Abstract
Background
Aedes albopictus and Ae. japonicus are two of the most widespread invasive mosquito species that have recently become established in western Europe. Both species are associated with the transmission of a number of serious diseases and are projected to continue their spread in Europe.
Methods
In the present study, we modelled the habitat suitability for both species under current and future climatic conditions by means of an Ensemble forecasting approach. We additionally compared the modelled MAXENT niches of Ae. albopictus and Ae. japonicus regarding temperature and precipitation requirements.
Results
Both species were modelled to find suitable habitat conditions in distinct areas within Europe: Ae. albopictus within the Mediterranean regions in southern Europe, Ae. japonicus within the more temperate regions of central Europe. Only in few regions, suitable habitat conditions were projected to overlap for both species. Whereas Ae. albopictus is projected to be generally promoted by climate change in Europe, the area modelled to be climatically suitable for Ae. japonicus is projected to decrease under climate change. This projection of range reduction under climate change relies on the assumption that Ae. japonicus is not able to adapt to warmer climatic conditions. The modelled MAXENT temperature niches of Ae. japonicus were found to be narrower with an optimum at lower temperatures compared to the niches of Ae. albopictus.
Conclusions
Species distribution models identifying areas with high habitat suitability can help improving monitoring programmes for invasive species currently in place. However, as mosquito species are known to be able to adapt to new environmental conditions within the invasion range quickly, niche evolution of invasive mosquito species should be closely followed upon in future studies.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-016-1853-2) contains supplementary material, which is available to authorized users.
Keywords: Asian bush mosquito, Asian tiger mosquito, Climate change, Invasive species, Species distribution modelling
Background
Climate change is assumed to generally promote the invasive success of introduced species. In addition to finding more suitable conditions, they can indirectly benefit from changed climatic conditions as some ecosystems might become less resistant to invasion [1]. Species distribution models are a useful and commonly applied tool to project climate change induced range shifts of species (e.g. [2]). Species distribution models can improve assessments of species’ invasive potential and guide management actions [3]. This is especially important for species that function as vectors, which may potentially pose a threat to human health.
Ecological niche modelling is a commonly used correlative approach to model the habitat suitability for a species under current and projected future climatic conditions. Based on a species’ presence/absence information, and environmental conditions at a particular geographical location, species-habitat relationships are estimated [4] by means of different statistical algorithms, providing data on relative habitat suitability. Based on the information where the species is present (and in addition ideally where the species is absent) and environmental conditions prevail there, the species-habitat-relationship is estimated [4] by means of different statistical algorithms. This modelled species-habitat relationship (niche function) can then be projected onto the study area, resulting in a habitat suitability map for the considered species within the considered area. This projection can be based on the data on current climatic conditions, thus reflecting the potential distribution under current climatic conditions. In addition, data on potential future climatic conditions, like those provided by the Intergovernmental Panel on Climate Change (IPCC), can be used, which would represent the potential future distribution of the considered species.
Projections on habitat suitability under future climatic conditions may help to assess the invasive potential of non-native species. Climate warming is assumed to yield a north eastwards shift and a shift to higher altitudes of areas with suitable habitat conditions for many species. Projections on habitat suitability for invasive species under future climatic conditions may help improving existing monitoring programmes as well as preventing negative consequences for ecosystems and human health in the future.
Aedes albopictus and Ae. japonicus are two of the most widespread invasive mosquito species worldwide. Native to Asia, these two species have been spreading rapidly across the globe with increasing transport of certain goods and facilitated by human activities such as travelling [5, 6]. Whereas Ae. albopictus originates in the forests of tropical regions of south-east Asia [5–7], Ae. japonicus has been originally restricted to Japan, north of the Ryukyu Islands (Hokkaido, Honshu, Shikoku, and Kyushu) and the Korean Peninsula, where it is common, even in large cities, but not particularly abundant [8]. Aedes japonicus does not occur in the tropics, but both species co-occur naturally in Japan and Korea [9].
Aedes albopictus is considered to be one of the world’s fastest-spreading invasive animal species [10]. Aedes japonicus has spread throughout North America and later into central Europe at a rate comparable to that of Ae. albopictus [8]. The rapid global spread of these species is certainly favoured by extrinsic factors such as globalization and climate change [11]. International as well as intercontinental trade (e.g. of used tyres that may act as breeding places) may have facilitated the introduction of these species [12]. Furthermore, the successful invasion of Ae. albopictus is assumed to be promoted by intrinsic factors (i.e. factors that act from within the species) such as strong ecological plasticity, which allows the species to get successfully established in a wide range of different habitats with different climatic conditions [12]. Both species produce desiccation-resistant eggs. This trait likely facilitated the transport and consequently, the successful introduction of Ae. albopictus and Ae. japonicus to many places worldwide [9, 13]. In addition, the eggs of both species can undergo diapause and thus overwinter in temperate climates despite the adult forms being unable to survive through this period (e.g. [14–16]).
Aedes albopictus as well as Ae. japonicus are known to function as competent vectors for a number of serious diseases [17, 18] including dengue fever, yellow fever, West Nile fever and Rift Valley fever [6] as well as Japanese encephalitis [19]. Due to this public health significance, the interest in investigating the establishment and spread of invasive mosquitoes in Europe has been on the rise for the past few years. Research including monitoring the current spread of these species, modelling the potential future spread and also studies on the species’ ecology is considered to be particularly important in order to contain their further spread [6].
In the present study, we focused on the spread of Ae. albopictus and Ae. japonicus in Europe. Both species are considered invasive in many countries worldwide, including North America and Africa. More recently, they have also become established in western Europe [13, 20, 21]. Due to ongoing introductions and projected climate change, both species are predicted to continue their spread in Europe and will therefore remain the subject of surveillance and monitoring programmes [22]. As a species adapted to warmer climatic conditions, Ae. albopictus is assumed to be strongly promoted by projected climate change (e.g. [17, 23]. Compared to Ae. albopictus, Ae. japonicus is assumed to be adapted to colder temperatures. Thus, one may hypothesize that Ae. japonicus will not particularly benefit from projected long-term global warming (or to a lesser extent) compared to Ae. albopictus. However, despite or even because of being able to withstand winter temperatures, and due to the recorded rapid spread in Switzerland, Ae. japonicus is projected to become more widely established in Europe in the following years [24].
We here used correlative species distribution modelling, based on ten commonly applied niche modelling algorithms (Ensemble forecasting), to project and compare the modelled habitat suitability for Ae. albopictus and Ae. japonicus in Europe under current and future climatic conditions. The first step comprised modelling the habitat suitability for the two species in Europe. Due to the assumed different climatic requirements, we expected the species to find suitable habitat conditions in different regions of Europe. We hypothesized that Ae. albopictus would clearly benefit from climate change resulting in an enlargement of the area with modelled suitable habitat conditions, but that Ae. japonicus would do so only to a lesser extent. As environmental variables we considered six variables covering temperature and precipitation conditions (mean temperature of coldest quarter, mean temperature of warmest quarter, temperature annual range, annual mean precipitation, annual precipitation as well as precipitation in warmest quarter).
To derive more specific information on the species’ ecology from the occurrence records in combination with environmental variables (temperature and precipitation), the second step was to compare the modelled MAXENT niches of the two species by means of so-called one-variable response curves. We expected that Ae. japonicus would show a smaller temperature niche with an optimum shifted to colder temperatures compared to Ae. albopictus. Considering Ae. albopictus and Ae. japonicus requirements of small aquatic habitats for breeding, we hypothesized that both species would have similar niches considering precipitation variables with regard to location and amplitude.
Methods
Occurrence data
Occurrence data are taken from Kraemer MUG et al. [25] and Koch et al. [17] for Ae. albopictus and from Schaffner et al. [26]; Huber et al. [27]; Huber et al. [19]; Krebs et al. [28]; Zielke et al. [29]; Melaun et al. [18] and Zielke et al. [30] for Ae. japonicus. The original coordinates were adjusted to the raster of the environmental variables (about 10 × 10 km).
The records were adjusted to the raster of the environmental variables. The records for Ae. albopictus date back to the period between 1980 and 2015 and the records for Ae. japonicus date back to the period between 2000 and 2015, and cover the area of the currently known distribution of both species. We modelled the habitat suitability for both species in Europe considering the following spatial extent: Latitude, 35°N-79°N; Longitude, 10°W-45°E; and worked at a spatial resolution of five arc min (~10 km). The maps were built using ESRI ArcGIS (Release 10.3, www.esri.com).
Environmental data (current and future climatic conditions)
Here, six climatic variables were taken into account, including mean temperature of coldest quarter, mean temperature of warmest quarter, temperature annual range (maximal temperature of warmest month - minimal temperature of coldest month), annual mean precipitation, precipitation in warmest quarter and precipitation seasonality (coefficient of variation). We thus accounted for temperature conditions during summer and winter, summer precipitation and annual precipitation as well as the variation of these two factors during the year. We decided to use annual precipitation instead of winter precipitation as the latter is assumed to be ecologically irrelevant for both species. This selection of variables from the 19 bioclimatic variables available from worldclim [31] (www.worldclim.org/) was based on a correlation analysis coupled with assumptions according the ecological relevance of the variables for the species. The considered variables are not strongly inter-correlated (Spearman correlation coefficient < 0.7, see Additional file 1: Table S1). Temperature in winter is regarded as critical for the survival of individuals (of different stages), summer temperature is assumed to influence the activity of individuals, temperature range may be important determining the east-west gradient of species in Europe, precipitation is considered crucial as both mosquito species require small aquatic habitats for breeding, whose general availability is assumed to be connected to precipitation conditions. Data on current climatic conditions were provided by worldclim. Data on future climatic conditions based on the fifth IPCC Assessment report (AR5) were taken from the International Centre for Tropical Agriculture (CIAT) and The CGIAR Research Program on Climate Change, Agriculture and Food Security (CCAFS) [32] (http://ccafs-climate.org/data/).
The IPCC is a scientific intergovernmental body that produces reports which have the agreement of leading climate scientists and the consensus of participating governments. Based on the IPCC reports a range of emission scenarios are derived for use in global climate models that project future climatic conditions under different socio-economic and emission scenarios. Socio-economic and emission scenarios are considered in climate research to provide plausible descriptions of how the future may evolve with respect to a range of variables including socio-economic change, technological change, energy and land use and emissions of greenhouse gases and air pollutants. They are used as input for climate model runs and as a basis for assessment of possible climate impacts and mitigation options and associated costs. In the IPCC AR5 four so-called ‘Representative Concentration Pathways’ (RCPs) are considered that represent a broad range of climate outcomes. The RCPs describe four possible climate futures, all of which are considered possible depending on how much greenhouse gases are emitted in the years to come.
RCP 2.6 is based on the assumption that the maximum of global annual greenhouse gas emissions takes place between 2010 and 2020 with a substantial decline thereafter. According to the RCP 4.5 and RCP 6.0, global annual greenhouse gas emissions will rise until 2040 and 2080, respectively, and then decline. In RCP 8.5, the global annual greenhouse gas emissions are assumed to rise throughout the 21st century [33].
We projected the habitat suitability for Ae. albopictus and Ae. japonicus for four time periods, including under current climatic conditions (based on measurements between 1950 and 2000) and under future climatic conditions (for three future time periods from 2021–2040, 2041–2060 and 2061–2080). We considered four different RCPs (5th Assessment report [34]) to account for uncertainty in data on future climatic conditions.
Ensemble forecasting
Modelling results often show a high variability, which can be ascribed to the sensitivity of correlative models to the data and the mathematical functions used to describe the species-habitat relationship [35]. Comparative studies using independent presence/absence data for evaluation (e.g. [36, 37]) could not verify the superiority of one single algorithm [35] although generating a lot of knowledge regarding modelling performance [38]. One way to deal with the uncertainty of SDM algorithms is the so-called Ensemble forecasting. Ensemble forecasting denotes the application of several alternative statistical algorithms [38]. The results of these different models are then integrated into one ‘consensus’ model, which provides more robust estimates [35]. Different methods exist to create the consensus model (see [38]) and here we chose to use weighted averages.
In order to yield habitat suitability maps for both mosquito species considered here, we run an Ensemble forecasting approach based on ten state-of-the-art algorithms (GLM, generalized linear models; GAM, generalized additive models; GBM, generalized boosted models; CTA, classification tree analysis; SRE, surface range envelope; ANN, artificial neural networks; FDA, flexible discriminant analysis; MARS, multivariate adaptive regression splines; RF, random forest; MAXENT, maximum entropy approach) implemented in the R package BIOMOD2 (version 3.3-7; [39]). For a short description of the used algorithms see [37] and [40].
Modelling performance and variable importance
The AUC-value (i.e. the area under the receiver operating characteristic curve value) is a threshold independent measure for modelling performance that ranges between 0 (low performance) and 1 (high performance). As a consensus model we considered the mean average of ten algorithms weighted by the AUC value [38] (required an AUC value of the single models of over 0.75). For binary modelling results, we applied the sensitivity equals specificity threshold [41].
Regarding environmental variables relevant for the potential distribution of the two mosquito species we compared the relative contributions of the six environmental variables to the MAXENT models for the two species. In order to calculate the MAXENT permutation variable importance as a measure of relative variable importance the values of each variable in turn are randomly permuted on training presence and background data. The model is re-evaluated on the permuted data, and the resulting drop in training AUC, normalized to percentages is then taken as a measure of relative variable importance.
MAXENT
In addition to providing habitat suitability maps for the two mosquito species under current and future climatic conditions, we aimed to further investigate the modelled species-habitat relationship with regard to a comparison of the modelled temperature and precipitation niches of the two species. For this, we focused on the maximum entropy niche modelling approach (MAXENT, [42, 43]) for the following reasons: the MAXENT approach is one of the most commonly employed algorithms to model species potential ranges (e.g. [44]), it scores well in comparative studies (e.g. [37]) and the modelled niche function is relatively easy to handle from a mathematically point of view [45]. According to Baldwin [46], the maximum entropy approach is relatively insensitive to spatial errors associated with location data, requires few locations to construct useful models and performs better than other presence only modelling algorithms. These may be especially important issues considering Ae. albopictus and Ae. japonicus as non-native invasive species in Europe. We used the Maximum entropy approach as implemented in the freeware MAXENT (version 3.3.3 k [42, 43]). We used the default setting with 20 replications but only linear, quadratic and product features (c.f. [45]). One-variable models were displayed as one-variable-response-curves using R (version 3.2.1, www.r-project.org) and compared between the two species. These curves reflect the dependence of predicted habitat suitability on each environmental variable. We only show response curves for variables for those the one-variable-models score an AUC value of at least 0.75 for both species.
Results
Ensemble forecasting under current and future climatic conditions
According to our modelling results, suitable habitat conditions for the two invasive mosquito species Ae. albopictus and Ae. japonicus can currently be found in distinct areas within Europe (Figs. 1 and 2). Suitable habitat conditions and thus potential distribution of Ae. albopictus was projected to be currently restricted to southern Europe, which can be characterized by Mediterranean climatic conditions (warm or hot, dry summers and mild or cool, wet winters). In contrast, Ae. japonicus is projected to find suitable conditions within the climatically more temperate regions in central Europe. Only in a few regions (the Upper Rhine Valley in Germany, France and Switzerland, parts of southern Germany, Switzerland and Slovenia as well as small regions in northern Italy, Austria and Croatia) both species are projected to find currently suitable climatic conditions, potentially leading to a co-occurrence in these regions (Fig. 2).
Fig. 1.
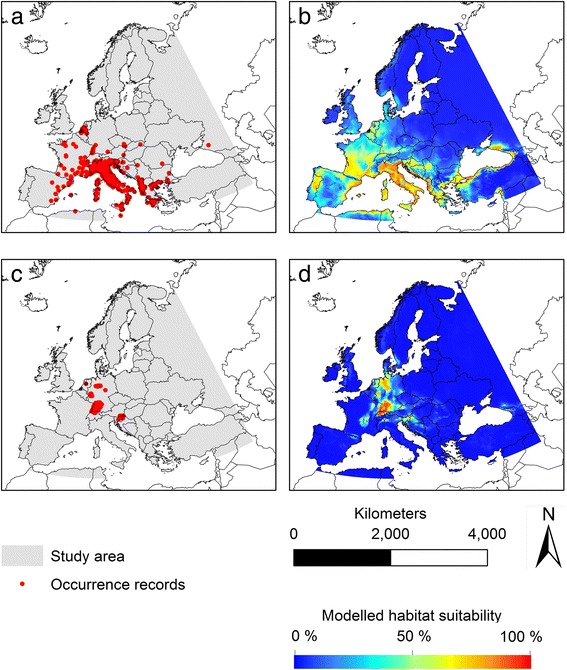
Observed and modelled European distribution for Aedes albopictus and Ae. japonicus. a Occurrence records for Ae. albopictus (n = 336). b Modelled habitat suitability for Ae. albopictus under current climatic conditions, Ensemble forecasting (AUC = 0.972). c Occurrence records for Ae. japonicus (n = 178). d Modelled habitat suitability for Ae. japonicus under current climatic conditions, Ensemble forecasting (AUC = 0.999)
Fig. 2.
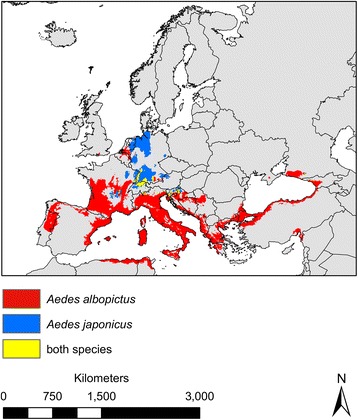
Area with modelled habitat suitability (Ensemble forecasting) in Europe for Aedes albopictus and Aedes japonicus and both mosquito species under current climatic conditions. Sensitivity equals specificity threshold was applied to yield binary modelling results
For both species the modelled habitat suitability (Fig. 1b, d) reflect the observed distribution (Fig. 1a, c) of both species quite well. The AUC values for the consensus models resulting from the Ensemble forecasts are high, with 0.972 for Ae. albopictus and 0.999 for Ae. japonicus. The AUC values for the single models are given in Additional file 2: Table S2.
Aedes albopictus is modelled to expand its potential range in Europe north-eastwards under future climatic conditions (Fig. 3), with large parts of France, the Benelux region and parts of Germany becoming climatically suitable for the species. Generally, the climatic suitability will increase area-wide across nearly whole Europe. These rises are consistent over all four RCP-scenarios. Under the RCP 2.6, which is associated to the lowest temperature increase in the face of climate change, the increase of projected habitat suitability for Ae. albopictus is comparably lowest, whereas the projected increase of habitat suitability is highest for the RCP 8.5 which is associated to the highest temperature increase.
Fig. 3.
Modelled habitat suitability (Ensemble forecasting) for Aedes albopictus under current and future climatic conditions
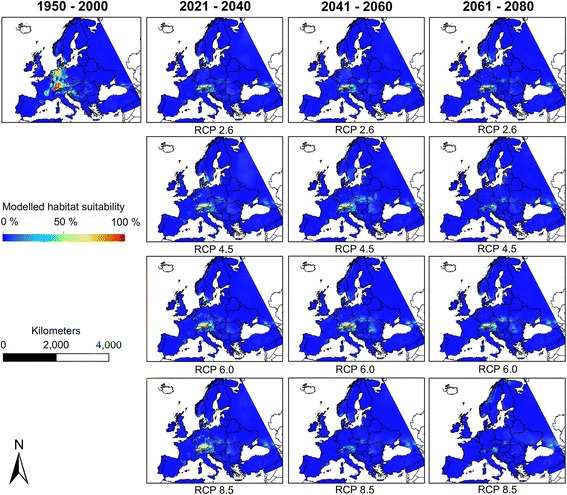
Compared to the increased habitat suitability for Ae. albopictus, Ae. japonicus is projected to find decreasingly suitable habitats under future climatic conditions (Fig. 4). Depending on the time period and RCP considered, Ae. japonicus is modelled to only find suitable habitat conditions in southern Germany and very small regions in the High Tatras.
Fig. 4.
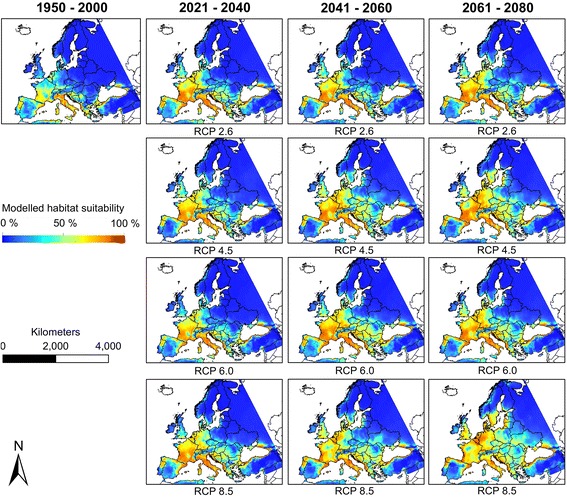
Modelled habitat suitability (Ensemble forecasting) for Aedes japonicus under current and future climatic conditions
Variable importance
Considering the permutation variable importance as a measure for the variables contribution to the MAXENT model (Table 1), the mean temperatures of coldest quarter showed for both species the highest values. In second place are mean temperature of warmest quarter for Ae. albopictus and annual precipitation for Ae. japonicus.
Table 1.
Relative variable importance (MAXENT) in %. The permutation importance provides estimates of relative contributions of the environmental variables to the MAXENT model
| Variables | Ae. albopictus | Ae. japonicus |
|---|---|---|
| Mean temperature of warmest quarter | 22.9 | 1.9 |
| Mean temperature of coldest quarter | 50.3 | 42.2 |
| Temperature annual range | 7.3 | 0.2 |
| Precipitation of warmest quarter | 6.6 | 11 |
| Annual precipitation | 9.2 | 39.5 |
| Precipitation seasonality | 3.6 | 5.2 |
One-variable response curves (MAXENT)
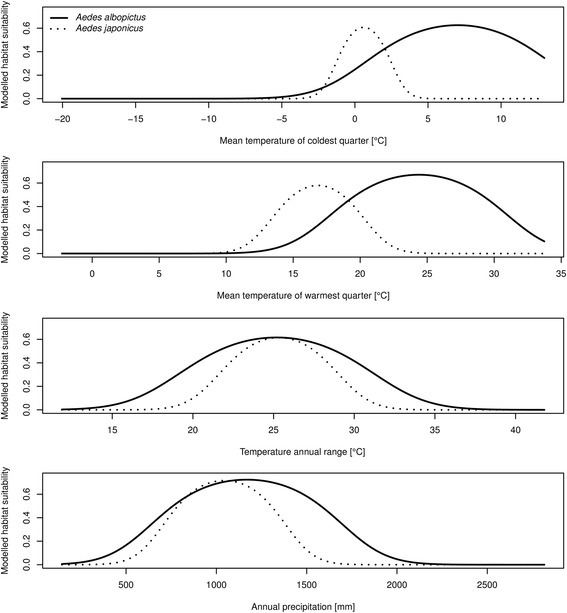
As depicted in the one-variable response curves (Fig. 5), the modelled niche function for Ae. japonicus shows a lower temperature optimum than that of Ae. albopictus; this is true for the variables mean temperature of the coldest quarter and mean temperature of the warmest quarter. Furthermore, Ae. japonicus is modelled to have a narrower niche considering these variables as well as for the variables temperature annual range and annual precipitation. For the latter two variables the modelled optima are rather similar for the both species. The response curves for the variables precipitation of warmest quarter and precipitation seasonality are not shown in Fig. 5 as the one-variable models for at least one of the two considered mosquito species has an AUC value of less than 0.75 and thus, the one-variable model is of low predictive power. The AUC values for the one-variable models for all six variables and the two species are given in Table 2.
Fig. 5.
One-variable-response-curves for Aedes albopictus (solid line) and Aedes japonicus (dotted line) considering the different environmental variables (required: AUC value for the one variable model for both species > 0.75, see Table 2)
Table 2.
AUC values for the one-variable models, mean average over 20 replications ± standard deviation. Response curves for the variables for which the one variable model yields an AUC value of at least 0.75 for both species are shown in Fig. 5
| Variable | Ae. albopictus | Ae. japonicus |
|---|---|---|
| Mean temperature of warmest quarter | 0.818 ± 0.002 | 0.840 ± 0.003 |
| Mean temperature of coldest quarter | 0.851 ± 0.001 | 0.924 ± 0.001 |
| Temperature annual range | 0.790 ± 0.001 | 0.915 ± 0.002 |
| Precipitation of warmest quarter | 0.593 ± 0.004 | 0.901 ± 0.002 |
| Annual precipitation | 0.764 ± 0.002 | 0.864 ± 0.002 |
| Precipitation seasonality | 0.554 ± 0.003 | 0.779 ± 0.002 |
Discussion
Mosquitoes can pose a serious threat to human health as they function as vectors for serious diseases. Facing risks from undeliberate introductions of non-native species together with range expansions facilitated by changing temperature regimes, modelling distribution of vector species is urgently needed. Here, we modelled the ecological niches and habitat suitability for the two mosquito species Ae. albopictus and Ae. japonicus within their invasive range under current and future climate in Europe using an Ensemble forecasting approach based on ten commonly applied niche modelling algorithms.
The results are broadly consistent with previous studies [17, 18, 23] despite different algorithms, different sets of explaining variables and different, more recent sets of occurrence data. This robustness of modelling indicated the reliability of modelling results.
The pattern of areas with modelled suitable habitat conditions as well as the one-variable response curves reflect the differences in temperature requirements of both species. Under current climate, Ae. albopictus, assumed to be adapted to warmer temperatures (Additional file 3: Table S3), is projected to find suitable conditions in the Mediterranean region, where generally higher temperatures prevail. Ae. japonicus, assumed to be adapted to comparably colder temperatures (Additional file 3: Table S3), is projected to find suitable habitat conditions in the more temperate regions in central Europe. These results are also reflected by a narrower modelled niche for Ae. japonicus with lower temperature optima compared to Ae. albopictus.
Potential distribution under current climatic conditions
Both species find suitable habitat conditions under current climate in distinct areas within Europe: Ae. albopictus within the Mediterranean regions in southern Europe and Ae. japonicus within the more temperate regions of central Europe. Only in a few regions do suitable habitat conditions overlap for both species. These results are in accordance with the currently observed distribution of the species (Additional file 4: Figure S1). The first European record for Ae. albopictus dates back to 1979, when the species was found in Albania. In 1990, Ae. albopictus was recorded for the first time in Italy. Since then, the species has successfully established in large parts of the Mediterranean region in southern Europe [24] (e.g. Italy [47–49], southern France [50] and Romania [51]). Aedes japonicus was recorded for the first time in 2000 in France, but was quickly eradicated [52]. Currently, Ae. japonicus is known from six European countries [30], including Belgium [26], The Netherlands [30], and is regarded as established in Switzerland [26], Austria and Slovenia [29]. Since 2008, Ae. japonicus has been continuously recorded in Germany, with potentially three established populations (in the German federal states North Rhine-Westphalia, Baden-Württemberg, Rhineland-Palatinate and Bavaria [18, 29, 53]). Although Ae. albopictus has repeatedly been trapped during the last few years in southern Germany (e.g. [22], together with Ae. japonicus [54]), it seems questionably if those individuals belong to established populations. In 2015, co-occurrence of Ae. albopictus and Ae. japonicus was observed in northern Italy [55].
Temperature and precipitation requirements
In addition to projecting the habitat suitability, we compared the modelled niche functions of the two invasive species by means of one-variable models using MAXENT. As has been suggested by others ([20, 56, 57]), Ae. albopictus seems to be adapted to warmer climates than Ae. japonicus. Therefore, we expected patterns of habitat suitability in Europe reflecting the species’ temperature requirements (higher temperatures in the south, higher habitat suitability for Ae. albopictus; lower temperatures in the north, higher habitat suitability for Ae. japonicus). This hypothesis is supported by the one-variable response curves regarding the two temperature variables. The response curves for Ae. albopictus show a higher temperature optimum considering the temperatures of coldest and warmest quarter, respectively. Aedes japonicus is known to be a cold tolerant mosquito species; however, it has been shown that higher temperature can positively affect the development of larvae of Ae. japonicus, at least to some extent, whereas temperatures exceeding a certain temperature may be inhibitory [8]. Thus, the southernmost limits of the European range for Ae. japonicus might be ascribed to limiting high temperatures.
The optimum of about 25 °C for the mean temperature of the warmest quarter matches the optimal temperature for adult longevity under laboratory conditions and field conditions stated by [58]. Delatte et al. [57] found the optimal temperature for immature stage development to be about 30 °C and is thus slightly above the optimum of 25 °C.
The response curves indicate a narrower temperature niche for Ae. japonicus compared to Ae. albopictus, which mirrors the formers much smaller native range. The geographically larger native range of Ae. albopictus comprises a much wider range of temperatures with higher mean temperatures (Additional file 3: Table S3). It must be kept in mind, however, that the native range niches of species may differ strongly from the invasive range niches (cf. [23]) due to adaptations during the invasion processes.
Considering the modelled precipitation niches the pattern is not as clear. Both species are reliant on the availability of small aquatic habitats for egg deposition, which requires a certain amount of precipitation during summer months. We hypothesized that precipitation requirements would be similar for both species. This assumption is reflected by both species showing quite similar niches considering the variable annual precipitation (i.e. similar requirements in terms of amplitude and optimum of annual precipitation). However, the one variable response curves do not account for interactions between variables. Thus, a certain amount of precipitation in a warmer or cooler climate leads to very different evaporation rates and therefore very different egg laying habitat compositions. It has been suggested that the role of human water supply to provide breeding sites for both species may be even more important than precipitation conditions [49] and it may thus be assumed that there is no clear relationship between the occurrence of Ae. albopictus and precipitation. This is in accordance with the comparably low contribution of the precipitation variables to the MAXENT model for Ae. albopictus. On the other hand, the relatively high contribution of the variable annual precipitation to the MAXENT model for Ae. japonicus which may be explained be the relatively high precipitation amounts within the northern Alpine foothills (Switzerland), where stable populations of Ae. japonicus are known (e.g. [19]).
Interspecific concurrence
Aedes albopictus has proven to be a superior competitor to Ae. japonicus in artificial container habitats, showing higher overwintering survival and securing more food resources in larval habitats [20, 59]. More specifically, Ae. albopictus larvae showed higher survivorship, shorter developmental time and higher estimated population growth rate compared to Ae. japonicus in competition experiments [20]. This can be partly compensated for by timing of the life-cycle stages of Ae. japonicus. Compared to larvae of Ae. albopictus, larvae of Ae. japonicus can be found earlier in the year [8]. Furthermore, the ability to establish earlier in spring and to remain active for longer in autumn is a characteristic, which, on the one hand, supports the assumed higher cold tolerance of the species, and secondly, allows Ae. japonicus to circumvent intense larval competition or avoid temporal overlap of larval stages with those of co-occurring species in the often highly density-dependent, resource-limited environment of the larval habitat [8]. However, compared to Ae. albopictus, Ae. japonicus generally seems to have a lower intrinsic capacity for population growth and is considered a weak larval competitor [8].
Assessment of modelling performance
Two key limitations should be kept in mind when interpreting the results of habitat suitability modelling for Ae. japonicus. First, the assumption that the distribution of Ae. japonicus in Europe is in equilibrium with its environmental conditions may be violated due to dispersal limitation as the species is considered to be currently in the process of spreading. Secondly, the time period of occurrence data does not match the time period of environmental variables. The occurrence records date from 2009 to 2015; however, empirical data on climatic conditions were not available for Europe for this time period, but only for 1950 up to 2000. Climatic conditions have changed in the interim.
The modelling of Ae. albopictus does not pose the same problems as the occurrence records used for modelling also date from 1950 to 2000, thus overlapping with the climate data. Differences in the patterns of projected habitat suitability as well as the modelled niche functions between Ae. albopictus and Ae. japonicus might not only be attributed to differences in the ecology of the two species but could be ascribed to a certain extent to different assumptions underlying the modelling processes. However, to test whether the same trends still hold true, the incorporation of more recent climatic data would be necessary but was not feasible at the moment.
The use of satellite imagery in species distribution modelling certainly yields valuable information (as recently shown by [60]). However, we here considered climatic effects determine species distribution or habitat suitability at a coarser spatial scale.
Future distribution
Aedes albopictus is considered to be the most invasive mosquito species in the world [6, 24]). Against the background of its vector competence, this species is assumed to become a major threat to public health in Europe [24]. According to our results, there is a clear expansion of the area with modelled habitat suitability for Ae. albopictus in Europe under projected climate change. The projected range expansion of Ae. albopictus in Europe is in accordance with the assumption that the species is adapted to warmer climatic conditions and will be thus promoted by global warming. In contrast, Ae. japonicus is assumed to be adapted to temperate climatic conditions [29]. Under future climatic conditions the potential range of this species (i.e. the area with modelled habitat suitability) seems to decrease. The projected range reduction is probably attributed to increasing temperatures as previously suggested ([8]). Moreover, Ae. japonicus is considered a low competing species, and its projected range restriction induced by climate change may additionally be decreased by further spread of Ae. albopictus in Europe in the face of climate change [8]. However, the projected decrease of the potential range for Ae. japonicus under climate change is based on the assumption that Ae. japonicus is not able to adapt to higher temperature [8]. Results from current monitoring suggest that Ae. japonicus tends to expand its current European range and will be able to colonise new territories in central Europe [53, 55], facilitated by human-mediated, passive transportation [53].
Both species are reliant on the availability of small aquatic habitats for egg deposition and thus on a certain amount of precipitation ([59]). The projected reduction of precipitation in the face of climate change (IPCC, [61]) is assumed to be less important for the two mosquito species compared to the projected raise in temperature in Europe. However, in the face of climate change, precipitation could become an important limiting factor driving the southernmost borders of the European ranges of these mosquito species.
Conclusions
Over the last few decades Aedes albopictus and Ae. japonicus have been accidentally introduced into many countries worldwide, and have shown a rapid and extensive range expansions beyond their native ranges [8]. Both species are assumed to be able to adapt to new climatic conditions outside their native range. Several characteristics of the species (high ecological plasticity, diverse larval habitats and desiccation resistance of eggs; [8]) together with extrinsic factors like increasing tourism and global trade might further promote their invasion success. Due to their vector relevance, further surveillance of the European spread for both species is necessary, focusing on regions where habitat suitability is predicted to be high under future climate scenarios. Although our models for Ae. albopictus and Ae. japonicus under future climate can be used for predictions, risk assessments and monitoring programmes, we still recommend continuously surveying the establishment and spread of the two vector species and potentially adjust models, e.g. considering the potential of niche evolution of mosquito species.
Acknowledgements
The present study was funded by the ERA-Net BiodivERsA, with the national funders DFG KL 2087/6-1, FWF I-1437 and ANR-13-EBID-0007-01 as part of the 2012–13 BiodivERsA call for research proposals and by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant number 2819105115.
Funding
The present study was funded by the ERA-Net BiodivERsA, with the national funders DFG KL 2087/6-1, FWF I-1437 and ANR-13-EBID-0007-01 as part of the 2012–13 BiodivERsA call for research proposals and by the German Federal Ministry of Food and Agriculture (BMEL) through the Federal Office for Agriculture and Food (BLE), grant number 2819105115.
The funding bodies had no role in the design of the study and collection, analysis, and interpretation of the data and in writing the manuscript.
Availability of data and material
Not applicable.
Authors’ contributions
SC, SK conceived and designed the study. SC, LKK compiled the data. SC performed the analysis. SC, LKK, JK, SK contributed to the writing of the manuscript. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Abbreviations
- ANN
Artificial neural networks
- AUC
Area under the receiver operating characteristic curve
- CTA
Classification tree analysis
- FDA
Flexible discriminant analysis
- GAM
Generalized additive models
- GBM
Generalized boosted models
- GLM
Generalized linear models
- IPCC
Intergovernmental Panel on Climate Change
- MARS
Multivariate adaptive regression splines
- MAXENT
Maximum entropy approach
- RF
Random forest algorithm
- SDM
Species distribution model
- SRE
Surface range envelope
Additional files
Spearman correlation coefficients of the six environmental variables provided by worldclim data (www.wordclim.org) within the study area: mean temperature of warmest quarter (bio10); mean temperature of coldest quarter (bio11); temperature range (bio07); precipitation of warmest quarter (bio18); annual precipitation (bio12); precipitation seasonality (bio15). (DOCX 14 kb)
AUC values for the single models as well as for the consensus model resulting from the Ensemble forecasting including all ten single models. (DOCX 13 kb)
Temperature conditions for Aedes albopictus and Ae. japonicus within the native range derived from the worldclim data (www.wordclim.org) (DOCX 15 kb)
Observed European distribution for Aedes albopictus and Ae. japonicus. Occurrence records as used for modelling: assembled by Kraemer MUG et al. [25] and Koch et al. [17] for Ae. albopictus and by Schaffner et al. [26]; Huber et al. [27]; Huber et al. [19]; Krebs et al. [28]; Zielke et al. [29]; Melaun et al. [18] and Zielke et al. [30] for Ae. japonicus. The records were adjusted to the raster of the environmental variables (about 10 × 10 km). (PNG 5199 kb)
Contributor Information
Sarah Cunze, Email: sarahcunze@gmail.com.
Lisa K. Koch, Email: Lisa.Koch@senckenberg.de
Judith Kochmann, Email: judith.kochmann@senckenberg.de.
Sven Klimpel, Email: Klimpel@bio.uni-frankfurt.de.
References
- 1.Walther G, Roques A, Hulme PE, Sykes MT, Pyšek P, Kühn I, et al. Alien species in a warmer world: risks and opportunities. Trends Ecol Evol. 2009;24(12):686–93. doi: 10.1016/j.tree.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Pompe S, Hanspach J, Badeck F, Klotz S, Thuiller W, Kühn I. Climate and land use change impacts on plant distributions in Germany. Biol Lett. 2008;4(5):564–7. doi: 10.1098/rsbl.2008.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guisan A, Tingley R, Baumgartner JB, Naujokaitis-Lewis I, Sutcliffe PR, Patrica R, et al. Predicting species distributions for conservation decisions. Ecol Lett. 2013;16(12):1424–35. doi: 10.1111/ele.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Model. 2009;135(2):147–86. [Google Scholar]
- 5.Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11(14–15):1177–85. [DOI] [PubMed]
- 6.Buhagiar J. A second record of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Malta. Eur Mosquito Bull. 2009;27:65–7.
- 7.Bonizzoni M, Gasperi G, Chen X, James AA. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol. 2013;29(9):460–8. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman MG, Fonseca DM. Invasion biology of Aedes japonicus japonicus (Diptera: Culicidae) Annu Rev Entomol. 2014;59:31–49. doi: 10.1146/annurev-ento-011613-162012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, Crepeau T, et al. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J Med Entomol. 2012;49(4):813–24. doi: 10.1603/ME11031. [DOI] [PubMed] [Google Scholar]
- 10.Boes KE, Ribeiro JMC, Wong A, Harrington LC, Wolfner MF, Sirot L. Identification and characterization of seminal fluid proteins in the Asian tiger mosquito, Aedes albopictus. PLoS Negl Trop Dis. 2014;8(6) doi: 10.1371/journal.pntd.0002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx Gómez J, Sonnenschein M, Vogel U, Winter A, Rapp B, Giesen N, editors. Oldenburg: BIS-Verlag. ISBN 978-3-8142-2317-9.
- 12.Ngoagouni C, Kamgang B, Nakouné E, Paupy C, Kazanji M. Invasion of Aedes albopictus (Diptera: Culicidae) into central Africa: what consequences for emerging diseases. Parasit Vectors. 2015;8:191. doi: 10.1186/s13071-015-0808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medlock JM, Snow KR, Leach S. Potential transmission of West Nile virus in the British Isles: an ecological review of candidate mosquito bridge vectors. Med Vet Entomol. 2005;19(1):2–21. doi: 10.1111/j.0269-283X.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 14.Becker N, Geier M, Balczun C, Bradersen U, Huber K, Kiel E, et al. Repeated introduction of Aedes albopictus into Germany, July to October 2012. Parasitol Res. 2013;112(4):1787–90. doi: 10.1007/s00436-012-3230-1. [DOI] [PubMed] [Google Scholar]
- 15.Kampen H, Werner D. Out of the bush: the Asian bush mosquito Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) becomes invasive. Parasit Vectors. 2014;7(1):1. [DOI] [PMC free article] [PubMed]
- 16.Pluskota B, Jöst A, Augsten X, Stelzner L, Ferstl I, Becker N. Successful overwintering of Aedes albopictus in Germany. Parasitol Res. 2016;115(8):3245–7. [DOI] [PubMed]
- 17.Koch LK, Cunze S, Werblow A, Kochmann J, Dörge DD, Mehlhorn H, et al. Modeling the habitat suitability for the arbovirus vector Aedes albopictus (Diptera: Culicidae) in Germany. Parasitol Res. 2015;115(3):957–64. doi: 10.1007/s00436-015-4822-3. [DOI] [PubMed] [Google Scholar]
- 18.Melaun C, Werblow A, Cunze S, Zotzmann S, Koch LK, Mehlhorn H, et al. Modeling of the putative distribution of the arbovirus vector Ochlerotatus japonicus japonicus (Diptera: Culicidae) in Germany. Parasitol Res. 2015;114(3):1051–61. doi: 10.1007/s00436-014-4274-1. [DOI] [PubMed] [Google Scholar]
- 19.Huber K, Schuldt K, Rudolf M, Marklewitz M, Fonseca DM, Kaufmann C, et al. Distribution and genetic structure of Aedes japonicus japonicus populations (Diptera. Culicidae) in Germany. Parasitol Res. 2014;113(9):3201–10. doi: 10.1007/s00436-014-4000-z. [DOI] [PubMed] [Google Scholar]
- 20.Armistead JS, Arias JR, Nishimura N, Lounibos LP. Interspecific larval competition between Aedes albopictusandAedes japonicus (Diptera. Culicidae) in Northern Virginia. J Med Entomol. 2008;45(4):629–37. [DOI] [PMC free article] [PubMed]
- 21.Kampen H, Zielke D, Werner D. A new focus of Aedes japonicus japonicus (Theobald, 1901) (Diptera, Culicidae) distribution in Western Germany: rapid spread or a further introduction event. Parasit Vectors. 2012;5(1):1–6. doi: 10.1186/1756-3305-5-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner D, Kronefeld M, Schaffner F, Kampen H. Two invasive mosquito species, Aedes albopictus and Aedes japonicus japonicus, trapped in south-west Germany, July to August 2011. Euro Surveill. 2012;17(4):20067. doi: 10.2807/ese.17.04.20067-en. [DOI] [PubMed] [Google Scholar]
- 23.Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob Planet Chang. 2011;78(1):54–64. doi: 10.1016/j.gloplacha.2011.05.008. [DOI] [Google Scholar]
- 24.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12(6):435–47. [DOI] [PMC free article] [PubMed]
- 25.Kraemer MUG, Sinka ME, Duda KA, Mylne A, Shearer FM, Barker CM, et al. Data from: The global compendium of Aedes aegypti and Ae. albopictus occurrence. Scientific Data. 2015. [DOI] [PMC free article] [PubMed]
- 26.Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23(4):448–51. doi: 10.1111/j.1365-2915.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 27.Huber K, Pluskota B, Jöst A, Hoffmann K, Becker N. Status of the invasive species Aedes japonicus japonicus (Diptera: Culicidae) in southwest Germany in 2011. J Vector Ecol. 2012;37(2):462–5. doi: 10.1111/j.1948-7134.2012.00252.x. [DOI] [PubMed] [Google Scholar]
- 28.Krebs T, Bindler P, L'Ambert G, Toty C, Perrin Y, Jourdain F. First establishment of Aedes japonicus japonicus (Theobald, 1901) (Diptera. Culicidae) in France in 2013 and its impact on public health. J Vector Ecol. 2014;39(2):437–40. doi: 10.1111/jvec.12119. [DOI] [PubMed] [Google Scholar]
- 29.Zielke DE, Werner D, Schaffner F, Kampen H, Fonseca DM. Unexpected patterns of admixture in German populations of Aedes japonicus japonicus (Diptera: Culicidae) underscore the importance of human intervention. PLoS One. 2012;9(7) doi: 10.1371/journal.pone.0099093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zielke DE, Ibáñez-Justicia A, Kalan K, Merdić E, Kampen H, Werner D. Recently discovered Aedes japonicus japonicus (Diptera. Culicidae) populations in The Netherlands and northern Germany resulted from a new introduction event and from a split from an existing population. Parasit Vectors. 2015;8(1):40. doi: 10.1186/s13071-015-0648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–78. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- 32.Ramirez J, Jarvis A. High resolution statistically downscaled future climate surfaces. Cali: International Center for Tropical Agriculture (CIAT), CGIAR Research Program on Climate Change, Agriculture, and Food Security (CCAFS); 2008. [Google Scholar]
- 33.Meinshausen M, Smith SJ, Calvin K, Daniel JS, Kainuma MLT, Lamarque J-F, et al. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim Change. 2011;109(1–2):213–41. doi: 10.1007/s10584-011-0156-z. [DOI] [Google Scholar]
- 34.IPCC . Climate Change 2013: The Physical Science Basis. Contribution of working group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC Fifth Assessment Report (AR5) New York: University Press; 2013. [Google Scholar]
- 35.Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007;22(1):42–7. [DOI] [PubMed]
- 36.Segurado P, Araujo MB. An evaluation of methods for modelling species distributions. J Biogeogr. 2004;31(10):1555–68. doi: 10.1111/j.1365-2699.2004.01076.x. [DOI] [Google Scholar]
- 37.Elith J, Graham C, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29(2):129–51. doi: 10.1111/j.2006.0906-7590.04596.x. [DOI] [Google Scholar]
- 38.Marmion M, Luoto M, Heikkinen RK, Thuiller W. The performance of state-of-the-art modelling techniques depends on geographical distribution of species. Selected Papers on Spatially Explicit Landscape Modelling. Current practices and challenges. 2009;220(24):3512–20. [Google Scholar]
- 39.Thuiller W, Georges D, Engler R. Package ‘biomod2’. Available from: https://cran.r-project.org/web/packages/biomod2/. Accessed Dec 2015.
- 40.Reiss H, Cunze S, König K, Neumann H, Kröncke I. Species distribution modelling of marine benthos. A North Sea case study. Mar Ecol Prog Ser. 2011;442:71–86. doi: 10.3354/meps09391. [DOI] [Google Scholar]
- 41.Liu C, White M, Newell G, Pearson R. Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr. 2013;4:778–89. doi: 10.1111/jbi.12058. [DOI] [Google Scholar]
- 42.Phillips SJ, Miroslav D, Schapire RE. Proceedings of the Twenty-First International Conference on Machine Learing. 2004. A maximum entropy approach to species distribution modeling. [Google Scholar]
- 43.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model. 2006;190(3):231–59. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- 44.Warren DL, Seifert SN. Ecological niche modeling in Maxent. The importance of model complexity and the performance of model selection criteria. Ecol Appl. 2011;21(2):335–42. doi: 10.1890/10-1171.1. [DOI] [PubMed] [Google Scholar]
- 45.Cunze S, Tackenberg O. Decomposition of the maximum entropy niche function - a step beyond modelling species distribution. Environ Model Softw. 2015;72:250–60.
- 46.Baldwin RA. Use of maximum entropy modeling in wildlife research. Entropy. 2009;11(4):854–66. doi: 10.3390/e11040854. [DOI] [Google Scholar]
- 47.Giorgio C, Romeo B, Marco C. Survey on Aedes albopictus (Skuse) (Diptera: Culicidae) infestation in Desenzano del Garda (Brescia province-Italy) Boll Ist Ent 'G Grandi' Univ Bologna. 1994;48:211–217. [Google Scholar]
- 48.Urbanelli S, Bellini R, Carrieri M, Sallicandro P, Celli G. Population structure of Aedes albopictus (Skuse): the mosquito which is colonizing Mediterranean countries. Heredity. 2000;84(3):331–7. doi: 10.1046/j.1365-2540.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 49.Roiz D, Rosa R, Arnoldi D, Rizzoli A. Effects of temperature and rainfall on the activity and dynamics of host-seeking Aedes albopictus females in northern Italy. Vector Borne Zoonotic Dis. 2010;10(8):811–6. [DOI] [PubMed]
- 50.Roche B, Léger L, L’Ambert G, Lacour G, Foussadier R, Besnard G, et al. The spread of Aedes albopictus in Metropolitan France: contribution of environmental drivers and human activities and predictions for a near future. PLoS One. 2015;10(5) doi: 10.1371/journal.pone.0125600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prioteasa LF, Dinu S, Fălcuţă E, Ceianu CS. Established population of the invasive mosquito species Aedes albopictus in Romania, 2012–14. J Am Moquito Contr. 2015;31(2):177–81. doi: 10.2987/14-6462R. [DOI] [PubMed] [Google Scholar]
- 52.Schaffner F, Chouin S, Guilloteau J. First record of Ochlerotatus (Finlaya) japonicus japonicus (Theobald, 1901) in metropolitan France. J Am Moquito Contr. 2003;19(1):1–5. [PubMed]
- 53.Zielke DE, Walther D, Kampen H. Newly discovered population of Aedes japonicus japonicus (Diptera: Culicidae) in Upper Bavaria, Germany, and Salzburg, Austria, is closely related to the Austrian/Slovenian bush mosquito population. Parasit Vectors. 2016;9(1):163. doi: 10.1186/s13071-016-1447-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Werner D, Kampen H. Aedes albopictus breeding in southern Germany, 2014. Parasitol Res. 2015;114(3):831–4. doi: 10.1007/s00436-014-4244-7. [DOI] [PubMed] [Google Scholar]
- 55.Seidel B, Montarsi F, Huemer HP, Indra A, Capelli G, Allerberger F, et al. First record of the Asian bush mosquito, Aedes japonicus japonicus, in Italy: invasion from an established Austrian population. Parasit Vectors. 2016;9(1):284. doi: 10.1186/s13071-016-1566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grim DC, Jackson BT, Paulson SL. Abundance and bionomics of Ochlerotatus j. japonicus in two counties in southwestern Virginia. J Am Moquito Contr. 2007;23(3):259–63. doi: 10.2987/8756-971X(2007)23[259:AABOOJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 57.Delatte H, Gimonneau G, Triboire A, Fontenille D. Influence of temperature on immature development, survival, longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of chikungunya and dengue in the Indian Ocean. J Med Entomol. 2009;46(1):33–41. doi: 10.1603/033.046.0105. [DOI] [PubMed] [Google Scholar]
- 58.Brady OJ, Johansson MA, Guerra CA, Bhatt S, Golding N, Pigott DM, et al. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors. 2013;6(1):351. doi: 10.1186/1756-3305-6-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medlock JM, Hansford KM, Versteirt V, Cull B, Kampen H, Fontenille D, et al. An entomological review of invasive mosquitoes in Europe. B Entomol. 2015;105(6):637–63. doi: 10.1017/S0007485315000103. [DOI] [PubMed] [Google Scholar]
- 60.Neteler M, Roiz D, Rocchini D, Castellani C, Rizzoli A. Terra and aqua satellites track tiger mosquito invasion: modelling the potential distribution of Aedes albopictus in north-eastern Italy. Int J Health Geogr. 2011;10:49. doi: 10.1186/1476-072X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pachauri RK, Mayer L. Climate change 2014. Synthesis report. Geneva: Intergovernmental Panel on Climate Change; 2015. p. 151. [Google Scholar]


