Abstract
RATIONALE
Sinoaortic denervated (SAD) and chemically sympathectomized (SNX) rats are characterized by a decrease in arterial distensibility without hypertension and would thus be relevant for analyzing arterial wall stiffening independently of blood pressure level. The fibronectin network, which plays a pivotal role in cell matrix interactions, is a major determinant of arterial stiffness. We hypothesized that in SAD and SNX rats, arterial stiffness is increased, due to alterations of cell-matrix anchoring leading to spatial reorganization of the extracellular matrix.
METHODS
The intrinsic elastic properties of the arterial wall were evaluated in vivo by the relationship between incremental elastic modulus determined by echotracking and circumferential wall stress. The changes of cell-extracellular matrix links in the abdominal aorta were evaluated by studying fibronectin, vascular integrins receptors and ultrastructural features of the aorta by immunochemistry.
RESULTS
In both experimental conditions wall stiffness increased, associated with different modifications of cell-extracellular matrix adhesion. In SAD rats, increased media-cross sectional area was coupled with an increase of muscle cell attachments to its extracellular matrix via fibronectin and its α5-β1 integrin. In SNX rats, reduced media-cross sectional area was associated with up-regulation of αv-β3 integrin and more extensive connections between dense bands and elastic fibers despite the disruption of the elastic lamellae.
CONCLUSION
In aorta of SNX and SAD rats, a similar arterial stiffness is associated to different structural alterations. An increase in αvβ3 or α5β1 integrins together with the already reported increase in the proportion of less distensible (collagen) to more distensible (elastin) components in both models, contribute to remodeling and stiffening of the abdominal aorta.
INTRODUCTION
Increased stiffness of large arteries is a significant and independent predictor of cardiovascular (CV) diseases [1]. Arterial stiffness is evaluated by the elastic properties of the artery as a whole measured by arterial distensibility and by the elastic properties of the arterial wall material measured by the incremental elastic modulus (Einc) [2]. Arterial distensibility and Einc are then 2 complementary parameters used to describe arterial stiffness. Until now, it remains difficult to separate the causal effects of blood pressure elevation from that of the mechanical and functional properties of the arterial wall that lead to alterations of distensibility and stiffness. We have previously shown that the spontaneously hypertensive rat (SHR) is characterized by a decreased distensibility at its operational pressure compared to its normentensive control [3]. Nevertheless evaluation of the arterial wall stiffness, assessed by the elastic modulus measurement, shows that for a given level of stress, SHR and Wistar rats have similar mechanical properties. These results indicate that the decrease of distensibility observed in SHR is related to hypertension, rather than to increased stiffness of the arterial wall. Therefore, other experimental models must be used to analyze the intrinsic stiffness of the aortic tissue. Sinoaortic denervated (SAD) rats and chemically sympathectomized (SNX) rats should help to investigate this issue. Both models are characterized by a decrease in arterial distensibility without hypertension compared to their respective control [4, 5], suggesting that they would be relevant for analyzing arterial wall stiffening independently of blood pressure level.
The fibronectin (Fn) network, which plays a pivotal role in cell matrix interactions, is a major determinant of arterial stiffness [3, 6]. Fibronectin controls deposition and organization of extra cellular matrix and modulates both cell proliferation and vascular smooth muscle cell (SMC) phenotype. Thus, by increasing cell-matrix anchoring through a α5-β1-integrin, aortic fibronectin accumulation may contribute to protect the arterial wall components from the increased mechanical loads associated with hypertension in young and old SHRs [3]. The accumulation of other integrins, such as αv-β3 integrin, has also been observed in the mesenteric artery of SHR [7]. These results suggest that cell-matrix interactions, which play a major role in SMC function, are also involved in the mechanical properties of the vascular wall. To our knowledge, cell-matrix interactions have never been described in non-hypertensive models related to arterial stiffness.
We hypothesized that in SAD and SNX rats, arterial stiffness is increased, and this may be due to alterations of cell-matrix anchoring leading to spatial reorganization of the extracellular matrix. We aim to determine in SAD and SNX rats (i) the intrinsic elastic properties of the arterial wall by evaluating in vivo the relationship between Einc and circumferential wall stress, and (ii) the changes of cell-extracellular matrix links in the abdominal aorta, by studying fibronectin, vascular integrins receptors and ultrastructural features of the aorta.
MATERIALS AND METHODS
Animals
Male Wistar rats (Iffa-Credo, Fresnes, France) were used. All procedures were in accordance with institutional guidelines for animal experimentation and conformed to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health.
Sino-aortic denervation and SNX were performed as previously described [4, 5]. In brief, SAD was performed at 10 weeks of age on anesthetized rats. Sham-operated rats were used as control of SAD rats. All the rats of these groups were examined and killed at 16 weeks of age. SNX rats were sympathectomized by subcutaneous injections of 50 mg/kg guanethidine sulfate for 12 weeks, 5 times a week, from day 5 after birth. The control (CO) rats received saline injections according to the same schedule, and both sets of rats were investigated during their 13th week of age. A total of 27 rats were used for the in vivo experiments and 38 rats for immunohistochemistry and electron microscopy.
Hemodynamic investigations
Mechanical properties of the abdominal aorta were assessed by circumferential wall stress (σ) and Einc. At the end of the treatment, under pentobarbital anesthesia (60 mg/kg ip), a catheter was introduced in the lower abdominal aorta via the femoral artery for blood pressure recording. A midline laparotomy was then performed and the probe of the ultrasonic device (NIUS-01, Asulab SA) positioned 1 cm above the aortic bifurcation for recording of internal arterial diameter. Blood pressure and internal arterial diameter were then simultaneously recorded. The relationship between pressure and lumen cross-sectional area was calculated by means of an arctangent function. σ and Einc were calculated with the above-mentioned parameters and medial cross-sectional area of the aorta determined by histomorphometry as previously described [3, 6, 8].
Antibodies
The antibodies used were monoclonal mouse antibodies (mAbs) reactive with an alternatively spliced form of fibronectin, EIIIA-Fibronectin (clone IST-9, Valbiotech, France) and all FN isoforms (Total-Fn, Valbiotech, France) [3], a mouse anti-vimentin monoclonal antibody (1/400, clone V9, Dako, France), a mouse anti-actin monoclonal antibody (1/500, clone 1A4, Dako, France), a rabbit anti-integrin αv polyclonal antibody (1/250, chemicon) a rabbit anti-integrin α5 subunit polyclonal antibody (Valbiotech, France) [3].
Immunohistochemical investigation
Immunohistochemical staining was performed on fresh unfixed freeze-dried suprarenal abdominal aorta [3]. We used the indirect immunoperoxidase technique as previously described for the determination of fibronectin, the EIIIA-Fibronectin isoform and the α5 integrin [3, 9]. The determination of actin, vimentin, αv integrin were performed on a Dako automate as described elsewhere [10]. No specific staining was observed when primary antibody was omitted from the protocol (negative control). The distribution and quantification of staining were determined by computer-directed color analysis performed with the Quant’Image software (Quancoul, Talence, France) [3].
Electron microscopy
The thoracic aortas (2 SAD, 2 SNX and 5 controls) were fixed in situ by transcardiac perfusion of fixative (4% glutaraldehyde and 1% formaldehyde in 0.1M Na-cacodylate). Thin (0.5–3.0 μm) and ultrathin (100 nm) sections were cut on a plane transverse to the thickness of the media and parallel to the length of the vessel, an approach that produced approximately transverse sections of the muscle cells. The sections were stained with uranyl acetate and lead citrate and viewed in a Philips 400 microscope. Photographic montages were made, covering the thickness of the media over a length of 150–400 μm, at a magnification of 8000x. On these montages the size of nucleated muscle cell profiles was measured as well as the percentage of the cell membrane displaying dense bands and the percentage of cell membrane in contact with lamellae of elastin [8]. Ultrastructure characterization and quantification were evaluated by counting tissue points of randomly selected photographic fields and a minimum of 6 nucleated cells per rat were quantified as previously reported [8]. Quantification of elastin and collagen have been presented elsewhere [4, 5].
Statistical Analysis
All values were averaged and expressed as mean ± SEM. Unpaired Student’s t tests were performed to compare SNX and SAD rats with their respective controls for arterial and immunohistochemical parameters [3]. For statistical comparison of Einc-σ curves between groups, Einc was log transformed to generate linear relationships. Calculating the r2 of the linear regression obtained with the new parameters for each individual checked the quality of the transformation. After this transformation, we calculated the mean slopes of the curves. If the slopes were not significantly different, we compared the curves by calculating the mean wall stress at 800 kPa of Einc (σ800), a value common to all groups [11]. Differences were considered significant at values of P<0.05.
RESULTS
Hemodynamic and aortic mechanics
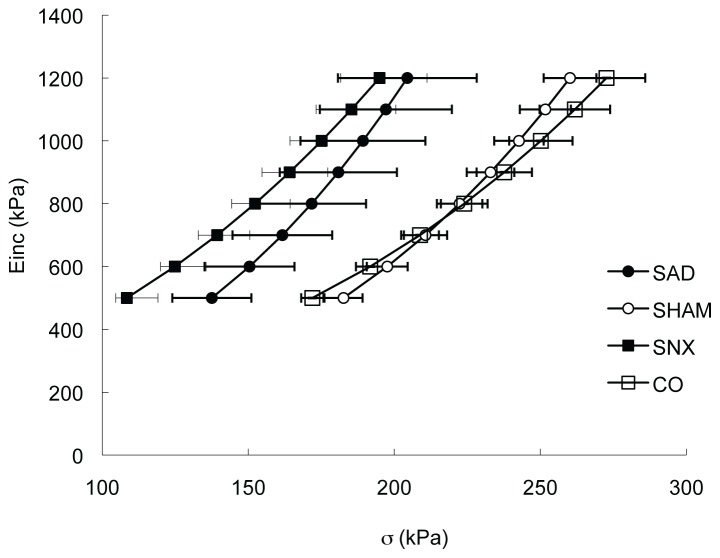
Body weight of SNX rats was significantly lower than that of control rats (303 ± 10 g vs. 353 ± 3 g, p<0.05). Likewise, SAD rats were significantly lighter than sham-operated rats (428 ± 13 g vs. 464 ± 7 g, p<0.05). Compared to their respective controls, heart rate (data not shown) and mean arterial pressure (MAP) were significantly reduced in SNX rats but remained unchanged in SAD rats. Media cross sectional area was significantly reduced in SNX rats compared to their controls; but was significantly increased in SAD rats compared to sham-operated rats. Therefore, SAD rats had significantly higher MCSA than SNX rats. Einc at MAP remained unchanged in all groups of rats. The circumferential wall stress was similar in SNX and SAD rats both at MAP and at 800 kPa of Einc but was significantly reduced when compared to their respective controls (Table 1). The Einc-wall stress curves of SNX and SAD rats were similarly shifted leftwards compared with their controls, indicating increased stiffness of the wall in both models (Figure 1).
Table 1.
Arterial properties of abdominal aorta in sinoaortic denervated (SAD) and in chemical sympathectomized (SNX) rats.
| Sham | SAD | CO | SNX | |
|---|---|---|---|---|
| Number | 8 | 8 | 6 | 5 |
| MAP, mmHg | 121 ± 2 | 116 ± 8 | 113 ± 3 | 70 ± 2*† |
| Media thickness, μm | 81 ± 1 | 79 ± 1 | 52 ± 2 $ | 44 ± 2*† |
| MCSA, mm2 | 0.31 ± 0.01 | 0.40 ± 0.03* | 0.22 ± 0.01 $ | 0.16 ± 0.01*† |
| Einc at MAP, kPa | 720 ± 80 | 760 ± 180 | 660 ± 40 | 570 ± 90 |
| σ at MAP, kPa | 208 ± 13 | 152 ± 20* | 203 ± 10 | 120 ± 12* |
| σ at Einc=800, kPa | 222 ± 8 | 172 ± 19* | 224 ± 8 | 152 ± 12* |
| Einc/σ | 204 ± 9 | 176 ± 32 | 265±32 | 228 ± 37 |
Values are mean ± SEM. CO, control of SNX rats; MAP, mean arterial pressure; MCSA, medial cross sectional area; Einc, incremental elastic modulus; σ, circumferential wall stress.
P<0.05 compared to Sham-operated or CO rats;
P<0.05 between Sham-operated rats and CO;
P<0.05 between SAD and SNX.
Figure 1.
Mean aortic Einc-wall stress curves in chronic sinoaortic denervated (SAD) and chronic sympathectomized (SNX) rats and their respective control (Sham and CO). Each point is the mean ± SEM.
Immunohistochemistry
Total-fibronectin and α5 integrin subunit staining were diffuse in the media of all control rats (Figures 2 and 3). Staining for both components was markedly increased in SAD rats compared to Sham-operated rats (Figure 2) whereas no accumulation of fibronectin and its α5 integrin were found in SNX rats compared to their controls (Figure 3).
Figure 2.
Aortic immunostaining of total fibronectin, EIIIA-fibronectin, α5 and α v integrins, smooth muscle alpha actin and vimentin of sinoaortic denervated (SAD) rats and their controls (Sham). Bottom panel presents the quantification of the immunostaning expressed in percent changes over Sham-operated rats. Each bar is the mean±SEM of 5–9 rats. * P<0.05 vs. SHAM.
Figure 3.
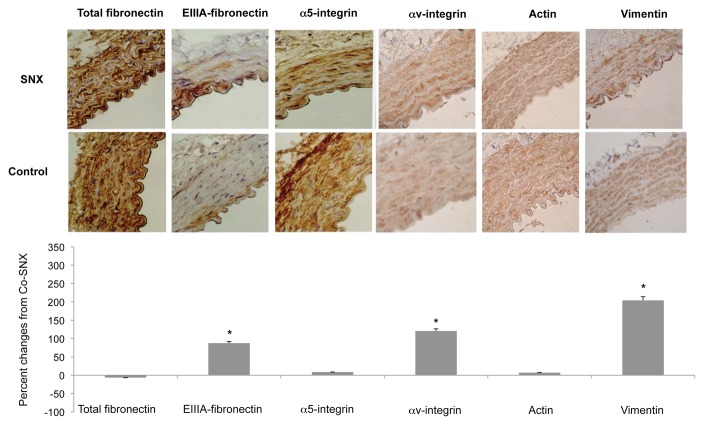
Aortic immunostaining of total fibronectin, EIIIA-fibronectin, α5 and α v integrins, smooth muscle alpha actin and vimentin of sympathectomized (SNX) rats and their controls. Bottom panel presents the quantification of the immunostaning expressed in percent changes from controls. Each bar is the mean±SEM of 5–9 rats. * P<0.05 vs. controls.
In Sham-operated and CO rats, immunoreactivity for cellular fibronectin (EIIIA-fibronectin), was observed in the inner part of the media. In SAD rats the EIIIA-fibronectin staining was equally intense but it involved the entire thickness of the media (Figure 2). A slight increased of EIII-A fibronectin was found in SNX rats compared to CO (Figure 3).
The endothelium highly expressed αv staining and the media showed relatively low but extensive expression in all control rats. In contrast with α5 integrin, αv and vimentin staining were increased in SNX rats (Figure 3) but unchanged in SAD rats (Figure 2) compared to their respective controls. Immunostaining for actin was equally intense in all conditions.
Electron Microscopy
The intima of the rats’ aortic wall is composed of a thin endothelium, sub-endothelium connective tissue and an inner elastic lamina. The endothelium of the thoracic aorta appeared similar in all groups.
The media, composed of elastic lamellae, with interposed layers of muscle cell and interconnected by elastic fibers and bundles of collagen fibrils, showed some alterations in the experimental conditions; however, the amount of elastic material in the wall appeared unchanged. In SNX rats some disruption of the elastic lamellae was observed, including the breaking up of some lamellae into large elastic bundles (Figure 4A). The muscle cells profiles, observed in transverse section, had an irregular contour, with large processes and invaginations, in all preparations. Numerous dense bands associated with actin bundles on the cytoplasmic side and with collagen and elastic fibers extracellularly, occupied the muscle cell membrane.
Figure 4.
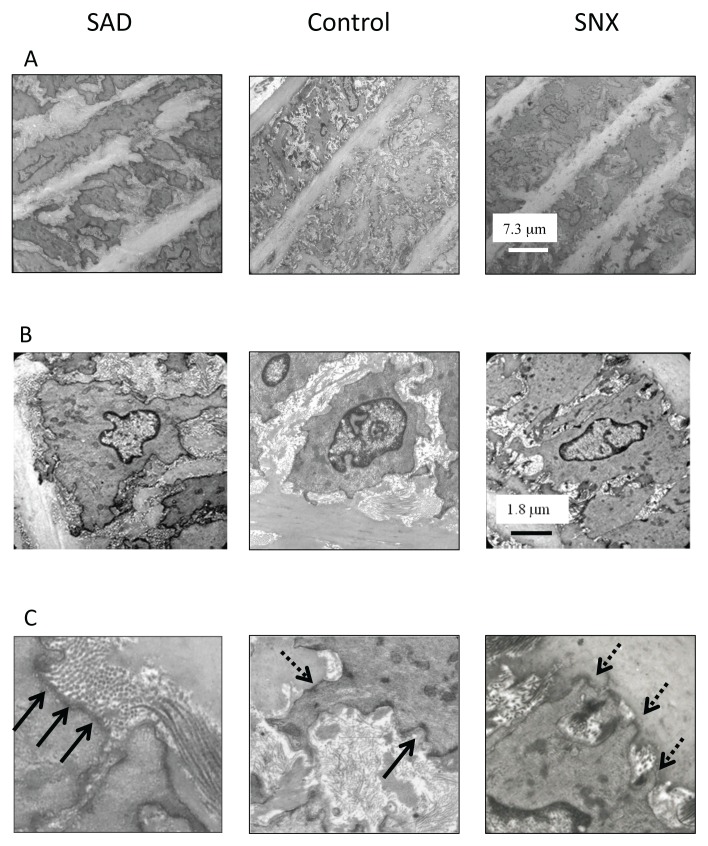
Electronic microscopy of elastic lamellae (A) and smooth muscle cell (B) of the aorta of sinoaortic denervated (SAD), control (data pooled from sham-operated and control of SNX rats) and sympathectomized (SNX) rats.
A- The elastic lamellae are bridged by elastic fibers and are separated by muscle cells and bundles of collagen fibrils. The interlamellar space, defined as the space between consecutive lamellae, is increased on average in SAD rats. The elastic lamellae are thinner and altered in SNX rats, in which some lamellae appear broken into large elastic bundles.
B- Dense bands are a prominent feature in the media of the rat aorta. In SAD rats, the percentage of cell surface occupied by dense bands is increased compared with sham-operated rats. Dense bands connected to elastic lamellae remain unchanged. In SNX rats, cell surface occupied by dense bands is well conserved. Nevertheless, the percentage of cell surface connected to the elastic lamellae is twice as high in SNX rats compared with their controls.
C- Characterization of Dense bands
Representative images of dense bands associated with collagen (full arrows) and elastic fibers (dashed arrows)
Despite the restricted number of animals used per groups, results observed were quite reproducible as already validated [8] and allow pooling data from sham-operated rats and control of SNX rats. In control rats, dense bands occupied 42 ± 2 % of the cell perimeter and half of it (20% ± 2 %) was connected to elastic fibers. The number of dense bands appeared obviously increased in SAD rats (57% ± 3 %; p<0.05) compared with controls but not in SNX rats (45% ± 2 %). In the latter there were more extensive connections between dense bands and elastic fibers than in controls (30 ± 2 %; p<0.05). The percentage of dense bands connected to elastic lamellae remained unchanged in SAD rats (Figure 4B).
DISCUSSION
In the present study, structural changes of the abdominal aorta were evaluated in SNX and SAD rats, two models of decreased arterial distensibility without hypertension. We observed an increase in wall stiffness in both experimental conditions, but different structural changes in the vessel wall. In SAD rats, aortic hypertrophy was coupled with an increase of muscle cell attachments to its extracellular matrix via fibronectin and its α5-β1 integrin. In SNX rats, aortic hypotrophy was associated with αv-β3 integrin up-regulation and alteration of elastin fibers.
In contrast to acute treatment, chronic treatment with guanethine significantly reduced blood pressure as already reported [5, 12]. This effect may be due to the succession of many hypotensive episodes, previously reported in this model [13]. A weight loss was also observed as previously reported in both models [12].
We have previously shown a similar reduction of carotid distensibility in SAD and SNX rats [4, 5]. While arterial distensibility is an indicator of the elastic properties of the artery as a hollow structure, the Einc expresses the elastic properties of the wall material that is independent of wall intima-media thickness [6]. Enhanced aortic stiffness is a significant and independent risk factor for all-cause and cardiovascular mortality [1], primarily coronary heart disease [14] and stroke [15] in human. Thus, elaboration of Einc/stress curves addresses arterial wall stiffness, independently of the wall thickness and of the pressure level. To our knowledge, stiffness of the arterial wall material had never been evaluated and compared in SNX and SAD rats. Therefore, the first new finding of the present experiments is that SNX and SAD rats are characterized by a similar increase in arterial wall stiffness (leftwards shift of the Einc-stress curves).
We have previously shown that in SHRs, the Einc of the aortic wall material, determined for a given level of circumferential wall stress, was not significantly different from that of Wistar rats. This indicates that arterial wall materials in SHR and its control strain have similar mechanical behavior [3]. In contrast, the increased stiffness of the arterial observed in the present study suggest that SNX and SAD rats seem pretty relevant models for analyzing arterial remodeling associated with stiffness. The second new finding of the present study derived from the characterization of extracellular matrix changes in both models. Extracellular matrix proteins determine the passive biomechanical properties, collagen providing tensile strength and elastin enabling vascular elasticity [16]. Indeed we and others have shown a strong relationship between decreased elastin/collagen ratio and arterial stiffness in both models indicating an alteration in the organization of the ECM [4, 5, 17]. However, because SNX is characterized by reduced MCSA (present results) with a predominant reduction in elastin [5], and SAD by an increased MCSA (present results) and a predominant increase in collagen [4], a different structure-function relationship is present in the two experimental conditions.
The dense bands of muscle cells provide a link between contractile apparatus and extracellular matrix, mediated by integrin receptors on the cell membrane [8, 18, 19]. In rat aorta, the major integrins ligands are fibronectin, a glycoprotein that plays an important role in the organization and assembly of the extracellular matrix, collagen and laminin. Accumulation of collagen in the aorta of SAD rats is associated with accumulation of total-fibronectin and its α5β1-integrin receptor, indicating an increased mechanical linking/coupling between muscle cells and extracellular matrix [19–21]. Alteration of cell-matrix attachments might thus contribute to increase arterial stiffness, as already reported in SHRs [3]. This result is strengthened by the obvious ultrastructural changes of the aorta shown in the present study where the number of dense bands per muscle cell profile is enhanced. In SAD rats, extracellular matrix composition is also characterized by an accumulation of EIIIA-fibronectin, up regulated during hypertension and aging [3, 21, 22], and closely associated with arterial stiffness [3, 6, 8, 23].
Despite the disruption of the elastic lamellae, we also observed an increase of cell-elastin connections and accumulation of αvβ3 integrin and vimentin in SNX rats. It is now well established that many αvβ3 integrin-rich focal are associated with vimentin intermediate filament cytoskeletons in parallel [24, 25]. Therefore, the accumulation of vimentin observed in SNX rats is in good agreement with αvβ3 integrin up-regulation. We and others have already observed enhancement of ultrastructural connections of smooth muscle with elastin in rat vessels, as reported in the present study [8, 26]. We suggest that αvβ3 integrin accumulation is mirrored by increase in the spatial density of dense bands observed. It should contribute to add strength to the structure of the vascular wall through focal attachments of vascular SMC with extracellular matrix. Aside from acting as a physical joint, αvβ3 integrins may also promote vascular remodeling. The isolated increase of EIIIA-fibronectin associated with αvβ3 and vimentin accumulation, already reported in hypertensive rats, is associated with eutrophic inward remodeling of small arteries [27]. It is well established that arterial total-fibronectin content increases with increased arterial pressure. Nevertheless, the small increase of EIII-A fibronectin observed is independent of the blood pressure level, as guanethine significantly reduced blood pressure in SNX rats compared to control. Our data support the concept that sympathectomy favors the expression of the immature phenotype of smooth muscle [28–30].
Beside hypertension and vascular disease such as atherosclerosis, increase blood pressure variability might be a possible mechanism of increase arterial stiffness as recently reported in human [31–33] and rats [15]. Indeed, we and other have shown that both models are characterized by an increase in blood pressure variability [4, 5, 12, 34]. Blood pressure variability leads to the mechanical process of fatigue, which might be buffered by modification in cell to matrix interactions. This contributes to the maintenance of aortic structure through morphological changes that take place in the vessel wall. The activation of the renin-angiotensin system and the central noradrenergic neurons described after long-term sino-aortic denervation [35], lead to vascular hypertrophy through fibronectin-α5 integrin complex. In the opposite, SNX rats are characterized by aortic catecholamine depletion after chemical sympathectomy [36]. Arterial wall hypotrophy is associated with serious alterations of the vessel integrity and elastin alteration as widely observed with aging [37], despite the up-regulation of αvβ3 integrin [38].
The presented data show the interplay between structure and mechanics of abdominal aorta in SNX and SAD rats. In the 2 models, increase in αvβ3 or α5β1 integrins together with the already reported increase in the proportion of less distensible (collagen) to more distensible (elastin) components plays a key role in remodeling and stiffening of the abdominal aorta.
Acknowledgments
We thank Carlos Labat, Huguette Louis and Véronique Reygnault for fruitful discussions. We also want to thank Marie Paul Boscher for her help and support for immunochemistry analysis.
Footnotes
Disclosure: No disclosure
Conflict of interest: None
References
- 1.Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001 May;37(5):1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 2.Pannier BM, Avolio AP, Hoeks A, Mancia G, Takazawa K. Methods and devices for measuring arterial compliance in humans. Am J Hypertens. 2002 Aug;15(8):743–53. doi: 10.1016/s0895-7061(02)02962-x. [DOI] [PubMed] [Google Scholar]
- 3.Bezie Y, Lamaziere JM, Laurent S, Challande P, Cunha RS, Bonnet J, et al. Fibronectin expression and aortic wall elastic modulus in spontaneously hypertensive rats. Arteriosclerosis, thrombosis, and vascular biology. 1998 Jul;18(7):1027–34. doi: 10.1161/01.atv.18.7.1027. [DOI] [PubMed] [Google Scholar]
- 4.Lacolley P, Bezie Y, Girerd X, Challande P, Benetos A, Boutouyrie P, et al. Aortic distensibility and structural changes in sinoaortic-denervated rats. Hypertension. 1995 Aug;26(2):337–40. doi: 10.1161/01.hyp.26.2.337. [DOI] [PubMed] [Google Scholar]
- 5.Lacolley P, Glaser E, Challande P, Boutouyrie P, Mignot JP, Duriez M, et al. Structural changes and in situ aortic pressure-diameter relationship in long-term chemical-sympathectomized rats. The American journal of physiology. 1995 Aug;269(2 Pt 2):H407–16. doi: 10.1152/ajpheart.1995.269.2.H407. [DOI] [PubMed] [Google Scholar]
- 6.Lacolley P, Labat C, Pujol A, Delcayre C, Benetos A, Safar M. Increased carotid wall elastic modulus and fibronectin in aldosterone-salt-treated rats: effects of eplerenone. Circulation. 2002 Nov 26;106(22):2848–53. doi: 10.1161/01.cir.0000039328.33137.6c. [DOI] [PubMed] [Google Scholar]
- 7.Intengan HD, Thibault G, Li JS, Schiffrin EL. Resistance artery mechanics, structure, and extracellular components in spontaneously hypertensive rats: effects of angiotensin receptor antagonism and converting enzyme inhibition. Circulation. 1999 Nov 30;100(22):2267–75. doi: 10.1161/01.cir.100.22.2267. [DOI] [PubMed] [Google Scholar]
- 8.Bezie Y, Lacolley P, Laurent S, Gabella G. Connection of smooth muscle cells to elastic lamellae in aorta of spontaneously hypertensive rats. Hypertension. 1998 Jul;32(1):166–9. doi: 10.1161/01.hyp.32.1.166. [DOI] [PubMed] [Google Scholar]
- 9.Louis H, Kakou A, Regnault V, Labat C, Bressenot A, Gao-Li J, et al. Role of alpha1beta1-integrin in arterial stiffness and angiotensin-induced arterial wall hypertrophy in mice. American journal of physiology. 2007 Oct;293(4):H2597–604. doi: 10.1152/ajpheart.00299.2007. [DOI] [PubMed] [Google Scholar]
- 10.Molinie V, Balaton A, Rotman S, Mansouri D, De Pinieux I, Homsi T, et al. Alpha-methyl CoA racemase expression in renal cell carcinomas. Human pathology. 2006 Jun;37(6):698–703. doi: 10.1016/j.humpath.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Mercier N, Osborne-Pellegrin M, El Hadri K, Kakou A, Labat C, Loufrani L, et al. Carotid arterial stiffness, elastic fibre network and vasoreactivity in semicarbazide-sensitive amine-oxidase null mouse. Cardiovascular research. 2006 Nov 1;72(2):349–57. doi: 10.1016/j.cardiores.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Johnson EM, Jr, O’Brien F. Evaluation of the permanent sympathectomy produced by the administration of guanethidine to adult rats. The Journal of pharmacology and experimental therapeutics. 1976;196(1):53–61. [PubMed] [Google Scholar]
- 13.Julien C, Kandza P, Barres C, Lo M, Cerutti C, Sassard J. Effects of sympathectomy on blood pressure and its variability in conscious rats. The American journal of physiology. 1990 Nov;259(5 Pt 2):H1337–42. doi: 10.1152/ajpheart.1990.259.5.H1337. [DOI] [PubMed] [Google Scholar]
- 14.Alarhabi AY, Mohamed MS, Ibrahim S, Hun TM, Musa KI, Yusof Z. Pulse wave velocity as a marker of severity of coronary artery disease. Journal of clinical hypertension (Greenwich, Conn. 2009 Jan;11(1):17–21. doi: 10.1111/j.1751-7176.2008.00061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke; a journal of cerebral circulation. 2003 May;34(5):1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- 16.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiological reviews. 2009 Jul;89(3):957–89. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li ZY, Xu TY, Zhang SL, Zhou XM, Xu XW, Guan YF, et al. Telemetric Ambulatory Arterial Stiffness Index, a Predictor of Cardio-Cerebro-Vascular Mortality, is Associated with Aortic Stiffness-Determining Factors. CNS neuroscience & therapeutics. 2013 May 22; doi: 10.1111/cns.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacolley P, Safar ME, Regnault V, Frohlich ED. Angiotensin II, mechanotransduction, and pulsatile arterial hemodynamics in hypertension. American journal of physiology. 2009 Nov;297(5):H1567–75. doi: 10.1152/ajpheart.00622.2009. [DOI] [PubMed] [Google Scholar]
- 19.Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovascular research. 2001 Dec;52(3):372–86. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- 20.Martinez-Lemus LA, Hill MA, Meininger GA. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda, Md. 2009 Feb;24:45–57. doi: 10.1152/physiol.00029.2008. [DOI] [PubMed] [Google Scholar]
- 21.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12(2):165–75. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takasaki I, Chobanian AV, Mamuya WS, Brecher P. Hypertension induces alternatively spliced forms of fibronectin in rat aorta. Hypertension. 1992 Jul;20(1):20–5. doi: 10.1161/01.hyp.20.1.20. [DOI] [PubMed] [Google Scholar]
- 23.Kakou A, Bezie Y, Mercier N, Louis H, Labat C, Challande P, et al. Selective reduction of central pulse pressure under angiotensin blockage in SHR: role of the fibronectin-alpha5beta1 integrin complex. Am J Hypertens. 2009 Jul;22(7):711–7. doi: 10.1038/ajh.2009.87. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, et al. Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. Journal of cell science. 2009 May 1;122(Pt 9):1390–400. doi: 10.1242/jcs.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuruta D, Jones JC. The vimentin cytoskeleton regulates focal contact size and adhesion of endothelial cells subjected to shear stress. Journal of cell science. 2003 Dec 15;116(Pt 24):4977–84. doi: 10.1242/jcs.00823. [DOI] [PubMed] [Google Scholar]
- 26.Clark JM, Glagov S. Structural integration of the arterial wall. I. Relationships and attachments of medial smooth muscle cells in normally distended and hyperdistended aortas. Laboratory investigation; a journal of technical methods and pathology. 1979 May;40(5):587–602. [PubMed] [Google Scholar]
- 27.Heerkens EH, Shaw L, Ryding A, Brooker G, Mullins JJ, Austin C, et al. alphaV integrins are necessary for eutrophic inward remodeling of small arteries in hypertension. Hypertension. 2006 Feb;47(2):281–7. doi: 10.1161/01.HYP.0000198428.45132.02. [DOI] [PubMed] [Google Scholar]
- 28.Hachani R, Dab H, Sakly M, Vicaut E, Callebert J, Sercombe R, et al. Influence of antagonist sensory and sympathetic nerves on smooth muscle cell differentiation in hypercholesterolemic rat. Auton Neurosci. 2010 Jun 24;155(1–2):82–90. doi: 10.1016/j.autneu.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Kacem K, Seylaz J, Issertial O, Aubineau P. Chemical sympathectomy favours vimentin expression in arterial smooth muscle cells of young rats. Journal of the autonomic nervous system. 1995 May 17;53(1):57–68. doi: 10.1016/0165-1838(94)00165-g. [DOI] [PubMed] [Google Scholar]
- 30.Damon DH. Sympathetic innervation promotes vascular smooth muscle differentiation. American journal of physiology. 2005 Jun;288(6):H2785–91. doi: 10.1152/ajpheart.00354.2004. [DOI] [PubMed] [Google Scholar]
- 31.Schillaci G, Bilo G, Pucci G, Laurent S, Macquin-Mavier I, Boutouyrie P, et al. Relationship between short-term blood pressure variability and large-artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012 Aug;60(2):369–77. doi: 10.1161/HYPERTENSIONAHA.112.197491. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Garcia A, Garcia-Ortiz L, Recio-Rodriguez JI, Patino-Alonso MC, Agudo-Conde C, Rodriguez-Sanchez E, et al. Relationship of 24-h blood pressure variability with vascular structure and function in hypertensive patients. Blood pressure monitoring. 2013 Apr;18(2):101–6. doi: 10.1097/MBP.0b013e32835ebc58. [DOI] [PubMed] [Google Scholar]
- 33.Giannattasio C, Failla M, Hennig M, Hollweck R, Laurent S, Mallion JM, et al. Different relation between 24-h blood pressure and distensibility at different peripheral arteries. Data from the European Lacidipine Study on Atherosclerosis (ELSA) Journal of hypertension. 2005 Mar;23(3):557–62. doi: 10.1097/01.hjh.0000160212.33232.3e. [DOI] [PubMed] [Google Scholar]
- 34.Krieger . Neurogenic hypertension in the rat. In: JD, editor. Handbook of Hypertension: Experimental and Genetic Models of Hypertension. New York: Elsevier; 1984. pp. 350–63. [Google Scholar]
- 35.Shan ZZ, Dai SM, Fang F, Su DF. Changes of central norepinephrine, beta-endorphin, LEU-enkephalin, peripheral arginine-vasopressin, and angiotensin II levels in acute and chronic phases of sino-aortic denervation in rats. Journal of cardiovascular pharmacology. 2004 Feb;43(2):234–41. doi: 10.1097/00005344-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Facemire CS, Banes AJ, Faber JE. Different alpha-adrenoceptors mediate migration of vascular smooth muscle cells and adventitial fibroblasts in vitro. American journal of physiology. 2002 Jun;282(6):H2364–70. doi: 10.1152/ajpheart.00858.2001. [DOI] [PubMed] [Google Scholar]
- 37.Hodis S, Zamir M. Mechanical events within the arterial wall: The dynamic context for elastin fatigue. Journal of biomechanics. 2009 May 29;42(8):1010–6. doi: 10.1016/j.jbiomech.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Yang JT, Hynes RO. Fibronectin receptor functions in embryonic cells deficient in alpha 5 beta 1 integrin can be replaced by alpha V integrins. Molecular biology of the cell. 1996 Nov;7(11):1737–48. doi: 10.1091/mbc.7.11.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]