Abstract
Mitral regurgitation (MR) is the second most common form of valvular disease requiring surgery. Correct identification of surgical candidates and optimising the timing of surgery are key in management. For primary MR, this relies upon a balance between the peri-operative risks and rates of successful repair in patients undergoing early surgery when asymptomatic with the potential risk of irreversible left ventricular dysfunction if intervention is performed too late. For secondary MR, recognition that this is a highly dynamic condition where MR severity may change is key, although data on outcomes in determining whether concomitant valve intervention is performed with revascularisation has raised questions regarding timing of surgery. There has been substantial interest in the use of stress echocardiography to risk stratify patients in mitral regurgitation. This article reviews the role of stress echocardiography in both primary and secondary mitral regurgitation and discusses how this can help clinicians tackle the challenges of this prevalent condition.
Keywords: stress echocardiography, mitral valve repair, mitral valve replacement, mitral regurgitation, timing of surgery
Introduction
Mitral regurgitation (MR) is the second most common type of valve disease requiring surgery in Europe (1). Despite a reduction in the incidence of rheumatic heart disease, the frequency of MR is increasing due to an ageing population (2). Improvements in the diagnosis, quantification and operative techniques for mitral valve (MV) repair now allow the restoration of normal life expectancy after surgery (3). The timing and type of surgery depend upon a number of factors, one of the most important being whether the MR is primary or secondary (Videos 1 and 2). The balance in each case then lies between facing the risks of early surgery and risking left ventricular (LV) dysfunction if intervention is performed too late. In addition, consideration has to be given to the risk of mitral valve replacement in case of immediate failure of repair and the risk of re-intervention in the situation of late failure of repair. Unfortunately, a significant proportion of those with severe MR who have apparently normal LV function pre-operatively (LVEF >60%) continue to present post-operatively with reduced ejection fraction and congestive cardiac failure (4). This article outlines the discussion surrounding the timing of surgery and highlights the importance of exercise stress echocardiography in the management of primary and secondary MR.
Primary mitral regurgitation with flail A2 scallop of the anterior mitral valve leaflet. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-1.
Download Video 1 (990KB, avi)
Secondary ischaemic mitral regurgitation (asymmetric). View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-2.
Download Video 2 (910KB, avi)
Controversy in the timing of surgery for primary MR
Class 1 indications for surgery in primary MR have been unchanged for many years (5). In recent years, this ‘conventional’ approach has been challenged by advocates for early MV repair who have come to regard standard class I triggers such as heart failure and LV dysfunction as promoting ‘rescue surgery’ (6). Data to support this view originated in operative series from the 1980s, which highlighted improved surgical outcomes in patients operated with NYHA class I–II rather than NYHA III–IV symptoms (3). In the modern era, several observational series have consistently demonstrated adverse outcomes for each of the individual components of the current class I surgical indications. In a prospective surgical series of 840 patients with MR amenable to repair, worsening NYHA classification was associated with a stepwise reduction in late survival rates 20 years after surgery (7). In a retrospective case registry, mortality was increased by 80% at 10-year follow-up once EF had fallen to 50–59% compared with those in whom LVEF remained above 60% (8). Furthermore, LV dilatation above 40 mm in end-systole in primary MR predicted excess mortality and was an independent predictor of permanent post op LV dysfunction (9). Summarising the risks of delay to repair, a retrospective of 1512 patients undergoing isolated primary MR at the Rochester Mayo clinic between 1990 and 2000 found long-term survival at 15 years was only 42 ± 2% for patients with a class I indication, compared with 53 ± 4% for those with a class II surgical indication including presence of atrial fibrillation or pulmonary hypertension, with the highest survival (70 ± 3%) observed in subjects receiving early operation based on the presence of a high probability of successful repair. Furthermore, operative mortality was only documented in those with a class I indication for surgery (6). These data have led authors to suggest that current class 1 recommendations are criteria that do not promote optimal outcomes for patients with severe primary MR.
Outcomes of mitral repair in primary MR
Registry studies have established that the results of MV repair are superior to replacement, even in the elderly (10). Prosthetic valve replacement is associated with higher operative mortality, reduced life expectancy, higher long-term risk of stroke and complications specific to valve replacement such as valve thrombosis and structural valve degeneration (11). If repair can be successfully performed before the onset of advanced symptoms, data from expert centres report equivalent long-term outcomes to age- and gender-matched controls at >20-year follow-up (7). Both prospective and registry data support early repair before class 1 indications are reached. A single-centre prospective study of 610 consecutive patients with asymptomatic severe MR diagnosed with quantitative echocardiography compared outcomes between those referred for early surgery (235 patients; 94% repair rate) and those referred with conventional class 1 indications (375 patients; 82% repair rate), with the decision made at the discretion of the referrer. At a follow-up of 12 years, the early surgery group had significantly lower cardiac mortality (HR 0.109; 95% confidence interval (CI) 0.014–0.836; P = 0.033) and cardiac event rates (HR 0.216; 95% CI 0.083–0.558; P = 0.002) (12). The multi-centre, multi-national Mitral Regurgitation International Database included 2097 consecutive patients with primary MR due to flail segments and found improved survival at 10 years with lower rates of heart failure for those proceeding with early mitral repair compared with those managed medically until class 1 guidelines were triggered (13).
Mitral repair as a class 2A indication for surgery
Given the consistency of these data, why do current guidelines (5) not emphasise the importance of earlier repair for all patients with severe, degenerative MR? First, the data supporting early surgery are mostly from single-centre, non-randomised studies and many findings are from retrospective registries. These have tended to be high-volume centres with specialised, experienced surgeons performing large numbers of MV repairs. Such data on rates of repair and lower perioperative morbidity and mortality cannot always be extrapolated to lower-volume centres (14, 15). Randomised prospective studies in mixed populations are lacking; however, trials are under way (16). Secondly, the benefits of such long-term outcomes are mainly reserved for those with primary degenerative disease (Carpentier mechanism type II) and outcomes are less consistent for other causes, including rheumatic disease (17). Thirdly, it can be more difficult to persuade an asymptomatic patient in clinic to undergo major cardiothoracic surgery – by definition, prophylactic surgery in asymptomatic individuals does not improve how they feel (although this is not an issue in conditions when mortality benefit is clear, for example aortic aneurysm surgery). Finally, there are also data that suggest careful outpatient care may deliver outcomes that are as good. In a study of 132 consecutive patients with asymptomatic severe MR, a programme of annual review with referral based on class 1 indications also delivered outcomes equivalent to the general population over a follow-up period of 62 ± 26 months – but with the added advantage that 55 ± 6% of the population were able to avoid surgery completely at 8-year follow-up without complication (18). Of equal importance was that surgical outcomes were also excellent, with no compromise in symptomatic status or LV outcome from delay.
Moreover, referral for early surgery may often not be a straightforward decision – even with quantitative echocardiography, grading severe MR is subject to significant variation between operators (19). When severe MR is confirmed, the ability to identify a reparable MV is not perfect. Although in the US there has been a significant improvement in rate of repair with promotion of the ‘mitral valve surgeon’ and discouragement of lower volume centres so that repair is performed in excess of 90% cases (20), this is not universal across all surgeons and in all countries. In the randomised prospective study of Kang and coworkers, recruiting only those with presumed reparable valves, repair was actually carried out in 94% (12). In the UK, audit data from the National Institute for Cardiovascular Outcomes Research (NICOR) identified that 1558 isolated first-time MV repairs were carried out in 2013, compared with 789 isolated MV replacements. The 1-year and 5-year mortality rates for isolated MV repair in 2013 were 1.1% and 11% respectively, but double for MV replacement at 2.4% and 20% (21). Such diverse surgical practice is of major concern, especially when considering the higher mortality and morbidity with MV replacement compared with MV repair and has prompted a call for defined centres of excellence for mitral valve repair in the UK with the presence of a dedicated heart valve team (22). Finally, MV repair is not risk free. In the UK, NICOR data reported mortality in 2013 for the first time, isolated MV repair at 1.09% and 2.79% when combined with coronary artery bypass grafting (CABG) (21). Furthermore, although actual survival far exceeds expected outcomes (5.19%), MR can still recur after repair. In the most recent series, rates of recurrent MR were 13.3 + 1.2% patients at 15-year follow-up, with a reoperation rate of 6.9 + 1.0% (23). Although serial data from this study suggest that rates of late failure are falling, possibly as a result of technical improvements such as routine use of ring annuloplasty and peri-operative 3D transoesophageal echocardiography, recurrence of MR after MV repair is associated with adverse LV remodelling and increased risk of death (23). For an asymptomatic patient to run this risk, there has to be certainty for that person that his or her own operative risk is low, that valve repair will be durable and that their own life expectancy will be long enough to benefit in the long term from any prognostic gain.
Stress echocardiography: improving risk stratification in primary MR
In addition to markers of adverse outcome that include the onset of atrial fibrillation (24), pulmonary hypertension (6) and left atrial dilatation (25), objective testing of symptom status is a critical step in decision making. Studies in those with severe aortic stenosis have emphasised that patients often minimise their symptoms by avoiding exercise and that objective testing may reveal limitations unsuspected on history alone. Similarly, in severe ‘asymptomatic’ MR, 20% have a sub-maximal functional capacity on cardiopulmonary exercise testing (26). Event-free survival is lower in those asymptomatic patients with severe primary MR who have a reduced exercise capacity despite good LV function and normal LV dimensions (27). In a large study of 884 consecutive patients undergoing exercise stress echocardiography, exercise capacity (lower than 100% age/sex predicted metabolic equivalents (METs) achieved), heart rate recovery (HRR, <18 beats within 1 min after exercise) after stress- and exercise-induced atrial fibrillation were strong independent markers of adverse outcome in primary MR (28). These data emphasise the importance of monitoring apparently asymptomatic patients using a regular exercise test to provide objective assessment of symptom status. In those with no exercise limitation according to age- and gender-based predicted metabolic equivalents, delay in surgery does not impair outcome at one year (29).
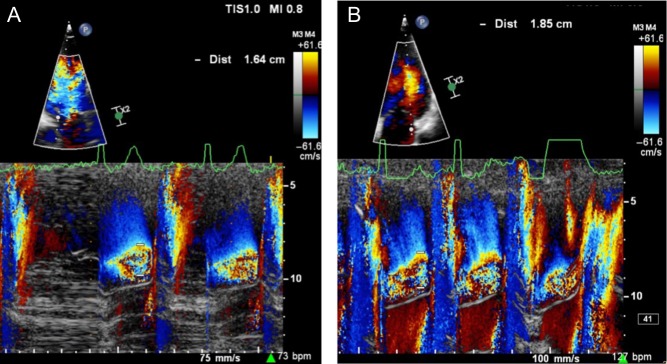
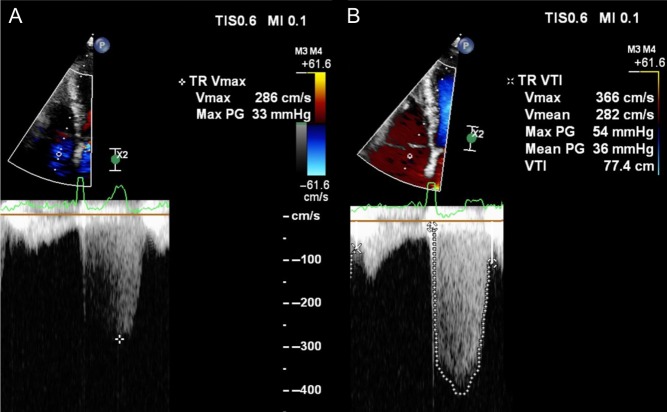
If echocardiography is added to the exercise test, what further information can be gleaned? First, a change in severity of primary MR with exercise is common and occurs in over 30% of those with asymptomatic moderate-to-severe MR (Fig. 1A and B) (30). From a small study of 61 asymptomatic patients, those who have an increase in effective regurgitant orifice area (EROA) of more than 10 mm2 during exercise have a lower symptom-free survival compared with those with no change. Secondly, patients with moderate-to-severe MR who develop pulmonary hypertension above 60 mmHg on exercise are at greater risk of symptoms and adverse outcomes (Fig. 2A and B) (30, 31). Thirdly, an assessment of LV function during stress echocardiography can also be an important marker of post-operative outcome. The onset of symptoms is not only governed by severity of MR and its effect on pulmonary pressure but also on the capacity of the LV to respond to volume-loading. Resting LV ejection fraction can be a poor marker of myocardial contractility as over a third of patients with a ‘normal’ pre-operative EF >60%, develop LV dysfunction below 50% after successful mitral repair (4). Latent contractile dysfunction can be predicted by measuring a systolic tissue velocity (<10.5 cm/s at rest) (Video 3 and Fig. 3) (32) and by quantifying global longitudinal strain (<−20%) (33). Improved outcomes can also be predicted by the LV response to exercise. In 71 consecutive asymptomatic patients with isolated moderate-to-severe primary MR, those in whom the LVEF failed to increase by ≥4%, had poorer symptom-free survival and worse outcomes after MV surgery (34). Similarly, a failure in global longitudinal strain to improve by ≥2% with exercise (Videos 4 and 5) appears to be a more sensitive marker of latent contractile dysfunction when indexing strain to end-systolic volume (35).
Figure 1.
Colour M-mode demonstrating worsening MR in a patient with normal LVEF. There is an increase in PISA from rest (A) to exercise (B) while cycling at 75 W.
Figure 2.
Continuous wave Doppler demonstrating an increase in severity of tricuspid regurgitation and increase in maximal velocity from rest (A) to exercise (B) while cycling at 75 W.
Figure 3.
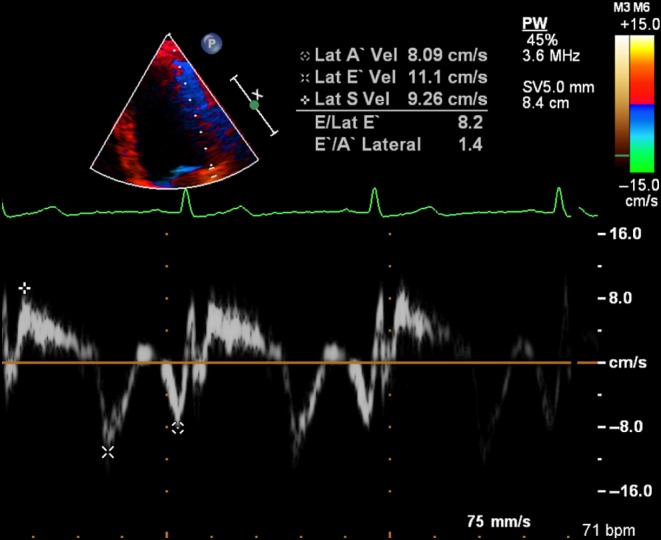
Example of latent contractile dysfunction with normal LVEF (Video 3), but systolic tissue velocity below 10.5 cm/s at rest.
Example of latent contractile dysfunction with normal LVEF but systolic tissue velocity below 10.5 cm/s at rest (Fig. 3). View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-3.
Download Video 3 (1.4MB, avi)
Example of reduction in GLS from rest in a patient with mitral regurgitation and LVEF >60%. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-4.
Download Video 4 (703.5KB, avi)
Example of reduction in GLS to exercise in a patient with mitral regurgitation and LVEF >60%. View Video 5 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-5.
Download Video 5 (434KB, avi)
Why does primary MR worsen during exercise?
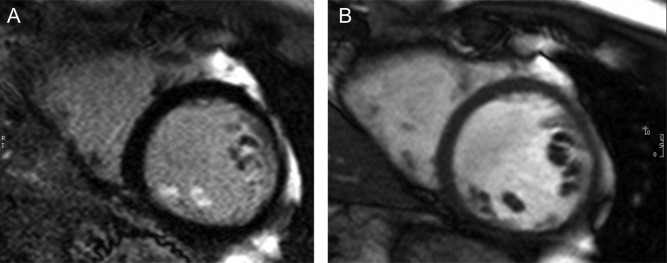
It has been suggested that exercise promotes an increase in systolic blood pressure, but pressure increase alone should not have a major impact on regurgitation without increase in regurgitant orifice as well as flow varies only with the square root of the pressure change between the LV and atrium (30). It is more likely that this may be due to changes in LV and annular geometry (36). Exercise-associated reduction in end-systolic volume could redefine the relationship between the papillary muscles and the zone of apposition of the leaflets (37). A further possibility is that the repetitive prolapse of a degenerate MV leads to papillary muscle traction, resulting in the fibrosis that has been documented on late enhancement with cardiovascular magnetic resonance imaging (Fig. 4) (38). Fibrosis in turn could promote a failure of the papillary muscles to respond to exercise, resulting in additional MR, a possibility suggested by differences in papillary muscle velocity and excursion in those with prolapse (39). These factors require further investigation as an understanding of the mechanisms of exercise-related MR may in turn further define those valves that need early repair.
Figure 4.
Late gadolinium enhancement of papillary muscle on cardiac MRI (A) and the corresponding cine short axis slice (B) in a patient with primary degenerative MR and normal LVEF.
Current indicators for stress echocardiography in primary MR
Current US guidelines support the use of exercise testing in the assessment of patients with asymptomatic severe valvular heart disease to help identify those with latent symptoms and to clarify prognosis (40). European guidelines support the addition of echocardiography to exercise stress testing in experienced hands for the assessment of patients whose symptoms or LV dysfunction appear disproportionate to the severity of MR at rest (5, 41). In the future, use of stress may be more widespread because it is possible that careful assessment of the patient with asymptomatic severe MR with normal cavity dimensions and good LV function may define two groups of patients – (1) those who have a good prognosis who can avoid surgery despite having a repairable valve and (2) those who are more likely to progress to symptoms and LV dysfunction who need surgery earlier. Unfortunately, there are as yet no randomised data that compare the outcome of such a strategy.
Secondary MR
Secondary MR is a dynamic condition where the degree of MR at rest does not predict the degree of MR on exertion. In a study of 70 consecutive patients with ischaemic LV dysfunction, MR decreased in 13 (19%), increased by less than 13 mm2 when measured by effective regurgitant orifice area (EROA) in 38 (54%) and increased more than this in the remaining 19 patients (42). The primary determinants of exercise-related deterioration in MR appear to be systolic annular area, degree of tenting of the valve and the associated wall motion abnormalities (42). These are dependent on the extent of ischaemic damage, exercise-induced dyssynchrony and the presence or absence of viability within the myocardium and papillary muscles; indeed, the severity of MR may reduce in patients with viable myocardium due to myocardial recruitment (43, 44, 45). Whether secondary MR improves or deteriorates on exercise is important as an increase in severity of MR with exercise by EROA ≥13 mm2 is associated with a five-fold increased risk of subsequent cardiac death (46). These and other data have led to support for the role of stress echocardiography in the investigation of patients with shortness of breath on exertion but who have less than severe secondary MR at rest. As yet, however, the role of stress echocardiography in the timing of mitral valve surgery in secondary MR remains controversial. In part, this is due to the lack of data, but in addition, it is not yet clear how best to manage patients with secondary MR.
Timing of surgery in chronic secondary MR
Secondary (ischaemic) MR is usually defined as the presence of MR either at rest or on exertion present more than 2 weeks after myocardial infarction. Although the frequency with which MR is detected may vary according to the method of imaging and the time at which this is carried out after MI, the presence of secondary MR confers a graded inverse relationship with risk of cardiovascular deaths (RR 1.88, 95% CI 1.23–2.86). Even those with mild ischaemic MR have a significantly worse survival (47). Although either the presence of MR and/or deterioration after exercise identifies a group of patients at particularly high risk, there continues to be controversy over whether surgical correction improves either life expectancy or quality of life (48). MV repair plus CABG was found to improve NYHA class, oxygen consumption on exercise testing and end-systolic volume index compared with CABG alone in a multi-centre study randomising 73 moderate MR patients, although mortality rates were similar in both groups (49). A similar finding was noted in a trial of 102 patients with an improvement in NYHA class after combined CABG and MV repair (50). In contrast, a further randomised study of 301 patients did not demonstrate concomitant MV repair led to any improvements in LV remodelling at 2-year follow-up but instead was associated with longer bypass time, hospital stay and more neurological events (48, 51). There is also controversy over whether MV repair or MV replacement produces the better outcome, with no apparent difference in end-systolic volume or mortality between approaches but less recurrent MR with replacement (52). These studies have generated a debate on whether secondary MR is only a marker of poor LV function rather than independently contributing to adverse outcome (53). Despite the inconsistency of evidence, it remains a class 2A indication to include MV repair at the time of CABG if MR is severe, whereas repair or replacement may be considered only if patients are severely symptomatic despite optimal medical therapy (class 2B) (40).
Stress echocardiography in secondary MR?
Although secondary MR may vary during exercise and diagnosis is associated with outcome, the question arises as to whether stress echocardiography could play a role in clarifying treatment strategy in symptomatic patients. In clinical practice, the main potential appears to be in two groups of patients. First, there are those whose symptoms of breathlessness appear to be disproportionate to the extent of LV impairment or the severity of MR. Exercise echocardiography can be used both to confirm objectively the extent of physical limitation (54), together with an imaging strategy that can define the extent of resting LV impairment, presence of ischaemia, development of dyssynchrony and alteration in the degree of MR during stress (5, 40, 42, 43). Secondly, the degree of secondary MR that is considered significant is relatively minor (EROA ≤13 mm2). In those in whom there is discussion as to whether percutaneous or surgical revascularisation should occur, demonstration of a significant exercise-related change in severity of MR or pulmonary hypertension would perhaps support surgery in those with symptoms (55). The limitation once again is that this diagnostic and intervention strategy is based on single-centre, non-randomised studies and large scale, randomised data are needed.
Performing stress echocardiography in mitral regurgitation
Stress echocardiography in MR should be preceded by a full transthoracic echocardiogram. This baseline echocardiogram should include an assessment of LV volumes and function, wall motion, aortic root, assessment of right ventricular dimensions and function, pulmonary pressure and assessment of all valves.
Most published studies have used upright or semi-supine bicycle exercise as the stressor because this permits continuous imaging at all stages of exercise. MR tends to resolve rapidly with rest, so that although treadmill exercise is useful for assessing symptoms and ischaemia, it is less so for quantifying change in MR. Moreover, bicycle stress provides more isometric stress than aerobic exercise, which may be more useful in evaluating MR. Usually, patients are asked to maintain a cadence of around 60/min with increments in workload of 25 W made at 2-min intervals, although protocols can be altered to younger patients using higher load (56). Data to be acquired are listed in Table 1 with a standard MR exercise echocardiography protocol shown in Fig. 5, illustrating the typical sequence of data acquisition. This generic protocol can of course be modified if there is a specific clinical question – such as more emphasis on LV function if there is an interest in LV viability. It is also useful to be aware of some of the limitations of quantitative echocardiography in MR, specifically the proximal isovelocity surface area (PISA) method for calculation of effective regurgitant orifice. First, the configuration or shape of PISA changes as the aliasing velocity changes – the convergence zone is flatter with higher aliasing velocities and becomes more elliptical with lower aliasing velocities. Secondly, the regurgitant orifice may vary during the cardiac cycle, occurring for example in the latter half of systole in MV prolapse. Colour M-mode can be used to assess variation during the cardiac cycle, but this is often not practical during stress. Thirdly, the PISA method for quantification of MR is based on the assumption that the MR jet is hemispheric proximal to the jet lesion, but this is not always the case. This is of greatest practical importance in secondary MR, when the PISA orifice may become less hemispheric and more ellipsoid, which leads to underestimation of severity. There are limited data regarding the use of pharmacological stress in MR.
Table 1.
Standard exercise echocardiography parameters for MR assessment, with key prognostic cut-off values for primary and secondary MR.
| Parameters | Key prognostic cut-off values in primary MR | Key prognostic cut-off values in secondary MR | |
|---|---|---|---|
| Resting quantitative assessment of disease severity | Resting BP and HR | ||
| EROA | Prospective 456 patients: highest survival with EROA <20 mm2. EROA >40 mm2 increases 5-year mortality rate (risk ratio 2.9) (57). | Prospective 303 patients: MR confers a graded inverse relationship with cardiac mortality (RR 1.88) (47). | |
| Regurgitant volume | Prospective 456 patients: adjusted mortality risk ratio increases by 1.15 per 10 mL increase in regurgitant volume (57). | ||
| Resting TR maximal velocity and calculation for PASP | Prospective 437 patients: resting PASP >50 mmHg is predictor of cardiovascular death (HR 2.21) (58). | ||
| LA volume | Prospective 492 patients: LA volume >60 mL/m2 reduces survival (HR 1.3), reversible with surgery (59). | ||
| Resting left ventricular assessment | LV internal dimensions/volumes | Class I indications for surgery: LVESD ≥45 mm in ESC or LVESD ≥40 mm in AHA/ACC guidelines (5, 40). | |
| Left ventricular ejection fraction | Observational 884 patients: LVEF <55% predicted mortality (28). | ||
| Wall motion score | |||
| Inferoseptal and anterolateral s′ and e′ tissue velocity | Retrospective 84 patients: resting systolic tissue velocity <10.5 cm/s predicts post-op reduction in EF (32). | ||
| Global longitudinal strain | Prospective 135 patients: resting GLS >−20% lowers event-free survival (33). | ||
| Exercise parameters | Exercise BP and HR | ||
| Heart rate recovery post-exercise | Observational 884 patients: HRR <18 bpm/min predicts adverse events (28). | ||
| Duration and extent of exercise | Observational 884 patients: <100% predicted METs predicts adverse events (28). | ||
| Symptoms on exercise | |||
| Quantitative MR severity | Prospective 61 patients: EROA increase of >10 mm2 or regurgitant volume >15 mL predicts symptom onset (30). | Prospective 98 patients: EROA during exercise ≥13 mm2 associated with increased cardiac mortality (60). | |
| Peak TR maximal velocity and calculation for PASP | Prospective 102 patients: exercise induced PASP >60 mmHg increased risk of post-op events (31). | ||
| Peak LVEF | |||
| LVEF | Prospective 71 patients: LVEF fail to improve by ≥4% have poorer prognosis (34). | Prospective 159 patients: exercise induced PASP >60 mmHg increased rate of cardiac events (HR 5.9) (55). | |
| Global longitudinal strain | Prospective 71 patients: GLS fail to improve by ≥1.9% predicts post-op EF reduction (35). |
Figure 5.
Mitral regurgitation exercise echocardiogram protocol. Reproduced, with permission, from Lancellotti P & Magne J, 2013, Stress echocardiography in regurgitant valve disease, Circulation: Cardiovascular Imaging, volume 6, pages 840–849 (56). Copyright 2013 Wolters Kluwer Health Inc.
Stress echocardiography in the assessment of patients with mitral valve disease is one of the most technically demanding skills. It is expected that image acquisition requires complete training in transthoracic echocardiography, with accreditation through the British Society of Echocardiography or the reciprocal European Association of Cardiovascular Imaging credentialing. Furthermore, the individual should then have a period of supervised experience in stress echocardiography, with US recommendations for accumulation of 100 cases under supervision. The British Society of Echocardiography has introduced the first formal accreditation process in stress echocardiography that involves a written exam, acquisition of 5 cases on video for submission and a logbook of 200 cases acquired within 2 years. Candidates will also be examined while acquiring images during exercise stress. Image interpretation likewise requires extensive experience in echocardiography and those involved should have specific training.
Summary
Although guidelines for timing of intervention in both primary and secondary MR have been established for several years, there continues to be controversy as to the appropriate timing of surgery. Quantitative exercise stress echocardiography may be a useful adjunct to the management of patients with both conditions: in primary MR, testing helps to select those asymptomatic patients with repairable valves to undergo early surgery while supporting those patients who may choose to delay intervention. In secondary MR, the role of stress echocardiography is more controversial but can help to identify the mechanism of regurgitation and target therapy in disproportionately symptomatic patients.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
Funding from BHF (grant no: PG/14/74/31056).
References
- 1.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Barwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, et al. 2003. A prospective survey of patients with valvular heart disease in Europe: the euro heart survey on valvular heart disease. European Heart Journal 24 1231–1243. ( 10.1016/S0195-668X(03)00201-X) [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. 2006. Burden of valvular heart diseases: a population-based study. Lancet 368 1005–1011. ( 10.1016/S0140-6736(06)69208-8) [DOI] [PubMed] [Google Scholar]
- 3.Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ, Frye RL. 1999. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation 99 400–405. ( 10.1161/01.CIR.99.3.400) [DOI] [PubMed] [Google Scholar]
- 4.Quintana E, Suri RM, Thalji NM, Daly RC, Dearani JA, Burkhart HM, Li Z, Enriquez-Sarano M, Schaff HV. 2014. Left ventricular dysfunction after mitral valve repair-the fallacy of ‘normal’ preoperative myocardial function. Journal of Thoracic and Cardiovascular Surgery 148 2752–2760. ( 10.1016/j.jtcvs.2014.07.029) [DOI] [PubMed] [Google Scholar]
- 5.Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, et al. 2012. Guidelines on the management of valvular heart disease (version 2012). European Heart Journal 33 2451–2496. ( 10.1093/eurheartj/ehs109) [DOI] [PubMed] [Google Scholar]
- 6.Enriquez-Sarano M, Suri RM, Clavel MA, Mantovani F, Michelena HI, Pislaru S, Mahoney DW, Schaff HV. 2015. Is there an outcome penalty linked to guideline-based indications for valvular surgery? Early and long-term analysis of patients with organic mitral regurgitation. Journal of Thoracic and Cardiovascular Surgery 150 50–58. ( 10.1016/j.jtcvs.2015.04.009) [DOI] [PubMed] [Google Scholar]
- 7.David TE, Armstrong S, McCrindle BW, Manlhiot C. 2013. Late outcomes of mitral valve repair for mitral regurgitation due to degenerative disease. Circulation 127 1485–1492. ( 10.1161/CIRCULATIONAHA.112.000699) [DOI] [PubMed] [Google Scholar]
- 8.Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. 1994. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation 90 830–837. ( 10.1161/01.CIR.90.2.830) [DOI] [PubMed] [Google Scholar]
- 9.Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, Ferlito M, Tafanelli L, Bursi F, Trojette F, et al. 2009. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. Journal of the American College of Cardiology 54 1961–1968. ( 10.1016/j.jacc.2009.06.047) [DOI] [PubMed] [Google Scholar]
- 10.Detaint D, Sundt TM, Nkomo VT, Scott CG, Tajik AJ, Schaff HV, Enriquez-Sarano M. 2006. Surgical correction of mitral regurgitation in the elderly – outcomes and recent improvements. Circulation 114 265–272. ( 10.1161/CIRCULATIONAHA.106.619239) [DOI] [PubMed] [Google Scholar]
- 11.Enriquez-Sarano M, Akins CW, Vahanian A. 2009. Mitral regurgitation. Lancet 373 1382–1394. ( 10.1016/S0140-6736(09)60692-9) [DOI] [PubMed] [Google Scholar]
- 12.Kang DH, Park SJ, Sun BJ, Cho EJ, Kim DH, Yun SC, Song JM, Park SW, Chung CH, Song JK, et al. 2014. Early surgery versus conventional treatment for asymptomatic severe mitral regurgitation. Journal of the American College of Cardiology 63 2398–2407. ( 10.1016/j.jacc.2014.02.577) [DOI] [PubMed] [Google Scholar]
- 13.Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, Barbieri A, Pasquet A, Huebner M, Rusinaru D, et al. 2013. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA 310 609–616. ( 10.1001/jama.2013.8643) [DOI] [PubMed] [Google Scholar]
- 14.Bolling SF, Li SA, O’Brien SM, Brennan JM, Prager RL, Gammie JS. 2010. Predictors of mitral valve repair: clinical and surgeon factors. Annals of Thoracic Surgery 90 1904–1912. ( 10.1016/j.athoracsur.2010.07.062) [DOI] [PubMed] [Google Scholar]
- 15.Gammie JS, O’Brien SM, Griffith BP, Ferguson TB, Peterson ED. 2007. Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation 115 881–887. ( 10.1161/CIRCULATIONAHA.106.634436) [DOI] [PubMed] [Google Scholar]
- 16.UK Early Mitral Surgery Trial 2015. British Heart Foundation. (available at: https://www.bhf.org.uk/research-projects/uk-early-mitral-surgery-trial) [Google Scholar]
- 17.Braunberger E, Deloche A, Berrebi A, Abdallah F, Celestin JA, Meimoun P, Chatellier G, Chauvaud S, Fabiani JN, Carpentier A. 2001. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation 104 I8–I11. ( 10.1161/01.CIR.104.suppl_1.I-8) [DOI] [PubMed] [Google Scholar]
- 18.Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, Schemper M, Maurer G, Baumgartner H. 2006. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation 113 2238–2244. ( 10.1161/CIRCULATIONAHA.105.599175) [DOI] [PubMed] [Google Scholar]
- 19.Biner S, Rafique A, Rafii F, Tolstrup K, Noorani O, Shiota T, Gurudevan S, Siegel RJ. 2010. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. JACC: Cardiovascular Imaging 3 235–243. ( 10.1016/j.jcmg.2009.09.029) [DOI] [PubMed] [Google Scholar]
- 20.Lapar DJ, Ailawadi G, Isbell JM, Crosby IK, Kern JA, Rich JB, Speir AM, Kron IL, Investigators Virginia C. 2014. Mitral valve repair rates correlate with surgeon and institutional experience. Journal of Thoracic and Cardiovascular Surgery 148 995–1003. ( 10.1016/j.jtcvs.2014.06.039) [DOI] [PubMed] [Google Scholar]
- 21.Blue Book Online 2013. The Society for Cardiothoracic Surgery in Great Britain and Ireland. Accessed 21/07/2016. (available at: http://bluebook.scts.org/) [Google Scholar]
- 22.Bonow RO, Adams DH. 2016. The time has come to define centers of excellence in mitral valve repair. Journal of the American College of Cardiology 67 499–501. ( 10.1016/j.jacc.2015.12.007) [DOI] [PubMed] [Google Scholar]
- 23.Suri RM, Clavel M-A, Schaff HV, Michelena HI, Huebner M, Nishimura RA, Enriquez-Sarano M. 2016. Effect of recurrent mitral regurgitation following degenerative mitral valve repair long-term analysis of competing outcomes. Journal of the American College of Cardiology 67 488–498. ( 10.1016/j.jacc.2015.10.098) [DOI] [PubMed] [Google Scholar]
- 24.Grigioni F, Avierinos JF, Ling LH, Scott CG, Bailey KR, Tajik AJ, Frye RL, Enriquez-Sarano M. 2002. Atrial fibrillation complicating the course of degenerative mitral regurgitation: determinants and long-term outcome. Journal of the American College of Cardiology 40 84–92. ( 10.1016/S0735-1097(02)01922-8) [DOI] [PubMed] [Google Scholar]
- 25.Le Tourneau T, Messika-Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, Suri R, Enriquez-Sarano M. 2010. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. Journal of the American College of Cardiology 56 570–578. ( 10.1016/j.jacc.2010.02.059) [DOI] [PubMed] [Google Scholar]
- 26.Messika-Zeitoun D, Johnson BD, Nkomo V, Avierinos JF, Allison TG, Scott C, Tajik AJ, Enriquez-Sarano M. 2006. Cardiopulmonary exercise testing determination of functional capacity in mitral regurgitation: physiologic and outcome implications. Journal of the American College of Cardiology 47 2521–2427. ( 10.1016/j.jacc.2006.02.043) [DOI] [PubMed] [Google Scholar]
- 27.Supino PG, Borer JS, Schuleri K, Gupta A, Hochreiter C, Kligfield P, Herrold EM, Preibisz JJ. 2007. Prognostic value of exercise tolerance testing in asymptomatic chronic nonischemic mitral regurgitation. American Journal of Cardiology 100 1274–1281. ( 10.1016/j.amjcard.2007.05.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naji P, Griffin BP, Asfahan F, Barr T, Rodriguez LL, Grimm R, Agarwal S, Stewart WJ, Mihaljevic T, Gillinov AM, et al. 2014. Predictors of long-term outcomes in patients with significant myxomatous mitral regurgitation undergoing exercise echocardiography. Circulation 129 1310–1319. ( 10.1161/CIRCULATIONAHA.113.005287) [DOI] [PubMed] [Google Scholar]
- 29.Naji P, Griffin BP, Barr T, Asfahan F, Gillinov AM, Grimm RA, Rodriguez LL, Mihaljevic T, Stewart WJ, Desai MY. 2014. Importance of exercise capacity in predicting outcomes and determining optimal timing of surgery in significant primary mitral regurgitation. Journal of the American Heart Association 3 e001010 ( 10.1161/JAHA.114.001010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magne J, Lancellotti P, Piérard LA. 2010. Exercise-induced changes in degenerative mitral regurgitation. Journal of the American College of Cardiology 56 300–309. ( 10.1016/j.jacc.2009.12.073) [DOI] [PubMed] [Google Scholar]
- 31.Magne J, Donal E, Mahjoub H, Miltner B, Dulgheru R, Thebault C, Pierard LA, Pibarot P, Lancellotti P. 2015. Impact of exercise pulmonary hypertension on postoperative outcome in primary mitral regurgitation. Heart 101 391–396. ( 10.1136/heartjnl-2014-306296) [DOI] [PubMed] [Google Scholar]
- 32.Agricola E, Galderisi M, Oppizzi M, Schinkel AF, Maisano F, De Bonis M, Margonato A, Maseri A, Alfieri O. 2004. Pulsed tissue Doppler imaging detects early myocardial dysfunction in asymptomatic patients with severe mitral regurgitation. Heart 90 406–410. ( 10.1136/hrt.2002.009621) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magne J, Mahjoub H, Pierard LA, O’Connor K, Pirlet C, Pibarot P, Lancellotti P. 2012. Prognostic importance of brain natriuretic peptide and left ventricular longitudinal function in asymptomatic degenerative mitral regurgitation. Heart 98 584–591. ( 10.1136/heartjnl-2011-301128) [DOI] [PubMed] [Google Scholar]
- 34.Lee R, Haluska B, Leung DY, Case C, Mundy J, Marwick TH. 2005. Functional and prognostic implications of left ventricular contractile reserve in patients with asymptomatic severe mitral regurgitation. Heart 91 1407–1412. ( 10.1136/hrt.2004.047613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donal E, Mascle S, Brunet A, Thebault C, Corbineau H, Laurent M, Leguerrier A, Mabo P. 2012. Prediction of left ventricular ejection fraction 6 months after surgical correction of organic mitral regurgitation: the value of exercise echocardiography and deformation imaging. European Heart Journal: Cardiovascular Imaging 13 922–930. ( 10.1093/ehjci/jes068) [DOI] [PubMed] [Google Scholar]
- 36.Flachskampf FA. 2010. Mitral regurgitation is incompletely characterized at rest. Journal of the American College of Cardiology 56 310–313. ( 10.1016/j.jacc.2010.02.052) [DOI] [PubMed] [Google Scholar]
- 37.Lee T-M, Su S-F, Huang T-Y, Chen M-F, Liau C-S, Lee Y-T. 1996. Excessive papillary muscle traction and dilated mitral annulus in mitral valve prolapse without mitral regurgitation. American Journal of Cardiology 78 482–485. ( 10.1016/0002-9149(97)00002-7) [DOI] [PubMed] [Google Scholar]
- 38.Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. 2008. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC: Cardiovascular Imaging 1 294–303. ( 10.1016/j.jcmg.2008.01.013) [DOI] [PubMed] [Google Scholar]
- 39.Han YC, Peters DC, Kissinger KV, Goddu B, Yeon SB, Manning WJ, Nezafat R. 2010. Evaluation of papillary muscle function using cardiovascular magnetic resonance imaging in mitral valve prolapse. American Journal of Cardiology 106 243–248. ( 10.1016/j.amjcard.2010.02.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. 2014. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 63 e57–e185. ( 10.1016/j.jacc.2014.02.536) [DOI] [PubMed] [Google Scholar]
- 41.Sharma V, Newby DE, Stewart RA, Lee M, Gabriel R, Van Pelt N, Kerr AJ. 2015. Exercise stress echocardiography in patients with valvular heart disease. Echo Research and Practice 2 89–98. ( 10.1530/ERP-15-0015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lancellotti P, Lebrun F, Piérard LA. 2003. Determinants of exercise-induced changes in mitral regurgitation in patients with coronary artery disease and left ventricular dysfunction. Journal of the American College of Cardiology 42 1921–1928. ( 10.1016/j.jacc.2003.04.002) [DOI] [PubMed] [Google Scholar]
- 43.Izumo M, Lancellotti P, Suzuki K, Kou S, Shimozato T, Hayashi A, Akashi YJ, Osada N, Omiya K, Nobuoka S, et al. 2009. Three-dimensional echocardiographic assessments of exercise-induced changes in left ventricular shape and dyssynchrony in patients with dynamic functional mitral regurgitation. European Journal of Echocardiography 10 961–967. ( 10.1093/ejechocard/jep114) [DOI] [PubMed] [Google Scholar]
- 44.Giga V, Ostojic M, Vujisic-Tesic B, Djordjevic-Dikic A, Stepanovic J, Beleslin B, Petrovic M, Nedeljkovic M, Nedeljkovic I, Milic N. 2005. Exercise-induced changes in mitral regurgitation in patients with prior myocardial infarction and left ventricular dysfunction: relation to mitral deformation and left ventricular function and shape. European Heart Journal 26 1860–1865. ( 10.1093/eurheartj/ehi431) [DOI] [PubMed] [Google Scholar]
- 45.Srichai MB, Grimm RA, Stillman AE, Gillinov AM, Rodriguez LL, Lieber ML, Lara A, Weaver JA, McCarthy PM, White RD. 2005. Ischemic mitral regurgitation: impact of the left ventricle and mitral valve in patients with left ventricular systolic dysfunction. Annals of Thoracic Surgery 80 170–178. ( 10.1016/j.athoracsur.2005.01.068) [DOI] [PubMed] [Google Scholar]
- 46.Lancellotti P, Gérard PL, Piérard LA. 2005. Long-term outcome of patients with heart failure and dynamic functional mitral regurgitation. European Heart Journal 26 1528–1532. ( 10.1093/eurheartj/ehi189) [DOI] [PubMed] [Google Scholar]
- 47.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. 2001. Ischemic mitral regurgitation – long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation 103 1759–1764. ( 10.1161/01.CIR.103.13.1759) [DOI] [PubMed] [Google Scholar]
- 48.Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, Acker MA, Hung JW, Chang HL, Perrault LP, et al. 2016. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. New England Journal of Medicine 374 1931–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan KMJ, Punjabi PP, Flather M, Wage R, Symmonds K, Roussin I, Rahman-Haley S, Pennell DJ, Kilner PJ, Dreyfus GD, et al. 2012. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation final results of the randomized ischemic mitral evaluation (RIME) trial. Circulation 126 2502–2510. ( 10.1161/CIRCULATIONAHA.112.143818) [DOI] [PubMed] [Google Scholar]
- 50.Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E, Calvaruso D, Ruvolo G. 2009. POINT: efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. Journal of Thoracic and Cardiovascular Surgery 138 278–285. ( 10.1016/j.jtcvs.2008.11.010) [DOI] [PubMed] [Google Scholar]
- 51.Smith PK, Puskas JD, Ascheim DD, Voisine P, Gelijns AC, Moskowitz AJ, Hung JW, Parides MK, Ailawadi G, Perrault LP, et al. 2014. Surgical treatment of moderate ischemic mitral regurgitation. New England Journal of Medicine 371 2178–2188. ( 10.1056/NEJMoa1410490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, Hung JW, Voisine P, Dagenais F, Gillinov AM, et al. 2016. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. New England Journal of Medicine 374 344–353. ( 10.1056/NEJMoa1512913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lancellotti P, Zamorano JL, Vannan MA. 2014. Imaging challenges in secondary mitral regurgitation unsolved issues and perspectives. Circulation: Cardiovascular Imaging 7 735–746. ( 10.1161/circimaging.114.000992) [DOI] [PubMed] [Google Scholar]
- 54.Szymanski C, Levine RA, Tribouilloy C, Zheng H, Handschumacher MD, Tawakol A, Hung J. 2011. Impact of mitral regurgitation on exercise capacity and clinical outcomes in patients with ischemic left ventricular dysfunction. American Journal of Cardiology 108 1714–1720. ( 10.1016/j.amjcard.2011.07.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lancellotti P, Magne J, Dulgheru R, Ancion A, Martinez C, Pierard LA. 2015. Clinical significance of exercise pulmonary hypertension in secondary mitral regurgitation. American Journal of Cardiology 115 1454–1461. ( 10.1016/j.amjcard.2015.02.028) [DOI] [PubMed] [Google Scholar]
- 56.Lancellotti P, Magne J. 2013. Stress echocardiography in regurgitant valve disease. Circulation: Cardiovascular Imaging 6 840–849. ( 10.1161/circimaging.113.000474) [DOI] [PubMed] [Google Scholar]
- 57.Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, Detaint D, Capps M, Nkomo V, Scott C, Schaff HV, Tajik AJ. 2005. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. New England Journal of Medicine 352 875–883. ( 10.1056/NEJMoa041451) [DOI] [PubMed] [Google Scholar]
- 58.Barbieri A, Bursi F, Grigioni F, Tribouilloy C, Avierinos JF, Michelena HI, Rusinaru D, Szymansky C, Russo A, Suri R, et al. 2011. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: a multicenter long-term international study. European Heart Journal 32 751–759. ( 10.1093/eurheartj/ehq294) [DOI] [PubMed] [Google Scholar]
- 59.Le Tourneau T, Messika-Zeitoun D, Russo A, Detaint D, Topilsky Y, Mahoney DW, Suri R, Enriquez-Sarano M. 2010. Impact of left atrial volume on clinical outcome in organic mitral regurgitation. Journal of the American College of Cardiology 56 570–578. ( 10.1016/j.jacc.2010.02.059) [DOI] [PubMed] [Google Scholar]
- 60.Lancellotti P, Troisfontaines P, Toussaint AC, Pierard LA. 2003. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation 108 1713–1717. ( 10.1161/01.CIR.0000087599.49332.05) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primary mitral regurgitation with flail A2 scallop of the anterior mitral valve leaflet. View Video 1 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-1.
Download Video 1 (990KB, avi)
Secondary ischaemic mitral regurgitation (asymmetric). View Video 2 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-2.
Download Video 2 (910KB, avi)
Example of latent contractile dysfunction with normal LVEF but systolic tissue velocity below 10.5 cm/s at rest (Fig. 3). View Video 3 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-3.
Download Video 3 (1.4MB, avi)
Example of reduction in GLS from rest in a patient with mitral regurgitation and LVEF >60%. View Video 4 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-4.
Download Video 4 (703.5KB, avi)
Example of reduction in GLS to exercise in a patient with mitral regurgitation and LVEF >60%. View Video 5 at http://movie-usa.glencoesoftware.com/video/10.1530/ERP-16-0019/video-5.
Download Video 5 (434KB, avi)



 This work is licensed under a
This work is licensed under a