Abstract
The study of inflammatory bowel disease, including Ulcerative Colitis and Crohn's Disease, has relied largely upon the use of animal or cell culture models; neither of which can represent all aspects of the human pathophysiology. Presented herein is a dual flow microfluidic device which holds full thickness human intestinal tissue in a known orientation. The luminal and serosal sides are independently perfused ex vivo with nutrients with simultaneous waste removal for up to 72 h. The microfluidic device maintains the viability and integrity of the tissue as demonstrated through Haematoxylin & Eosin staining, immunohistochemistry and release of lactate dehydrogenase. In addition, the inflammatory state remains in the tissue after perfusion on the device as determined by measuring calprotectin levels. It is anticipated that this human model will be extremely useful for studying the biology and testing novel interventions in diseased tissue.
INTRODUCTION
Despite intensive research into inflammatory bowel disease (IBD) i.e., Crohn's Disease (CD) and Ulcerative Colitis (UC), the pathogenic mechanisms remain unclear.1–3 It is thought that a combination of environmental and genetic influences alter the bacterial composition of the intestines causing an uncontrolled immune response in the intestinal mucosa and leading to a breakdown of the epithelial barrier. This local reaction leads to an increased influx of intestinal material into the cell wall creating an inflammatory response within the gut.4–6 IBD is a chronic disease without medical cure and requires lifetime care with a possibility of surgery, as well as the increased risk of colorectal cancer.7–10
Current research models into IBD either require inducing the disease in murine models or using cell culture, both which fail to fully replicate the extent of the disease seen in humans.11 Despite these drawbacks, the models have helped to decipher the underlying mechanisms of IBD pathogenesis as well as to evaluate a number of potential therapeutics. The most commonly used animal model is to induce a mouse into a “colitis like” state through the addition of a water soluble, negatively charged, sulphated polysaccharide known as dextran-sulphate sodium (DSS) into their diet.11,12 The mechanism by which DSS induces intestinal inflammation is unclear but it is likely the result of damage to the epithelial monolayer lining the large intestine which allows the dissemination of pro-inflammatory intestinal contents, e.g., bacteria and their products, into the underlying tissue.11,12 The colitis model induced by DSS remains very popular in IBD research due to its speed, simplicity, and reproducibility.1,13 However, this method of studying IBD does have some drawbacks, such as significant differences between both the mouse and human immune systems and the microbiota found in their respective intestines, as well as the associated costs with the upkeep and housing of animals.
On the other hand, cell culture models are commonly used which typically utilise a single cell type grown in a monolayer, whereas cells in vivo grow in 3D structures consisting of many different cell types arranged in a unique spatial arrangement interweaved with extracellular matrix (ECM).14,15 It is this interaction between neighbouring cells which guides the developmental processes central for functioning; monolayer cell culture models cannot replicate this.16 Development of an in vitro, living, cell-based model of the intestine that mimics the mechanical, structural, absorptive, transport, and pathophysiological properties of the gut along with its crucial microbial symbionts would accelerate pharmaceutical development and potentially reduce the numbers of animals used in research and drug testing.15,17
An alternative method of studying intestinal tissue as a whole is the Ussing chamber which provides a valuable, time-proven method for the measurement of electrolyte, nutrient and drug transport across epithelial tissues.18,19 The method was developed over 50 years ago by Hans Ussing as a means to understand the phenomenon of active NaCl transport, i.e., the capacity of cells to move ions or nutrients against an electrical and/or concentration gradient, which had previously been demonstrated by isotopic tracer experiments.18,19 Ussing chamber studies of intestinal mucosa have provided many of the key observations that moved our understanding of trans-epithelial transport processes toward a molecular basis. The Ussing chamber uses a 1–3 mm3 biopsy of human intestinal tissue immersed in a cell culture medium that is commonly kept alive between 6–8 h.18,19 This allows for cell-cell interactions and tissue characteristics to be maintained, mimicking a more in vivo environment. However, this method has some well-known drawbacks including: holding the tissue in place by pressure plates which crush the biopsy at the edges; the media containing the nutrients for the tissue is static and thus the waste products build up; in addition to the complexity and time required to establish each model.
With the recognition that precise 3D structures are important for normal or pathophysiological function, complex cell cultures are becoming more popular research tools, e.g., spheroids/organoids.20 An example of such research was highlighted by Materne et al.37 who developed a four organ microfluidic device which used a 3D spheroid, equivalent to ten liver lobules, to mimic liver function along with 2D cell cultures to mimic kidney, skin, and intestine behaviour in the model.21 However, such cell spheroid models are complicated, cumbersome, and expensive when compared to the 2D culture methods. Another approach, pioneered by Ingber and colleagues, was to develop a thermally bonded microfluidic device composed of two sides separated by a porous flexible membrane made of polydimethylsiloxane (PDMS) coated with extracellular matrix (ECM) and lined by human intestinal Caco-2 epithelial cells to mimic the complex structure of a living intestine.15,17 The “gut on a chip” then had tubing attached to provide a source of media together with a vacuum chamber to mimic the constant muscular motions of the intestinal tissue.15,17
The advancement of using a microfluidic device means that it is now possible to maintain biopsies or slices of actual organs in a viable state e.g., artery on a chip.22 The approach is similar in nature to the Ussing chamber but overcomes many of the technical issues associated with this method. Using a human biopsy removes the problem of not being sure whether the tissue is a true replica of the in vivo tissue, however, the major concern of this approach is how to maintain the viability. A number of studies have shown that full thickness biopsies of various human and animal tissues can be maintained in an in vitro device that mimics the in vivo environment in a relatively simple manner.23–26
Developed herein is a dual flow microfluidic device, designed to mimic the transport of intestinal material inside of the gut (luminal surface) through one side of the device and mimic the blood supply on the “outside” of the tissue (serosal surface) providing the necessary nutrients to maintain the viability of the tissue for a number of days after removal from a patient. The dual flow device uses a similar approach as the Ussing chamber with improvements, as well as the tissue is not crushed to be held in place, and it is not a static environment but undergoing continuous perfusion with fresh nutrients whilst simultaneously removing the waste products thereby better mimicking the in vivo environment. This device can be used to investigate pathophysiological mechanisms, microbe-tissue interaction and various treatments which have been known or suspected to aid in the treatment of IBD.
MATERIALS AND METHODS
Full thickness intestinal sections were removed, following informed consent, from patients undergoing small bowel or large bowel resection (REC 13/YH/0173) and transported in media on ice to the laboratory. The dual flow device fabricated from glass (1.5 cm × 1.5 cm) with a chamber milled using a CNC machine (1 cm × 0.5 cm × 200 μM) was created in-house (University of Hull, Chemistry Department). PDMS (Dow Corning, UK) was prepared at various thicknesses (dependent on the thickness of the tissue biopsy) and cut to the size of the glass chip before been trimmed in alignment with the milled chamber of the glass chip with a 5 mm punch biopsy needle (Harvard, UK). The tissue was cut with the same punch biopsy needle to ensure an exact fit, without sustained pressure as required in the Ussing chamber. The microfluidic components of tubing and connectors (Kinesis and Mengel Engineering respectively) allowed continuous perfusion of cell culture media {non-phenol red and phenol red Dulbecco modified Eagles media (DMEM) both supplemented with 10% v/v FBS, 1% v/v penicillin/streptomycin, 1.5% v/v 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES buffer) and 1% v/v minimal essential amino acids (MEM), (SLS, UK)} across the tissue to maintain viability. Double-sided adhesive was used to hold the glass to the PDMS, keeping the tissue in a known orientation (Fig. 1). The device was constructed in a sterile class II biological safety cabinet before being housed in a pre-heated incubator for the duration of the environment at 37 °C, with a flow rate of 4 μl/min using a syringe pump (Harvard, UK). The effluent was collected over time and stored until analysis. All the components (except the tubing) were autoclaved before use. Household petroleum jelly (Vaseline™) was applied to the edges of the tissue to seal any gaps between the tissue and PDMS, ensuring that perfusion is only possible across the tissue.
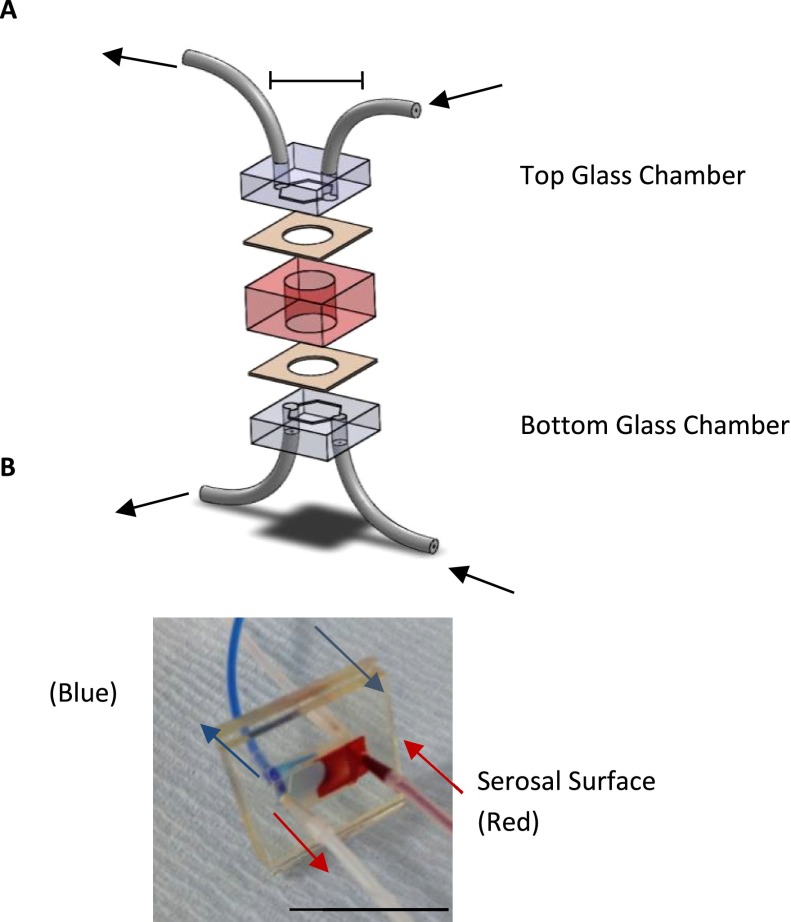
FIG. 1.
The dual flow device. Panel A: Schematic of the dual flow device used to hold the human gut tissue in a known orientation. Panel B: Coloured food dye depicting the dual flow in the microfluidic device.
The Lactate Dehydrogenase (LDH) colourimetric assay (Roche) was used according to the standard protocol on aliquots of effluent (50 μl per sample analysed in triplicate in a 96 well plate). Tissue was lysed with a 10% v/v lysis solution from the kit at 68 h to determine the level of viability that was maintained for the duration of the experiment. A calibration curve using known concentrations of LDH (measured in Units of activity) was created and values derived were finally divided by the wet weight of the tissue biopsy (mg) to allow comparison between samples.
Tissue for sectioning was fixed in 4% w/v paraformaldehyde solution (Sigma Aldrich) for 24 h before dehydration in alcohol and paraffin embedding (VWR). Tissue blocks were then sectioned at 10 μm and stained using Haematoxylin and Eosin Stain (H&E staining; Sigma Aldrich) before mounting with histomount (Sigma Aldrich). Sections of tissue were used for immunohistochemical staining of Ki-67 (MIB1, Dako) following standard microwave antigen retrieval to determine proliferating cells in the tissue.27
Permeation studies of the intestinal mucosal layer were determined using two differently sized fluorescein isothiocyanate (FITC)-dextran molecules: 2 000 000 and 50 000 molecular weight (Sigma Aldrich). Individually each dextran-FITC species was added to the culture media at 10% w/v and applied to the luminal surface of the tissue. After different lengths of time, the tissue was snap frozen and sectioned using a cryostat (Leica CM 1950) before imaging by confocal microscopy. Controls were completed simultaneously using either a PDMS and petroleum jelly “plug” or petroleum jelly alone both of which acted as an impermeable barrier across the device. Previous work has already demonstrated that petroleum jelly is compatible with human tissue as commonly used to aid in wound healing.28
Calprotectin, also known as s100a8, an inflammatory marker, was measured in the collected effluents using a duo set ELISA (enzyme linked immunosorbent assay) (RnD Systems Europe) following the manufacturer's protocol. The minimal detectable level was 31.3 pg/ml. Concentrations were expressed per mg wet weight of the tissue biopsy.
RESULTS AND DISCUSSION
Once the sample had been taken from a patient undergoing a bowel resection it took a maximum of 1.5 h to establish the devices. This time involved transport and preparation using previously sterilised and prepared devices. If the devices were operated in the same facility as the surgical procedure it would be possible to establish devices under 20 min, as the tissue processing is minimal and simple.
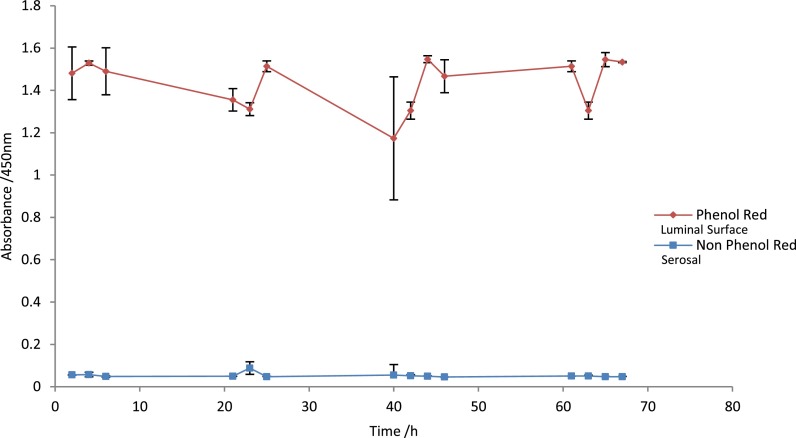
Initial experiments were done to ensure that the tissue could be held in place in the PDMS layer of the device correctly, and that the transfer of media from one side to the other occurred through the tissue by perfusion not leaking around the edges. Phenol Red and non-phenol red media were perfused for 70 h as depicted in Fig. 1 through the device, and the effluents were sampled at multiple 2 h time points over 4 days; absorbance was measured at 450 nm to detect the presence of phenol red (Fig. 2). Five independent tissue biopsies were tested in this way and demonstrated the continued separation of the media streams, ensuring the device does not leak.
FIG. 2.
Two differently coloured media red and clear (phenol red and non phenol red) were continuously flowed (4 μl/min) over the tissue to maintain viability over the duration of the experiment (70 h), n = 5, errors bars show a standard deviation (S.D). The luminal surface was maintained via the phenol red media (red) and the stromal surface maintained with the non phenol red media (clear).
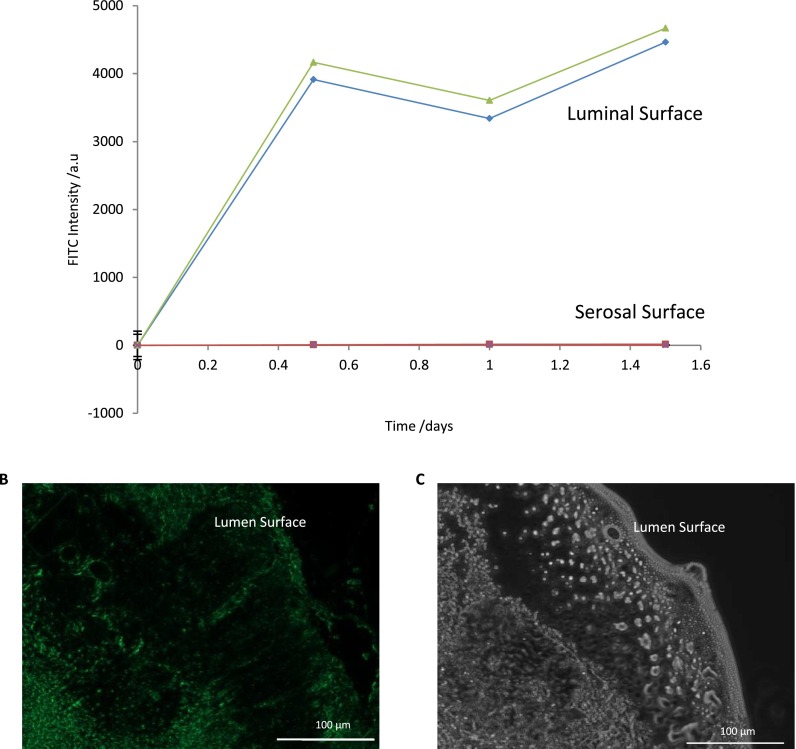
The permeability of tissue is known to increase in IBD leading to an influx of bacteria into the lumen surface of the gastrointestinal tract which causes the inflammatory immune response.4–6 When either FITC-dextran species was added to the luminal media they passed through the tissue within 25 min (data not shown). Biopsies from five independent patients were studied, and no obvious differences were observed in perfusion kinetics. Similarly, when devices were set up with only petroleum jelly and a PDMS plug filling the chamber as in Fig. 2, no perfusion of the fluorescent dextran was observed over the 68 h period (Fig. 3) supporting the fact that perfusion only occurs through the tissue. The relatively rapid nature of the perfusion is in contrast to some other models of intestinal mucosa, e.g., a porcine model developed by Westerhout et al.29 who reported <0.5% leakage of FITC-dextran of 4000 molecular weight over a 3 h time frame. One potential explanation for this difference is that the previous work was not a disease model and, as has been stated above, IBD is well known to cause an increase in mucosa permeability.
FIG. 3.
FITC Dextran flowed through the device (without tissue in Panel A) using a PDMS plug and petroleum jelly to ensure a thorough seal (Panel A, green line) and without petroleum jelly to determine leakage (Panel A, blue line). Panel A: Shows the FITC flowed through the device ensuring FITC remains on the luminal side of the device only. Panel B: A frozen section of tissue used to show fluorescence passing through the tissue. Panel C: Dark field image of tissue to determine structure without FITC.
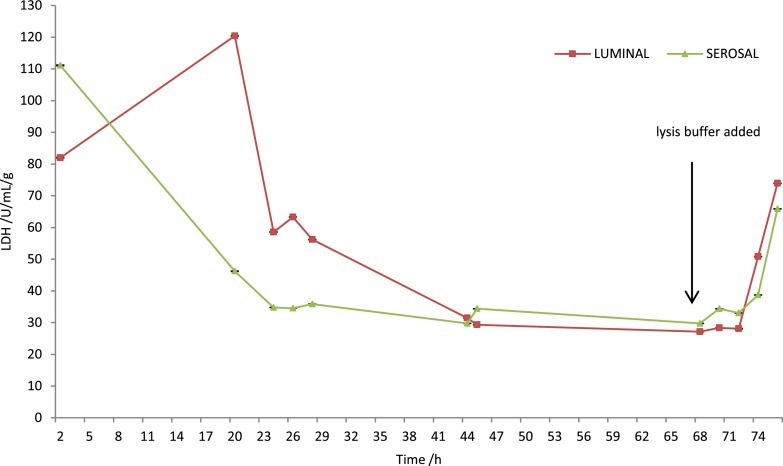
Further characterisation of the device shows that the tissue is maintained at viable levels by measuring cell death via LDH which is released from the dead and dying cells throughout the length of experiment. To show that viable tissue was present at the end of the maintenance period (68 h) tissue was lysed by the addition of buffer containing detergent (Fig. 4). In addition to the spike of LDH release following introduction of a detergent-containing solution, a relatively high release of LDH is seen within the first 24 h of the tissue being placed in the microfluidic device; this pattern of release has been observed previously when studying other tissues such as rat liver, human tumours (ovarian and head and neck carcinomas) in similar microfluidic devices. The initial high levels of LDH release are thought to be due to unavoidable damage caused by the handling of the tissue from the resection and then preparation for insertion into the device.23–26,30,31
FIG. 4.
LDH Assay of the collected effluent, lysis buffer added at 68 hours to purposely kill the tissue and ensure viability remains in tissue during the time in the microfluidic device, (n = 5).
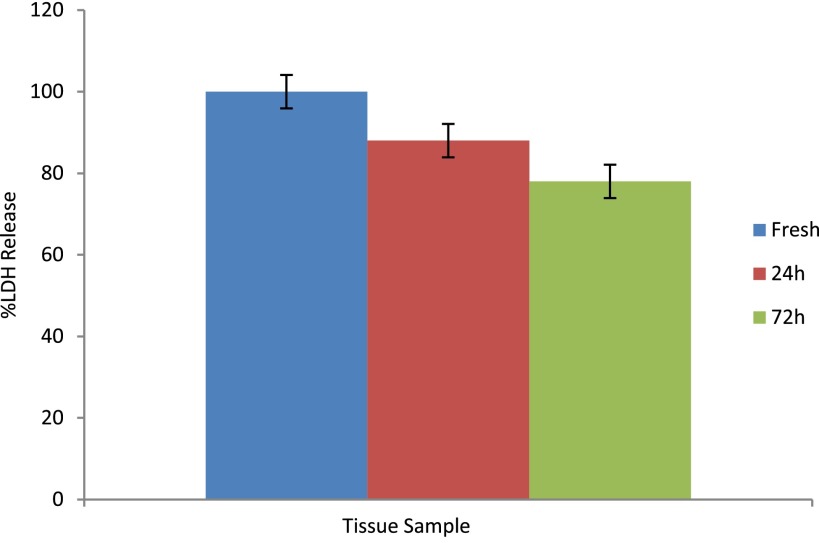
To quantify the proportion of viable cells that remained in the tissue throughout the duration of the experiment, a portion of the tissue taken from patients was immediately put into 10% v/v lysis solution for 24 h, and the total LDH release was measured. Similarly, tissue which had been in the device for 24 or 72 h was also treated in this way, and the total amounts released were quantified (Fig. 5). The LDH release given after 24 and 72 h was expressed as a percentage of the initial concentration. These experiments show that almost 80% of the tissue remains viable having been maintained in the dual flow device for 3 days.
FIG. 5.
Total LDH release comparing fresh tissue (blue) taken from patient, 24 h (red) in dual device and 72 h (green) in dual flow device (n = 5).
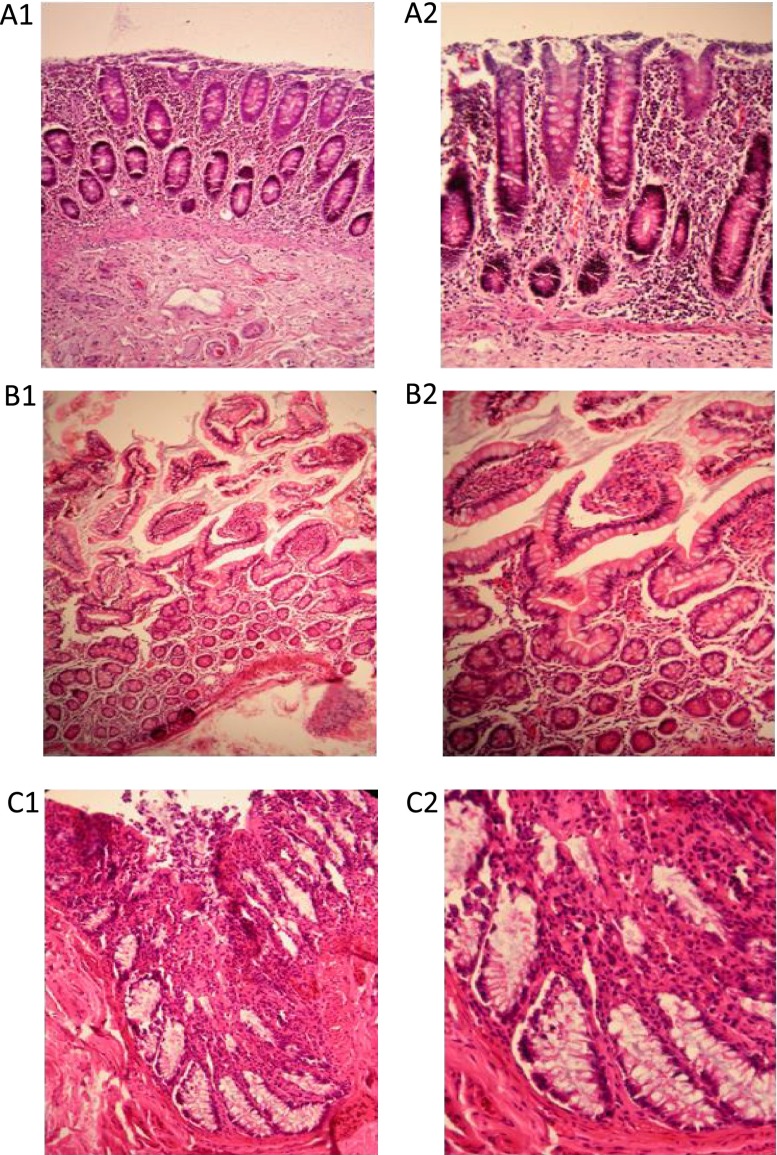
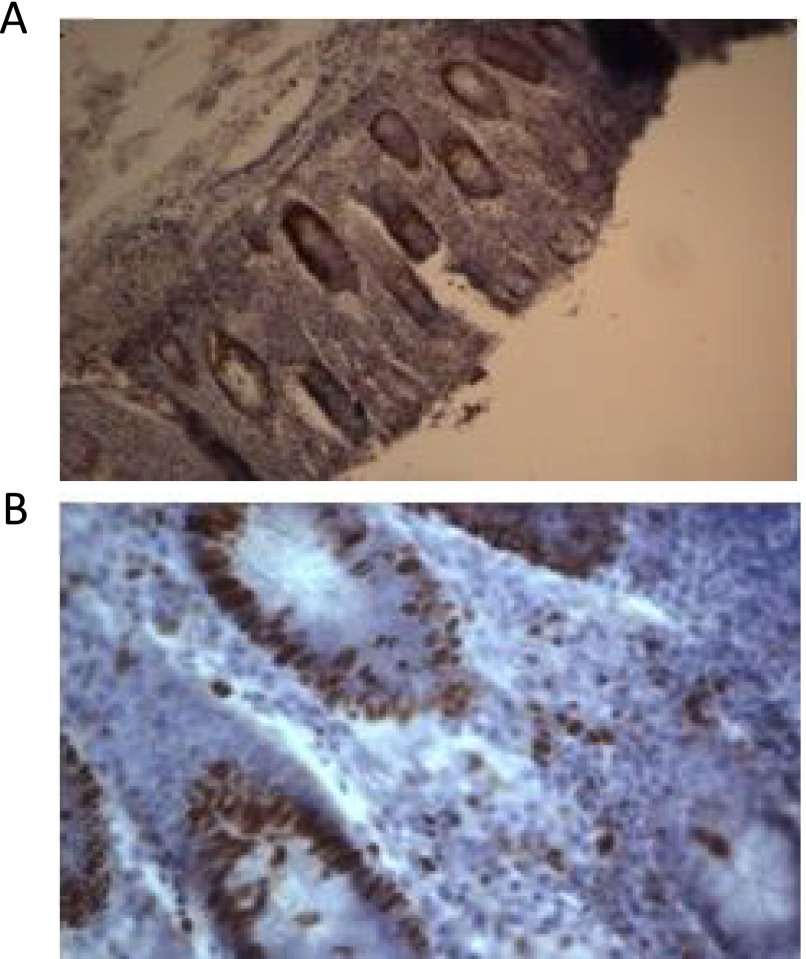
Viability was further evaluated by studying the architecture of the tissue, before and after being maintained in the device using H&E staining. A Consultant Histopathologist (LK) reviewed the stained sections taken immediately from the resection specimen (fresh) and after 72 h of on-chip maintenance. The crypts and goblet cells in the lumen of the tissue were found intact in both small and large bowel and overall relatively little changes were seen; it was still possible to histologically report the inflammation using the biopsy recovered from the device (Fig. 6). In addition to demonstrating retention of architecture for the duration of the time spent in the device, cell proliferation via Ki-67 staining was also studied with the proliferating cells possessing a brown-staining nuclei. This analysis again showed that the structures of the large bowel remained identifiable and also that proliferating cells were found in the tissue after 72 h of perfusion on the device (Fig. 7).
FIG. 6.
H&E staining of IBD small and large bowel tissue stained prior to (A1 and A2) and after maintenance for 72 h (B1, B2 and C1, C2) in the dual flow device. All tissues were fixed and sectioned (5 μm), (representative of 10 independent patient samples). A1: IBD large bowel tissue (fresh; immediately fixed) ×20, A2: IBD large bowel tissue (fresh; immediately fixed) ×40, B1: IBD small bowel tissue ×20 magnification (on-chip), B2: IBD small bowel tissue ×40 (on-chip), C1: IBD large bowel tissue ×40 (on-chip), C2: IBD large bowel tissue ×100 (on-chip).
FIG. 7.
Ki-67 Staining of tissues before being in the device (Panel A) ×20 and after being in the device for 72 h (Panel B) ×100 (representative of 4 independent samples) to show the remains identifiable and also proliferation (shown by staining as brown spots) is still present in the tissue after having been in the device for 72 h.
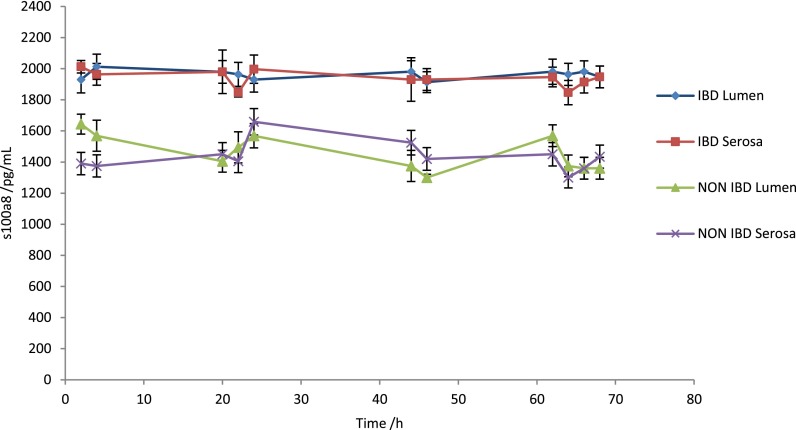
IBD is characterised by chronic inflammation and therefore having shown that biopsies remained viable in the device an analysis of the effluent was undertaken for the determination of the concentration of Calprotectin (s100a8). This factor is commonly used as a marker for inflammation in serum or plasma. Increased levels of calprotectin were seen in all the IBD samples over a 70 h period when compared with the control samples, i.e., healthy bowel tissue (Fig. 8). Interestingly, no difference in levels was observed over time, and the levels were similar in both the luminal and stromal effluents.
FIG. 8.
ELISA analysis measured off-chip of calprotectin (s100a8) levels measured in the effluents collected from the dual flow device over a 70 h period, (n = 5).
A common question with any new model is its robustness and reliability. The dual-flow device described herein can be set-up within 20 min and has a 79% success rate (36/46 devices with samples from 22 patients) of generating data, which could be used clinically e.g., the s100a8 data, LDH release for maintenance of the tissue and the staining methods all aid in diagnosis of disease. Current methods which use human or animal intestinal tissue rely on methods which do not fully incorporate the complex system of the gut e.g., lumen-microbe interaction.32 The Ussing chamber's main limitation is that it can only maintain tissue outside of the human body for a few hours.29 However, the use of microfluidics in maintaining tumour tissue has shown that such perfused systems can potentially extend this time frame with >7 days being routine in some devices.23–26,33
As the causes of IBD remain largely unknown, many patients with the disease may develop complications which are potentially life-threatening including an increased risk of colorectal cancer.10 There is a significant morbidity and mortality in active young people requiring elective or emergency surgery for IBD, which has a significant impact on the quality of life of sufferers. However, in some cases, the disease can be effectively managed especially if enhanced prognostic tools are available. Additionally, treatment for IBD is life-long as there is currently no cure, at a cost to the NHS of about £720 million per annum; an average cost of £3000 per year per patient.34,35 It is hoped that this new microfluidic platform will allow future treatments to be more quickly tested and translated to patients. The work presented here is focused on mimicking the human system in microfluidic devices to better understand the disease and the various ways by which we can deliver targeted drug therapies. It is probable that the development of novel organ- or human-on-a-chip,36–38 such as that described here will open up new ways of testing the downstream effects seen, for example, in the kidneys or liver.39
CONCLUSIONS
In conclusion, we have developed a device which can maintain the intestinal tissue according to LDH release, H&E and Ki-67 staining for up to 72 h, after removal from a patient undergoing a bowel resection, in a functional and “normal” anatomical state. This novel device provides a human-based model for the in vitro study of IBD and the investigation of therapeutic interventions providing a better understanding of the disease which current methods of cell culture and animal work fail to replicate fully.
ACKNOWLEDGMENTS
Special thanks to Mr. Paul Hawkin, Miss Gemma Solon, and the theatre staff at Castle Hill Hospital, Cottingham, Kingston upon Hull, UK for the recruitment of patients into the study.
References
- 1. Cader M. Z. and Kaser A., “ Recent advances in inflammatory bowel disease: Mucosal immune cells in intestinal inflammation,” Gut (11), 1653–1664 (2013). 10.1136/gutjnl-2012-303955 [DOI] [PubMed] [Google Scholar]
- 2. Danese S., “ New therapies for inflammatory bowel disease: From the bench to the bedside,” Gut (6), 918–932 (2012). 10.1136/gutjnl-2011-300904 [DOI] [PubMed] [Google Scholar]
- 3. Nugent S. G., Kumar D., Rampton D. S., and Evans D. F., “ Intestinal luminal pH in inflammatory bowel disease: Possible determinants and implications for therapy with aminosalicylates and other drugs,” Gut (4), 571–577 (2001). 10.1136/gut.48.4.571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanauer S. B., “ Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities,” Inflammatory Bowel Dis. (5), S3–S9 (2006). 10.1097/01.MIB.0000195385.19268.68 [DOI] [PubMed] [Google Scholar]
- 5. Shanahan F., “ Probiotics and inflammatory bowel disease: Is there a scientific rationale?,” Inflammatory Bowel Dis. (2), 107–115 (2000). 10.1097/00054725-200005000-00007 [DOI] [PubMed] [Google Scholar]
- 6. Tsilingiri K., Barbosa T., Penna G. et al. , “ Probiotic and postbiotic activity in health and disease: Comparison on a novel polarised ex-vivo organ culture model,” Gut (7), 1007–1015 (2012). 10.1136/gutjnl-2011-300971 [DOI] [PubMed] [Google Scholar]
- 7. Ananthakrishnan A. N., Cheng S. C., Cai T. et al. , “ Serum inflammatory markers and risk of colorectal cancer in patients with inflammatory bowel diseases,” Clin. Gastroenterol. Hepatol. (8), 1342-8.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boleij A. and Tjalsma H., “ Gut bacteria in health and disease: a survey on the interface between intestinal microbiology and colorectal cancer,” Biol. Rev. (3), 701–730 (2012). 10.1111/j.1469-185X.2012.00218.x [DOI] [PubMed] [Google Scholar]
- 9. Itzkowitz S. H. and Present D. H. “ Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease,” Inflammatory Bowel Dis. (3), 314–321 (2005). [DOI] [PubMed] [Google Scholar]
- 10. Triantafillidis J. K., Nasioulas G., and Kosmidis P. A., “ Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies,” Anticancer Res. (7), 2727–2737 (2009). [PubMed] [Google Scholar]
- 11. Chassaing B., Aitken J. D., Malleshappa M., and Vijay-Kumar M., “ Dextran sulfate sodium (DSS)-induced colitis in mice,” Curr. Protoc. Immunol. (15.25), 15.25.1–15.25.14 (2014). 10.1002/0471142735.im1525s104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleiveland C. R., Hult L. T. O., Spetalen S. et al. , “ The noncommensal Bacterium Methylococcus capsulatus (Bath) ameliorates dextran sulfate (sodium salt)-induced ulcerative colitis by influencing mechanisms essential for maintenance of the colonic barrier function,” Appl. Environ. Microbiol. (1), 48–56 (2013). 10.1128/AEM.02464-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dulai P. S., Siegel C. A., Colombel J.-F., Sandborn W. J., and Peyrin-Biroulet L., “ Systematic review: Monotherapy with antitumour necrosis factor α agents versus combination therapy with an immunosuppressive for IBD,” Gut (12), 1843–1853 (2014). 10.1136/gutjnl-2014-307126 [DOI] [PubMed] [Google Scholar]
- 14. Bettinger C. J. and Borenstein J. T., “ Biomaterials-based microfluidics for engineered tissue constructs,” Soft Matter (20), 4999–5015 (2010). 10.1039/c0sm00247j [DOI] [Google Scholar]
- 15. Kim H. J. and Ingber D. E., “ Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation,” Integr. Biol. (9), 1130–1140 (2013). 10.1039/c3ib40126j [DOI] [PubMed] [Google Scholar]
- 16. Ponder A. and Long M. D., “ A clinical review of recent findings in the epidemiology of inflammatory bowel disease,” Clin. Epidemiol. , 237–247 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim H. J., Huh D., Hamilton G., and Ingber D. E., “ Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow,” Lab Chip (12), 2165–2174 (2012). 10.1039/c2lc40074j [DOI] [PubMed] [Google Scholar]
- 18. Clarke L. L., “ A guide to Ussing chamber studies of mouse intestine,” Am. J. Physiol. Gastrointest. Liver Physiol. (6), G1151–G1166 (2009). 10.1152/ajpgi.90649.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lomasney K. W. and Hyland N. P., “ The application of Ussing chambers for determining the impact of microbes and probiotics on intestinal ion transport,” Can. J. Physiol. Pharmacol. (9), 663–670 (2013). 10.1139/cjpp-2013-0027 [DOI] [PubMed] [Google Scholar]
- 20. Rodrigues E., Slobbe L., Schultz M., and Butt G., “ Chronic exposure to microbial stimuli affects the development of the intestinal epithelium in human colonic enteroids,” FASEB J. , 26–28 (2015). [Google Scholar]
- 21. Maschmeyer I., Lorenz A. K., Schimek K. et al. , “ A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents,” Lab Chip (12), 2688–2699 (2015). 10.1039/C5LC00392J [DOI] [PubMed] [Google Scholar]
- 22. Baker M., “ Tissue models: A living system on a chip,” Nature (7340), 661–665 (2011). 10.1038/471661a [DOI] [PubMed] [Google Scholar]
- 23. Hattersley S., Sylvester D., Dyer C., Stafford N., Haswell S., and Greenman J., “ A microfluidic system for testing the responses of head and neck squamous cell carcinoma tissue biopsies to treatment with chemotherapy drugs,” Ann. Biomed. Eng. (6), 1277–1288 (2012). 10.1007/s10439-011-0428-9 [DOI] [PubMed] [Google Scholar]
- 24. Hattersley S. M., Dyer C. E., Greenman J., and Haswell S. J., “ Development of a microfluidic device for the maintenance and interrogation of viable tissue biopsies,” Lab Chip (11), 1842–1846 (2008). 10.1039/b809345h [DOI] [PubMed] [Google Scholar]
- 25. Hattersley S. M., Greenman J., and Haswell S. J., “ Study of ethanol induced toxicity in liver explants using microfluidic devices,” Biomed. Microdevices (6), 1005–1014 (2011). 10.1007/s10544-011-9570-2 [DOI] [PubMed] [Google Scholar]
- 26. Hattersley S. M., Greenman J., and Haswell S. J., “ The application of microfluidic devices for viral diagnosis in developing countries,” Methods Mol. Biol. , 285–303 (2013). 10.1007/978-1-62703-134-9_19 [DOI] [PubMed] [Google Scholar]
- 27. Cuevas E., Jones D. B., and Wright D. H., “ Immunohistochemical detection of tumour growth fraction (Ki-67 antigen) in formalin-fixed and routinely processed tissues,” J. Pathol. (4), 477–478 (1993). 10.1002/path.1711690415 [DOI] [PubMed] [Google Scholar]
- 28. Najafi R. and Bernard S. M., “Use of physiologically balanced, ionized, acidic solution in wound healing,” U.S. patent US6426066 B1 (30 July 2002).
- 29. Westerhout J., van de Steeg E., Grossouw D. et al. , “ A new approach to predict human intestinal absorption using porcine intestinal tissue and biorelevant matrices,” Eur. J. Pharm. Sci. , 167–177 (2014). 10.1016/j.ejps.2014.07.003 [DOI] [PubMed] [Google Scholar]
- 30. Webster A., Dyer C. E., Haswell S. J., and Greenman J., “ A microfluidic device for tissue biopsy culture and interrogation,” Anal. Methods (8), 1005–1007 (2010). 10.1039/c0ay00293c [DOI] [Google Scholar]
- 31. Woods J., Docker P. T., Dyer C. E., Haswell S. J., and Greenman J., “ On-chip integrated labelling, transport and detection of tumour cells,” Electrophoresis (22), 3188–3195 (2011). 10.1002/elps.201100172 [DOI] [PubMed] [Google Scholar]
- 32. Schaubeck M., Clavel T., Calasan J. et al. , “ Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence,” Gut (2), 225–237 (2016). 10.1136/gutjnl-2015-309333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sylvester D., Hattersley S. M., Stafford N. D., Haswell S. J., and Greenman J., “ Development of microfluidic-based analytical methodology for studying the effects of chemotherapy agents on cancer tissue,” Current Analyt. Chem. (1), 2–8 (2013). 10.2174/1573411011309010002 [DOI] [Google Scholar]
- 34. Rubin D. T., “ The rationale and growth of advanced training in inflammatory bowel disease,” Gastroenterology (4), 696–700 (2015). 10.1053/j.gastro.2015.02.036 [DOI] [PubMed] [Google Scholar]
- 35. Ng S. C., Bernstein C. N., Vatn M. H. et al. , “ Geographical variability and environmental risk factors in inflammatory bowel disease,” Gut (4), 630–649 (2013). 10.1136/gutjnl-2012-303661 [DOI] [PubMed] [Google Scholar]
- 36. Materne E.-M., Maschmeyer I., Lorenz A. K. et al. , “ The multi-organ chip - A microfluidic platform for long-term multi-tissue coculture,” J. Vis. Exp. , e52526 (2015). 10.3791/52526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van der Meer A. D. and van den Berg A., “ Organs-on-chips: breaking the in vitro impasse,” Integr. Biol. (5), 461–470 (2012). 10.1039/c2ib00176d [DOI] [PubMed] [Google Scholar]
- 38. Williamson A., Singh S., Fernekorn U., and Schober A., “ The future of the patient-specific body-on-a-chip,” Lab Chip (18), 3471–3480 (2013). 10.1039/c3lc50237f [DOI] [PubMed] [Google Scholar]
- 39. Loskill P., Marcus S. G., Mathur A., Reese W. M., and Healy K. E., “ μOrgano: A Lego™ like plug & play system for modular multi-organ-chips,” PLoS One (10), e0139587 (2015). 10.1371/journal.pone.0139587 [DOI] [PMC free article] [PubMed] [Google Scholar]