.88 (95% CI, 0.56–1.39) with > 1 and ≤ 3 years of use, and 0.74 (95% CI, 0.43–1.27) with more than 3 years of use (Figure 1). Similarly, compared to non-users, the aHRs of surgery were 0.53 (95% CI, 0.29–0.99) with ≤ 300 DDDs, 0.83 (95% CI, 0.52–1.33) with > 300 and ≤ 900 DDDs, and 0.94 (95% CI, 0.60–1.47) for > 900 DDDs of use (Figure 1).
Figure 1. Oral Contraceptive Use and Risk of Surgery§.
* Abbreviations: confidence interval (CI), hazard ratio (HR), multivariable (MV), and person-year (P-Y). ¶ Adjusted for age (years), age at diagnosis (categories based on Table 1), number of encounters, education (≤ 9, 10–12, ≥ 13 yrs), and health care region of residence (categories based on Table 1).
Oral Contraceptives and Risk of Steroid Prescription
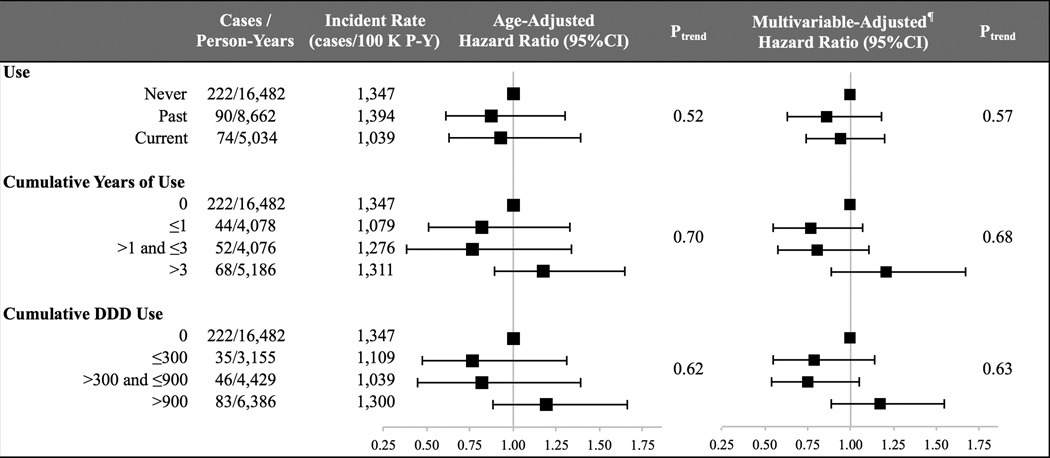
We also explored the association between OC use and risk of steroid use. Compared to non-users, the age-adjusted HRs of receiving a steroid prescription were 0.87 (95% CI, 0.67–1.14) for past users and 0.93 (95% CI, 0.70–1.24) for current users. These estimates were not significantly altered in multivariable analyses; aHRs of steroid prescription were 0.86 (95% CI, 0.66–1.12) among past users and 0.94 (95% CI, 0.71–1.26) among current users (Ptrend = 0.57) (Figure 2). In addition, longer duration of use and increased number of prescriptions of OC were not associated with increased risk of steroid prescription (Ptrend = 0.68 and 0.63, respectively) (Figure 2). Specifically, compared to the non-users, the aHRs of steroid prescription were 1.21 (95% CI, 0.89–1.67) among UC women with greater than 3 years of OC use and 1.17 (95% CI, 0.89–1.55) for greater than 900 DDD use of OC.
Figure 2. Oral Contraceptive Use and Risk of Steroid Prescription*.
* Abbreviations: confidence interval (CI), hazard ratio (HR), multivariable (MV), and person-year (P-Y). ¶ Adjusted for age (years), age at diagnosis (categories based on Table 1), number of encounters, education (≤ 9, 10–12, ≥ 13 yrs), and health care region of residence (categories based on Table 1).
Types of Oral Contraceptives and Risk of Steroid Prescription and Surgery
We explored the possibility that the association between OC and disease progression may differ according to the type of OC use and therefore evaluated the independent effect of combination-type and progestin-only OC on risk of UC complications. Similar to our primary analyses, we did not observe an association between progestin or combination-type OC and risk of surgery. Specifically, compared to non-users, the aHRs of surgery among women who had used OC for over two years were 0.84 (95% CI, 0.54–1.31) for combination-type OC and 1.02 (95% CI, 0.58–1.80) among progestin-only containing contraceptives. In addition, the risk of surgery did not increase with higher number of DDD use of combination-type OC (Ptrend = 0.53) or the progestin-only type (Ptrend = 0.94). Similar to our main analyses, longer duration of combination or progestin-only containing types of OC use was not associated with increased risk of steroid prescriptions (Ptrend = 0.35 and 0.89, respectively).
Exploratory and Sensitivity Analyses
Since use of anti-TNF therapy also represents a significant escalation of treatment for patients with established UC, we explored the association between OC use and risk of anti-TNF initiation in the Stockholm county, where detailed and updated information on infliximab infusions were available starting in 2007 (shortly after approval of infliximab for UC in Europe). Data on other injectable anti-TNF therapy including adalimumab use was obtained from prescription registry. Compared to non-users, the aHR of anti-TNF use was 0.83 (95% CI, 0.59 – 1.18) among current OC users. In addition, longer duration or cumulative DDD use of OC were not associated with increased risk of anti-TNF use (Ptrend = 0.97 and 0.98, respectively).
As voluntary childlessness may alter women’s decision to use OC, we performed stratified analyses according to parity (nulliparious vs. multiparous) and observed no effect modification (Pinteraction = 0.85). Since there were significant differences in age of diagnosis according to OC status likely due to introduction of prescription registry in 2005 and variations in use according to age even among premenopausal women, we assessed the effect of OC on UC progression according to strata defined by age of diagnosis (e.g. age at second UC-related encounter) and observed no effect modification (Pinteraction = 0.55). Specifically, the aHR of surgery among current OC users in women with age of diagnosis less than 33 years (mean age of diagnosis and typical age with highest prevalence of OC use) was 1.02 (95% CI, 0.50–2.10). Similarly, we observed no effect modification by age at second UC-related encounter on the risk of steroid prescription with OC use (All Pinteractions > 0.29). Specifically, the aHR of steroid prescription among current OC users in women with age of diagnosis less than 33 years was 0.94 (95% CI, 0.63–1.39).
We considered the possibility that women who were OC users in 2005 may have been using for varying durations (e.g. left truncation bias) and thus conducted sensitivity analysis in which we included only women who were diagnosed with UC after 2005 (both first and second UC-related encounters occurred after 2005). In addition, we excluded women who were users during the first year the prescription registry was started (July 2005 – July 2006) and restricted analyses to follow up after July 2006 and obtained similar results. Compared to non-users, the aHRs of surgery and steroid prescriptions among current OC users were 0.84 (95% CI, 0.47–1.48) and 1.05 (95% CI, 0.71–1.54), respectively.
Discussion
In a nationwide cohort study, we found no association between OC use and risk of UC progression(8). Specifically, we did not identify an association between OC use and risk of surgery and steroid or anti-TNF prescription among women with established UC.
In animal models, exogenous estrogen modulates intestinal barrier function (23, 24). In addition, endogenous estrogen, levels of which are altered by OC, enhances cellular proliferation and the humoral immune system(25) and likely contributes to thrombosis, which may lead to multifocal gastrointestinal infarction (26). Although the precise pathophysiology of UC and CD remains largely unknown, these estrogen-mediated pathways may play a key role in etiology and progression of IBD. Epidemiologic studies have consistently shown an association between OC and risk of CD(10). Recently, we also demonstrated an association between OC use, particularly the combination type, and risk of surgery among women with established CD(8). In contrast, more recent studies indicate that there does not appear to be a link between OC use and risk of UC (9, 10). Several older case series have reported acute severe self-resolving UC among younger women with recent initiation of OC(27–29). However, our study is the first to systematically assess the link between OC prescription and UC progression.
Our study has several strengths. First, the nationwide coverage of Swedish inpatient and outpatient and prescription registries offers an opportunity to determine a more precise estimate of the association between OC use and risk of UC progression. Second, data were collected prospectively and this minimizes the risk of recall and selection biases that are typically evident in many case-control and cross-sectional studies. Finally, in our analyses, OC information was updated every month, which enabled us to account for significant changes in the type or use of OC among younger women over time.
We acknowledge several limitations. First, despite our large sample size, we may not have had sufficient statistical power to examine more modest associations between OC use and UC progression. However, most of our risk estimates were less than unity (inverse association); a larger sample size would be highly unlikely to yield positive associations. Moreover, even if weak positive associations were present, their clinical significance would likely be minimal. Second, voluntary childlessness among women with more severe disease may alter patterns of OC use. However, such a bias would have likely led to spurious associations between short-term use of OC and risk of surgery and/or steroid prescription. In contrast, in our primary analyses we did not observe any differences in risk of surgery or steroid prescription comparing OC users to non-users. Third, we used surgery and steroid or anti-TNF prescriptions as a proxy for disease flare and/or complication and were unable to specifically evaluate the short-term effect of OC use on disease activity using other clinical and endoscopic activity indices. In addition, steroid prescription data may not fully capture all episodes of UC flares. Fourth, we did not have information on smoking, which has previously been linked to poor outcomes in UC (30, 31). However since smoking is likely a negative confounder of the association between OC use and risk of UC complication (i.e. OC users are less likely to be current smokers), adjusting for smoking would have been unlikely to change the direction of the observed non-significant inverse association. Lastly, information on OC use was only available after July of 2005 and therefore we were unable to fully capture cumulative use. However, our results were consistent across several sensitivity analyses. Specially starting our follow up in June of 2006 (one year after prescription registry was started) did not materially alter our effect estimates. In addition, stratified analyses according to age, which could be a proxy for duration of OC use prior to July of 2005 not captured by prescription registry (e.g. younger women less likely to have been on OC for long period of time compared to older women) yielded similar risk estimates according to age groups.
In conclusion, we did not find an association between OC use and risk of surgery and steroid or anti-TNF use among women with established UC. Our findings provide reassurance that OC use does not negatively impact long-term progression of UC.
Supplementary Material
What is already known about the topic
Oral contraceptive use has been consistently linked to increased risk of inflammatory bowel disease.
Long-term use of oral contraceptives in patients with established Crohn’s disease is associated with increased likelihood for surgery.
A specific role of oral contraceptives on progression, rather than the etiology of ulcerative colitis is unclear.
What are the new findings
Oral contraceptives are not associated with increased risk of surgery in patients with established ulcerative colitis.
Oral contraceptive use is not associated with increased rates of steroids prescription or use of anti-TNF therapy in patients with established ulcerative colitis.
The effect does not appear to differ according to the type of oral contraceptives.
Our data offer reassurance regarding the safety of oral contraceptives assessed by its effect on risk of surgery and steroid or anti-TNF use in women with established UC.
Acknowledgments
Grant Support: Funded by K23 DK099681. Dr. Chan is supported by a senior investigator grant from the Crohn’s and Colitis Foundation of America (CCFA) and K24 DK098311. Dr. Khalili is supported by a career development award from the American Gastroenterological Association (AGA) and by National Institute of Diabetes and Digestive and Kidney Diseases (K23 DK099681). This work was also supported by a travel grant from the American College of Gastroenterology (ACG), the Swedish Foundation for Strategic Research, Stockholm County Council (ALF), and the Bengt Ihre research fellowship in gastroenterology, and the Mjölkdroppen foundation.
Financial Disclosures: Dr. Khalili has received consulting fee from Abbvie, Inc. Dr. Chan has served as a consultant for Bayer Healthcare, Pfizer Inc., and Aralez Pharmaceuticals.
Abbreviations
- OC
Oral contraceptives
Footnotes
Conflict of Interest: None to declare
Ethical Approval: The study was approved by the regional ethics committee at Karolinska Institutet (Stockholm, Sweden).
Authors Contributions
HK - study concept and design; statistical analysis; interpretation of data; drafting of the manuscript
MN - acquisition of data; critical revision of the manuscript.
AE - study concept and design; acquisition of data; critical revision of the manuscript.
JFL - critical revision of the manuscript
JA - study concept and design; critical revision of the manuscript
ATC- study concept and design; critical revision of the manuscript
OO - study design; acquisition of data; critical revision of the manuscript
REFERENCES
- 1.Samuel S, Ingle SB, Dhillon S, Yadav S, Harmsen WS, Zinsmeister AR, et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm Bowel Dis. 2013;19(9):1858–1866. doi: 10.1097/MIB.0b013e31828c84c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinesen LC, Walsh AJ, Protic MN, Heap G, Cummings F, Warren BF, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010;4(4):431–437. doi: 10.1016/j.crohns.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Edwards FC, Truelove SC. The Course and Prognosis of Ulcerative Colitis. Gut. 1963;4:299–315. doi: 10.1136/gut.4.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalili H, Higuchi LM, Ananthakrishnan AN, Manson JE, Feskanich D, Richter JM, et al. Hormone therapy increases risk of ulcerative colitis but not Crohn's disease. Gastroenterology. 2012;143(5):1199–1206. doi: 10.1053/j.gastro.2012.07.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn's disease. Gut. 2014;63(5):776–784. doi: 10.1136/gutjnl-2013-305304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng SC, Bernstein CN, Vatn MH, Lakatos PL, Loftus EV, Jr, Tysk C, et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut. 2013;62(4):630–649. doi: 10.1136/gutjnl-2012-303661. [DOI] [PubMed] [Google Scholar]
- 8.Khalili H, Granath F, Smedby KE, Ekbom A, Neovius M, Chan AT, et al. Association Between Long-term Oral Contraceptive Use and Risk of Crohn's Disease Complications in a Nationwide Study. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalili H, Higuchi LM, Ananthakrishnan AN, Richter JM, Feskanich D, Fuchs CS, et al. Oral contraceptives, reproductive factors and risk of inflammatory bowel disease. Gut. 2013;62(8):1153–1159. doi: 10.1136/gutjnl-2012-302362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornish JA, Tan E, Simillis C, Clark SK, Teare J, Tekkis PP. The risk of oral contraceptives in the etiology of inflammatory bowel disease: a meta-analysis. Am J Gastroenterol. 2008;103(9):2394–2400. doi: 10.1111/j.1572-0241.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- 11.Socialstyrelsen. English - the National Patient Register. 2011 [Google Scholar]
- 12.Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. doi: 10.1007/s10654-009-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josefsson A, Wirehn AB, Lindberg M, Foldemo A, Brynhildsen J. Continuation rates of oral hormonal contraceptives in a cohort of first-time users: a population-based registry study, Sweden 2005–2010. BMJ Open. 2013;3(10):e003401. doi: 10.1136/bmjopen-2013-003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg M, Foldemo A, Josefsson A, Wirehn AB. Differences in prescription rates and odds ratios of antidepressant drugs in relation to individual hormonal contraceptives: a nationwide population-based study with age-specific analyses. Eur J Contracept Reprod Health Care. 2012;17(2):106–118. doi: 10.3109/13625187.2012.658925. [DOI] [PubMed] [Google Scholar]
- 15.Rodstrom K, Bengtsson C, Milsom I, Lissner L, Sundh V, Bjourkelund C. Evidence for a secular trend in menopausal age: a population study of women in Gothenburg. Menopause. 2003;10(6):538–543. doi: 10.1097/01.GME.0000094395.59028.0F. [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Busch K, Ludvigsson JF, Ekstrom-Smedby K, Ekbom A, Askling J, Neovius M. Nationwide prevalence of inflammatory bowel disease in Sweden: a population-based register study. Aliment Pharmacol Ther. 2014;39(1):57–68. doi: 10.1111/apt.12528. [DOI] [PubMed] [Google Scholar]
- 18.Wettermark B, Hammar N, Fored CM, Leimanis A, Otterblad Olausson P, Bergman U, et al. The new Swedish Prescribed Drug Register--opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. doi: 10.1002/pds.1294. [DOI] [PubMed] [Google Scholar]
- 19.StatisticsSweden. Background Facts, Labor and Education Statis- tics, integrated database for labor market research. 2009 [Google Scholar]
- 20.Ludvigsson JF, Almqvist C, Bonamy AE, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016 doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 21.The Swedish Medical Birth Register: a summary of content and quality. 2003 Nov [Google Scholar]
- 22.Nilsson AC, Spetz CL, Carsjo K, Nightingale R, Smedby B. Reliability of the hospital registry. The diagnostic data are better than their reputation. Lakartidningen. 1994;91(7):598, 603–605. [PubMed] [Google Scholar]
- 23.Braniste V, Jouault A, Gaultier E, Polizzi A, Buisson-Brenac C, Leveque M, et al. Impact of oral bisphenol A at reference doses on intestinal barrier function and sex differences after perinatal exposure in rats. Proc Natl Acad Sci U S A. 2010;107(1):448–453. doi: 10.1073/pnas.0907697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-beta signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300(4):G621–G626. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 25.Cutolo M, Capellino S, Straub RH. Oestrogens in rheumatic diseases: friend or foe? Rheumatology (Oxford) 2008;47(Suppl 3):iii2–iii5. doi: 10.1093/rheumatology/ken150. [DOI] [PubMed] [Google Scholar]
- 26.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010;6(5):339–346. [PMC free article] [PubMed] [Google Scholar]
- 27.Laszlo S, Albert FI. Acute ulcerative colitis with spontaneous regression. A new side effect of contraceptives? Orv Hetil. 1976;117(49):2987–2991. [PubMed] [Google Scholar]
- 28.Bonfils S, Hervoir P, Girodet J, Le Quintrec Y, Bader JP, Gastard J. Acute spontaneously recovering ulcerating colitis (ARUC). Report of 6 cases. Am J Dig Dis. 1977;22(5):429–436. doi: 10.1007/BF01071891. [DOI] [PubMed] [Google Scholar]
- 29.Simon L, Figus AI. Spontaneously regressive ulcerative colitis: the result of contraceptive agents? Gastroenterol Clin Biol. 1978;2(4):442–443. [PubMed] [Google Scholar]
- 30.Dias CC, Rodrigues PP, da Costa-Pereira A, Magro F. Clinical predictors of colectomy in patients with ulcerative colitis: systematic review and meta-analysis of cohort studies. J Crohns Colitis. 2015;9(2):156–163. doi: 10.1093/ecco-jcc/jju016. [DOI] [PubMed] [Google Scholar]
- 31.Hoie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, et al. Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102(8):1692–1701. doi: 10.1111/j.1572-0241.2007.01265.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.