Abstract
Background
Intermittent preventive treatment of malaria in pregnancy with 3+ doses of sulfadoxine-pyrimethamine (IPTp-SP) reduces maternal mortality and stillbirths in malaria endemic areas. Between December 2014 and December 2015, a project to scale up IPTp-SP to all pregnant women was implemented in three local government areas (LGA) of Sokoto State, Nigeria. The intervention included community education and mobilization, household distribution of SP, and community health information systems that reminded mothers of upcoming SP doses. Health facility IPTp-SP distribution continued in three intervention (population 661,606) and one counterfactual (population 167,971) LGAs. During the project lifespan, 31,493 pregnant women were eligible for at least one dose of IPTp-SP.
Methods
Community and facility data on IPTp-SP distribution were collected in all four LGAs. Data from a subset of 9427 pregnant women, who were followed through 42 days postpartum, were analysed to assess associations between SP dosages and newborn status. Nominal cost and expense data in 2015 Nigerian Naira were obtained from expenditure records on the distribution of SP.
Results
Eighty-two percent (n = 25,841) of eligible women received one or more doses of IPTp-SP. The SP1 coverage was 95% in the intervention LGAs; 26% in the counterfactual. Measurable SP3+ coverage was 45% in the intervention and 0% in the counterfactual LGAs. The mean number of SP doses in the intervention LGAs was 2.1; 0.4 in the counterfactual. Increased doses of IPTp-SP were associated with linear increases in newborn head circumference and lower odds of stillbirth. Any antenatal care utilization predicted larger newborn head circumference and lower odds of stillbirth. The cost of delivering three doses of SP, inclusive of the cost of medicines, was US$0.93–$1.20.
Conclusions
It is feasible, safe, and affordable to scale up the delivery of high impact IPTp-SP interventions in low resource malaria endemic settings, where few women access facility-based maternal health services.
ClinicalTrials.gov Identifier NCT02758353. Registered 29 April 2016, retrospectively registered
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-016-1578-x) contains supplementary material, which is available to authorized users.
Keywords: Malaria in pregnancy, IPTp-SP, Scale up, Integrated MNH, Primary health care, Human-centered design, Community engagement, Community-based health workers, Sokoto State, Nigeria
Background
In 2015, there were an estimated 214 million malaria cases and 438,000 malaria deaths in the world [1]. Sub-Saharan Africa accounted for 89% of global malaria cases and 91% of malarial deaths [2]. Nigeria alone accounted for almost 25% of malarial deaths in Africa [3]. In 2009, the Nigeria Federal Ministry of Health estimated that malaria was a direct contributor to 11% of overall maternal mortality, 25% of infant mortality and 30% of under-five mortality [3].
In Nigeria–with its estimated 6.35 million annual births in 2015—and in malaria-endemic settings, pregnant women, primipara in particular, are vulnerable to malarial infection [4–6]. On account of weakened immune systems, pregnant women are four times more likely than non-pregnant adults to suffer from symptomatic forms of malaria [6]. Furthermore, social factors such as unequal balance of power between women and men, constrain women’s equitable access to financing and health. This double burden undermines women’s ability to respond appropriately, and access prevention and treatment for malaria in pregnancy, even when services are available [7].
WHO-recommended strategies for the prevention and management of malaria during pregnancy comprise a three-pronged approach: (1) use of insecticide treated nets, (2) intermittent preventive treatment (IPTp), and (3) effective case management of malarial illness [8]. This approach was nuanced in 2012 with a call for countries to scale up IPTp [9]. WHO recommends the use of sulfadoxine-pyrimethamine (IPTp-SP), with a prescribed minimum of three doses during focused antenatal care visits. Each dose of IPTp-SP is expected to be recorded to enhance the monitoring of pregnant women that receive successive doses (i.e., IPTp-1, IPTp-2, IPTp-3, IPTp-4, etc.).
Malaria and fetal growth
Malaria in pregnancy (MiP) has been shown to have an impact on fetal growth and birth outcomes [6, 10, 11]. Small size at birth has implications for newborn survival and long-term implications for a child’s growth and development [19]. Most studies on the impact of placental malaria on fetal growth have relied on birthweight as the outcome. In resource poor settings where many women give birth at home, mechanisms are scarce to weigh newborns [12–15], Sreeramareddy et al. have argued that in contexts where home births are prevalent, surrogate measurement approaches that do not require a weighing scale to determine low birth weight, are recommended for implementation [12].
Malaria and stillbirths
There were an estimated 2.6 million (2.4–3.0 million) stillborn babies in the world in 2015 [16]. Ninety-eight percent of stillbirths occurred in low and middle income countries; Africa and Asia accounted for 77% of the global stillbirth burden [16]. With an estimated 40.1 stillbirths per 1000 live births, Nigeria has the second highest number of stillbirths in the world [17]. Nonetheless, the true magnitude of stillbirths in Nigeria is difficult to estimate in the absence of standardized national policies or protocols that enjoin facilities and communities to record, audit or to review causes of stillbirth [18].
Van Geertruyden et al. in a systematic review of 117 malarial studies conducted between 1948 and 2002 found that placental malaria was significantly associated with an increased odds of stillbirth (OR 2.19; 95% CI 1.49–3.22) [19]. However, Radeva-Petrova et al. noted that there were too few publications that studied a direct association between IPTp-SP and stillbirths [20]. The authors concluded that published studies were too “underpowered to collect clinically important differences” [20].
IPTp-SP and ITNs for the prevention of malaria in pregnancy in Nigeria
Nigeria adopted the IPTp-SP strategy in 2005 [21]. SP was included in the national essential list of medicines as an over-the-counter medicine in 2005 [22]. Nigeria’s efforts to deliver IPTp-SP services during ANC care have not been successful or impactful [23]. In 2013, only 23% of women who had given birth in the two years preceding a survey received any dose of IPTp-SP when pregnant [24]. About 15% had received two doses of and just 6% received three doses of SP (SP3) [24]. In effect, the majority of pregnant women and their unborn babies in Nigeria are not adequately protected from placental malaria and its consequences [25].
A concern expressed in a 2013 WHO Consensus Statement on MiP, equally applicable to Nigeria, was that “despite clear global gains in malaria control, the delivery of MiP interventions remains suboptimal in most endemic countries” [9]. Too many women that attended antenatal care did not receive at least two doses of SP before delivery. There is now an urgent global call for new approaches and interventions to reduce missed opportunities and, in particular, to substantially increase the coverage of SP among pregnant women [9]. In 2013, the WHO Policy Brief for the implementation of IPTp called for research on “innovative strategies to improve the delivery of IPTp-SP and malaria case management among pregnant women at the primary health centre level”, and for “innovative community strategies that do not detract from ANC services to increase IPTp coverage (such as community-based ANC outreach, promotion or distribution of IPTp)” [26]. The policy brief also called for research into “methods for using health system information systems for routine monitoring of IPTp-SP implementation and effectiveness” [26].
A recent study in Southeastern Nigeria that utilized community-based workers to successfully distribute IPT-SP in community settings, reported that women in intervention areas were significantly more likely to ingest at least two doses of IPTp-SP [27]. However, the study did not test for the scalability of community-based distribution, nor did it track for associated newborn outcomes.
The Sokoto State malaria in pregnancy project (MIPP)
MiPP was a yearlong project funded by Bill and Melinda Gates Foundation and implemented by JSI Research & Training Institute, Inc. that ended in December 2015. The project was undertaken in three local government areas (LGAs) of Sokoto state, Nigeria. The default IPTp-SP programme was a facility-only activity operated as part of its focused antenatal care services based on national guidelines [21]. The MiPP programme, to the best of our knowledge, is the first project in Nigeria and elsewhere, to include community-based delivery approaches and to track at scale, the number of IPTp-SP doses ingested by pregnant women, and associated newborn outcomes.
Sokoto State
Sokoto State is located in the North West Zone of Nigeria between longitude 11′′ 30–13′′ 50 and latitude 4′′–6′′. It borders Niger Republic to the north and Benin Republic to the northwest, Kebbi State to south and Zamfara State to the east. It has a land mass area of about 32,000 sq km, and consists of 23 local government areas and 244 political wards. Ward Development Committees (WDCs) are the smallest unit of governance which typically manage a revolving drug fund, supervise a cadre of community-based health volunteers (CBHVs), and oversee primary health facilities in their jurisdiction [28]. The population is predominantly rural, Muslim and consists almost entirely of Hausa/Fulani ethnic groups.
Sulfadoxine-pyrimethamine and insecticide-treated net use profile in Sokoto State
Malaria is seasonal in Sokoto State, with peak transmission from May to December. The highest point prevalence of parasitaemia—which mirrors rainfall patterns—is in August (59.5%), and the lowest is in March (9.18%) [29]. In 2013, the state prevalence rate of MiP was 9% and it ranged from 35% in the highest burden LGA to 1% in the lowest burden [30]. In 2013, 80% of women in the state did not use antenatal care in their last pregnancy—the largest non-use of ANC services by a state in Nigeria [24]. Of the 17.4% of women that obtained ANC from a skilled attendant, three in five received no SP whatsoever [24]. Less than 5% and less than 1% of all pregnant women in the state received SP2 and SP3 respectively in 2013 [24].
Study overview
The study objectives were to:
Examine scale-up mechanisms that enable increased SP coverage through community-based primary health care delivery, without reducing facility uptake of SP.
Examine community acceptance of SP and the likelihood of long-term community-sustained demand.
Document associations, if any, between increased SP3 coverage and improved intrauterine conditions for newborn, as measured by head circumference increments and declines in still birth rates.
Estimate the costs of delivering SP at scale per woman for a three doses or higher regimen.
The MiPP intervention
The intervention consisted of house-to-house distribution of SP to eligible pregnant women—administered through directly observed treatment (DOTs)—by trained community-based health volunteers (CBHVs). Community distribution of SP was limited to the first three doses of SP; SP4 and higher doses were intentionally designated for administration in a health facility, to promote the use of facilities. The MiPP intervention was twinned with an ongoing facility-based SP distribution as well as case management of suspected cases of malaria in health centers in intervention and counterfactual LGAs. Similarly, established and ongoing LLIN distribution continued in health facilities in both intervention and counterfactual LGAs.
Outcomes tracked
The programme tracked three principal outcomes. The first was the percentage IPTp-SP coverage among all pregnant women between April and November 2015 and by number of SP doses ingested. Head circumferences of live newborns was tracked, as a measure of intrauterine growth function. Head circumference was measured in centimetres, to the 10th of a centimetre, using a standardized protocol. A tape measure was placed above the ears and midway between eyebrows and the hairline to the occipital prominence at the back of the head. CBHV supervisors, all of whom were literate and numerate, carried out the measurements. Newborn head circumference, as a measure of intrauterine growth was tracked instead birthweight because it was considered less susceptible to the obesogenic effects of pregnancy [31]. Newborn head circumference has also been shown to be inversely related to and sensitive to malaria-associated lesions of the placenta [13, 31, 32]. Finally, in the context of Sokoto State, the measurement of head circumference was less intrusive and more readily managed by community health workers with adequate training.
Head circumference measurements were limited to live newborns whose mothers resided in one of four project LGAs, that were delivered—home or facility births—during the intervention period. Head circumference measurements were not undertaken in newborns whose mothers (a) recently migrated into the intervention area and had not been exposed to the intervention (b) older than seven days postpartum at the time of measurement, and (c) stillborn babies. Head circumference measurements were limited to those obtained within seven days postpartum to reduce temporal biases in our head circumference measurements. It was not culturally permissive to measure the head circumference of stillborn babies.
The third outcome was the incidence of stillbirths. Stillbirths were defined as deliveries that occurred after 7 months of gestation in which a baby was birthed without any signs of life (no breathing, no movement, and no sound) as reported by the mother or an informed family member.
Intervention LGAs and the counterfactual LGA selection
Dange Shuni Goronyo and Silame (combined 2015 population, according to official Sokoto State estimates = 661,606) LGAs were purposively selected as intervention LGAs, and Yabo LGA, the fourth (2015 population, according to official Sokoto State estimates = 167,971), was selected as the counterfactual LGA. The selection criteria were that all LGAs had a high prevalence of malaria in pregnancy and that at one LGA each in the intervention group, was selected from each of the State’s three senatorial zones. Such political balance was considered important for the potential future adoption of study recommendations across Sokoto state. Figure 1 shows a mapping of the intervention and counterfactual LGAs.
Fig. 1.
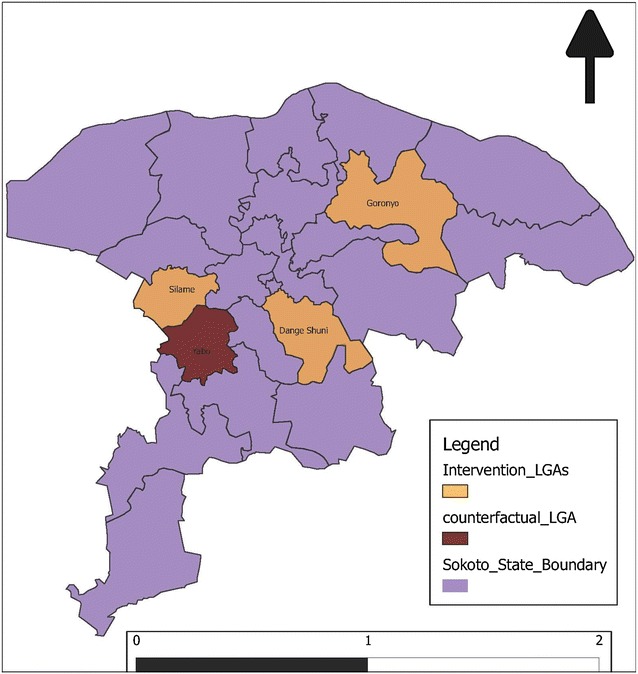
Map of intervention and counterfactual LGAs
Mapping households and identifying pregnant women in the community
In the three intervention LGAs, households were mapped and all women of reproductive age (WRA) were enumerated and registered in a household registration system (HRS) database. Designed with a household as the unit of analysis, the database contained compound numbers, household numbers, the number of WRA, pregnant women, and children under five. It also identified each woman—at the time of mapping exercise—that was pregnant, that was eligible for SP, when she got what SP dose, and where she resided. Any changes in these parameters in a given household were recorded into the Household Register Book (HRB), which was used to update the HRS on a monthly basis. Households were not mapped in the counterfactual LGA. Instead, official census projections were used to estimate the expected number of eligible pregnant women during the study period.
Implementing the malaria in pregnancy project
The IPTp-SP distribution built upon an existing community based distribution system introduced in 2013 by Sokoto State government with technical support from JSI/USAID. The community-based health volunteer system is comprised of 10 CBHVs per ward, which formed a cadre of 2440 CBHVs throughout the 244 wards in the State. CBHVs had already been trained to deliver the key counseling messages prescribed by the WHO and Nigeria’s National Primary Health Care Development Agency, and were assigned the additional task of delivering SP to women at home. CBHVs already had a system in place to implement an on-demand distribution of chlorhexidine gel 7.1% digluconate and misoprostol tablets to women that delivered their babies in home settings, which is shown in Fig. 2 [24, 33]. The study added five additional CBHV per ward to increase the geographic coverage of the intervention LGAs. With these additions, the number of CBHVs per 10,000 residents in the intervention LGAs of Silame, Dange Shuni and Goronyo were 10, 7 and 6, respectively. It was 7 per 10,000 in Yabo, the counterfactual LGA.
Fig. 2.
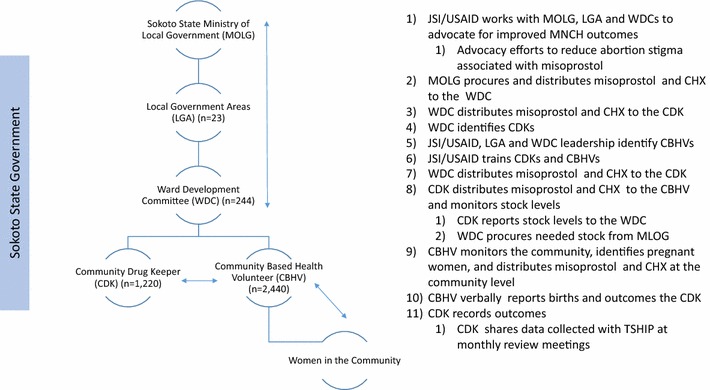
Sokoto State chlorhexidine–misoprostol distribution system
The medicine delivery component of the CBHV system had an inbuilt data collection system managed by a community drug keeper (CDK) and a supervising facility-based health worker to monitor distribution at the community level. Data captured in the outcome form included the condition of the newborn and mother at birth, of the newborn at birth—stillbirth or live birth—at days 7, 14 and 28 postpartum. The study team modified the outcome form to capture the number of SP doses a woman received in the intervention LGAs and data collection was managed by CBHV supervisors. In the counterfactual LGA, the outcome form was revised to exclude intervention-related questions that did not apply. It nonetheless inquired a pregnant woman’s primipara status, ANC status, SP doses taken, gestation at time of delivery, the state of newborns, and head circumference measurements. In the place of CBHV supervisors in the counterfactual LGA, 10 data collectors were recruited. These data collectors were titled as “home visitors” as they had no role in CBHV supervision. See Additional file 1 for examples of the modified outcome forms, for the intervention and counterfactual LGAs.
SP commodity logistics
In every ward in the intervention LGAs, one designated health facility served as a supply hub for SP intended for community distribution. The health facilities were selected based on their central proximity in a ward, possessing a good volume of clients, the availability of skilled service providers, and satisfactory medicines storage capacity. These health facilities were part of a long established statewide supply grid for malaria commodities including artemisinin-based combination therapy (ACT) medicines, rapid diagnostic testing kits (RDT), (LLIN) and SP. These commodities were provided by US Presidential Malaria Initiative (PMI) through the USAID/JSI DELIVER Project. Health facilities in the counterfactual LGAs were similarly supplied with identical malaria commodities. Two service providers from each designated health facility were co-trained with CBHV supervisors on the community distribution process and on the Nigeria National Malaria policy guidelines—on SP dosing, malaria case management—referrals and documentation of services rendered into registers and commodity stock books. Health facilities supplied SP tablets to CHBV supervisors who in turn distributed them to the CBHVs in the wards. Within each ward, a CBHV regularly covered a defined catchment of compounds/households.
SP distribution process
The scale-up of community distribution of SP was trialed in three wards (one ward per LGA) for 1 week, and reached all known pregnant women eligible for SP (n = 264). During the trial, it was learned that the system used to identify households caused avoidable delays in tracking women. It did not work for the CBHVs, most of whom were unable to read or write. It was successfully replaced at scale up by the use of simple pictograms that uniquely identified households. Additional file 2 provides a sample of the pictograms used by the CBHV’s. CBHVs and their supervisors met weekly, and updated their lists of eligible pregnant women for IPTp–SP, and made quantifications of SP doses needed.
At the household level, CBHVs counselled pregnant women and inquired about any symptoms of malaria, the gestational age of pregnancy and presence of quickening, history of SP administration within last 4 weeks, a history of reaction to sulphur-containing drugs. CBHVs counseled women on the benefits of IPTp-SP, of LLIN utilization, good nutrition and the importance of ANC visits. Eligible and healthy pregnant women were given a dose of SP administered via DOTs and told the date of their next monthly dose. CBHVs issued a colour-coded card linked to a specific dosage administered—white for 1st dose, yellow for 2nd dose and green for the 3rd dose. Each SP card was pre-recorded with a given woman’s compound number, household number and her unique number. Mothers were advised to bring their SP cards to ANC clinic visits. This setup enabled more reliable data exchange with health workers in health facilities on what doses a woman had received at home. Examples of the cards used to track SP doses can be found in Additional file 3. Figure 3 shows the decision tree for IPTp-SP dosage and referral protocol applied in the MiPP project.
Fig. 3.
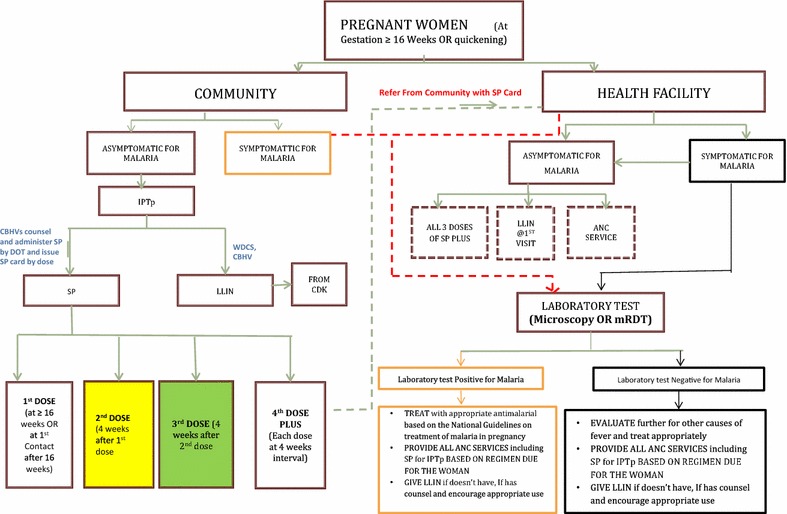
Decision tree for SP dosage and referral
Review meetings
Two sets of review meetings—a monthly one with CBHVs and their supervisors and a bi-monthly one with LGA officers—were regularly conducted. At monthly ward meetings, CBHVs with their supervisors, service providers and WDC members, LGA RBM officer, LGA M&E officer and project team members reviewed the month’s work and exchanged experiences. Three wards were clustered per meeting for cross-learning. One outcome of such meetings, at the behest of community leaders, was the introduction of outreach ANC services to underserved hard-to-reach areas. ANC clinics run by community health extension workers and midwives were held at least thrice there so that three doses of SP were delivered.
At LGA bi-monthly review meetings, feedback was provided to LGA level stakeholders, and lessons for future use beyond the project, were drawn. Participants at these meetings included the LGA chairman or his designate, the LGA primary health care director and team (the MCH coordinator, RBM officer and M&E officer), district heads from the LGA, WDC representatives, representatives of the State Primary Health Care Development Authority, representatives of the Ministry of Local Government, representatives of facility-based service providers, three representative CBHV supervisors and the project staff. Figure 4 shows the planning schema and participants for the MiPP review meetings.
Fig. 4.
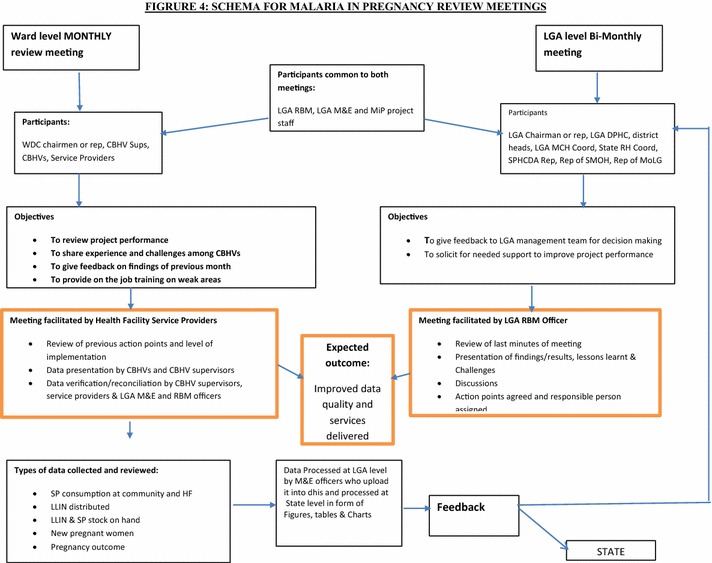
Planning schema for MiPP project review meetings
Monitoring the safety of IPTp-SP delivery
During the project, all service providers searched proactively for reports of adverse reactions to SP use in pregnant women. All CBHVs and their supervisors were trained on how to ask about any adverse reactions and how to document them if they occured. The imperative that there be a clear cut history of quickening as a prerequisite for SP use in pregnant women, was reinforced in trainings and throughout all review meetings and supervision encounters at ward, facility and LGA levels. WDC members were also asked to report any suspected adverse reactions to SP in their communities. At the health facility where such practices were the norm, service providers regularly checked for suspected reactions for onward referral to a secondary referral health facility. As found in other studies, IPTp-SP was well tolerated; there were no reported cases of severe adverse reaction to SP [34].
Ensuring data quality on SP consumption and reporting at facility and community levels
Data quality checks of SP doses administered were performed on health facilities’ and CBHV Supervisors’ records. At the facility level, daily SP services are typically summarized into a monthly summary form (MSF), which is entered into a cloud-based national DHIS2 database that constitutes Nigeria’s national monthly summary health indicators reporting system. The MSF was used as the standard with which to measure accuracy. Within the DHIS2, each facility that reports data is named and so it is possible to compare a facility’s DHIS2 data with what was reported in the same facility’s MSF. Between June and November, monthly reviews of the DHIS2 reported data in a given facility was cross-checked with the MSF. Inconsistencies were compiled and shared with a team of the LGA Monitoring and Evaluation Officer (LGA M&E Officer), the LGA Roll Back Malaria Officer (LGA RBM Officer) and the team leader of health facilities, for resolution. For example, the problem of non-reporting by some facilities was traced to insufficient coordination between the LGA RBM Officer and M&E Officers and to lack of transportation to reach out to distally located facilities. Health facilities in the intervention LGAs added up SP doses served in their catchment communities to the facility data.
In the intervention LGAs, the HRB master list, an aggregation of individual household HRB, contained the most up-to-date information of WRA, pregnant women and SP doses given. HRB was the source of information for CBHVs and their supervisors to prepare and regularly update lists of eligible women. CBHVs and their supervisors documented their list of women reached with SP in the HRB with which dose and when. CBHV Supervisors prepared monthly summary forms from these activities. The HRB master list was compared each month with the CBHV Supervisors’ monthly summary form. Discrepancies were addressed by CBHV supervisors at follow up home visits.
Tracking birth reporting, birth outcomes and head circumference measurements
Twelve teams of four data quality auditors, independent of other project staff, were recruited to track data quality. Each team comprised of three females and one supervisor. Over the life of the project, the teams visited all the women recorded with at least one birth—that occurred during the project—in the 42 wards of the three intervention and one counterfactual LGAs. Information on omitted mothers and births was sought for and collected. Mothers—or an informed family member in the event of a maternal death—if a CBHV and CBHV Supervisor visited, the status of newborns, alive or stillborn, and if head circumference was measured within seven days among live births. With mothers’ responses as the gold standard, births, status of births, and confirmed HC measurements were verified. Overall, a 3.7% error rate was found with facility-based SP records, 2.5% error rate with community-based SP records. There was a 17% underreporting of live births and 2.1% error rate with the correct recording of births and outcomes. These were all addressed prior to data analysis.
Methods
Data and methodology for the coverage and scale of SP doses
From the HRS database, 25,572 women who were eligible for SP between April and November in the three intervention LGAs, were identified. Based on actual numbers, 94% of pregnant women in the intervention LGAs became eligible for SP1 between April and November. In the intervention LGAs, the number of pregnant women eligible to receive SP1, was used as a denominator to calculate SP1, SP2 and SP3+ coverage (Table 4).
Table 4.
Determining the number of women eligible for SP1 between April and November 2015, and differences between project mapping and official projections
| LGA | Official projected population in 2015 | Percentage pregnant women per annum (assuming 0.05 of Pop) | Estimated number of pregnant women over 8 month period | Enumerated pregnant women from the MiPP database | % Difference between MiPP database and projected population of pregnant women | Women that became eligible for SP1 Between April and November 2015 | % Eligible for SP1 between April and November in MiPP database (%) |
|---|---|---|---|---|---|---|---|
| Dange Shuni | 255,908 | 12,795 | 9597 | 9550 | 0% | 9136 | 96 |
| Goronyo | 257,984 | 12,899 | 9674 | 11,231 | 14% | 10,850 | 97 |
| Silame | 147,715 | 7386 | 5539 | 6251 | 11% | 5586 | 89 |
| Yabo | 167,971 | 8399 | 6299 | NA | NA | 5921b | 94a |
| Total | 829,577 | 41,479 | 31,109 | 27,032 | 8% | 31,493 | 94 |
aMean percentage of women becoming eligible for SP1 in the intervention LGA’s
bEstimated using the mean percentage of pregnant women that became eligible for SP1 in the intervention LGAs
In the counterfactual group, the number of pregnant women eligible to receive SP1 over the life of the project was estimated. Using the official population estimate for Yabo LGA, from the Sokoto State Government (167,971) and assuming that 5% of the population was pregnant, there were an estimated 8399 pregnant women in Yabo, in 2015. Prorating that number for the 8 months of the project, there were an estimated 6299 pregnant women in Yabo between April and November 2015. Assuming Yabo would have the same percentage of women eligible for SP1, as found in the intervention areas (94%) it was estimated that there was a total of 5921 women eligible for SP1 during the project lifespan (Table 4). This estimate as the denominator to calculate SP1 and SP2 coverage.
Univariate and bivariate analyses were performed to compare intervention and counterfactual LGAs on the number of SP doses and source of SP. Analyses were conducted in Excel spreadsheet®.
Newborn head circumference data and methodology
Programmatic data extracted from outcome forms were available for 9241 live births in intervention and counterfactual LGAs between April and November. Head circumference (analyzed in mm) of live newborns measured within 7 days of birth, was available for 6720 (73%) of live births. Head circumference data were missing for 2521 live newborns. Of these, 1721 (19%) mother-newborn dyads missed initially, were identified during the data quality review in October. For the remaining 800 newborns, head circumferences were not measured within 7 days postpartum.
Independent variables used in this analysis were guided by prevailing epidemiological evidence base and by what was feasible to collect by CBHV supervisors. These included the sex of newborn, gravidity of mothers, successive doses of SP, exposure to ANC, month of delivery and gestational age at birth. Globally, female newborns have smaller head circumferences than male newborns [15]. Primigravida women are at higher risk for placental malarial infection, [35]. Table 1 presents how each of these variables was coded in the analyses.
Table 1.
Variables used to understand the impact of SP interventions on head circumference among 6720 live newborns, born between May and November 2015
| Variable name | Definition | |
|---|---|---|
| Head_circum | Head circumference, at birth, in mm | n = 6720 Continuous |
| Girl | 1 = Female | n = 6712 |
| 0 = Male | ||
| SPFREQ | 0,1,2, 3 + doses of SP | n = 6720 |
| Intervention | 1 = DANGE SHUNI, Goronyo or Silame LGA, where community based SP distribution occurred | n = 6720 |
| 0 = Yabo, where no community based SP distribution occurred | ||
| Primi | 1 = Primigravida | n = 6711 |
| 0 = Multigravida | ||
| Month of birth | April–November 2015 | In the unadjusted analyses, all earlier months are compared to November. In the adjusted analyses, this variable was used as a continuous variable n = 6720 |
| Gestational age at birth (months) | 2 = 10 | n = 6719 |
| 1 = 9 | ||
| 0 = 8 | ||
Univariate analyses tested for any associations between a given independent variable and the mean head circumference of live newborns. Unadjusted t tests were used to assess any differences within each predictor variable and newborn head circumference. Unadjusted tests of correlations between mean head circumference and doses of IPTp-SP, and month of birth, were performed. Mean newborn head circumferences were used to assess correlations in the number of IPTp-SP doses over the project period in the intervention and counterfactual LGAs. A multivariate linear regression model was used to test for the impact of SP doses and other variables on head circumference. Analyses were performed with Excel® and SAS v. 9.4.
Stillbirth data and methodology
Data extracted from outcome forms were available for 9453 term births in both the intervention and control LGAs between April and November. To examine the impact of IPTp-SP doses on the incidence of stillbirths, all confirmed pregnancies that ended in miscarriage and abortion, or were delivered before 8 months of gestational age (n = 99) were excluded. If the newborn was stillborn, it was coded as “1”; if it was a live birth, it was coded as “0.”
Stillbirth rates (SBR), per 1000 births, and correlations between SBR and doses of SP were calculated. Unadjusted and adjusted logistic regression modelling was used to predict the odds with 95% confidence intervals, of having a stillbirth among women who ingested different doses of SP, according to exposure to intervention, those who attended at least one ANC visit, gravidity of mothers, those who gave birth in a facility vs those who gave birth at home, and those who gave birth later (July–November) vs. those who gave birth earlier (April–June). Multivariate logistic regression analysis was used to assess whether these associations would hold after controlling for other variables in the model. Analyses were conducted in Excel® and SAS v9.4. Table 2 presents each variable as coded in the analyses.
Table 2.
Variables used to understand the associations between SP interventions and stillbirths between April and November 2015 (n = 9453)
| Variable Name | Definition | N and notes |
|---|---|---|
| Stillbirth | 1 = Stillbirth | A stillbirth was defined as an infant born at least 8 months or more of estimated gestational age, who showed no signs of life at birth |
| 0 = Live birth | Births that occurred before 8 months gestation were coded as missing (miscarriage/abortion) | |
| SPFREQ | 0,1,2, 3+ doses of SP | |
| Intervention | 1 = DANGE SHUNI, Goronyo or Silame LGA, where community based SP distribution occurred | |
| 0 = Yabo | ||
| Any ANC | 1 = Yes | |
| 0 = No | ||
| Primi | 1 = Primigravida | n = 9438 |
| 2 = Multi | ||
| Place of delivery | 1 = Health facility (hospital, health center/post) | |
| 0 = Home | ||
| Month of birth | 1 = July–November | Month of birth |
| 0 = May, June |
Costing data and methodology
Nominal cost and expense data in 2015 Nigerian Naira (NGN) directly related to community and facility distribution of SP in the intervention and counterfactual LGAs were obtained from project records and other sources. The cost estimates obtained are what it would cost the state government and LGAs as de jure providers of primary health care in Nigeria, to deliver SP-related services at both the community and facility level, including start-up costs. Estimates were limited to a 12-month horizon.
Different degrees of contributions by each service component at facility and community levels, towards the delivery of SP at facility and community levels, were assumed. Table 3 lists the assumptions about the magnitude of contributions by each level of care to SP distribution. For this purpose, six cost centers were included in the analysis: health facility, LGA technical administration, CBHV supervisors, WDC, CBHV, and logistics for SP distribution. Table 3 provides a summary of each cost center, and their relative contribution towards SP distribution the activities involved; these were costed. Twenty-two work days per month were assumed. Published government salary schedules were used to compute government officers’ salary costs. Governments officers’ salary costs included time spent at monthly LGA level review meetings in each LGA attended by representatives of wards. At the community level, costs were attributed to WDCs and to CBHVs. There was a WDC and one CBHV supervisor in each ward. WDCs supported CBHVs in the distribution of SP and supervised CBHV, as well as ad hoc community meetings that were called to tackle issues that could undermine the demand or supply sides of the programme. CBHV-related costs also included level of effort, transportation for SP distribution and monthly rentals of meeting rooms for ward-level CBHV review meetings also attended by LGA officials. Costs associated with the transportation and distribution of SP to 42 health facilities were captured in central storage costs.
Table 3.
Cost centers by level of care and magnitude of their component costs associated with SP distribution
| Cost center | Level | Description | Components costed (magnitude) |
|---|---|---|---|
| Health facility | Health Facility | SP distributed through ANC. Hub for community distribution and receipt of community consumption data | Rent and utilities (10%) M&E documentation tools (10%) Two health staff that operate ANC (50%) In-facility storage (10%) |
| Local Government Area Council | LGA-wide, all levels | Supervision and program oversight including quality assurance, data management and coordination | Meeting venue (100%) LOE for nine key personnel at all levels who contribute supervisory and oversight roles and collaborate in the program (5% time) |
| CBHV Supervisor | Community/ Facility |
Technical supervision of CBHV, assuring SP availability to CBHV and data-driven accountability to WDC and HF | LOE (50%) Transport costs of SP from HF-CBHV (100%) Monitoring and Supervision (100%) |
| CBHV | Community | Oversight and coordination of community-level operations | CBHV LOE (50%) Transport cost for SP distribution (100%) Transport costs for home visits (100%) |
| Ward Development Committees | Community | Community mobilization and sustaining program momentum | Meeting attendance costs for WDC member (30%) Meeting attendance cost of CBHV supervisor of ward (30%) Supervision and monitoring of CBHV (100%) Meeting/dialogue (100%) Meeting venue (100%) |
Two ratios were calculated: cost per dose and cost per woman served, disaggregated by number of SP doses in the intervention and counterfactual group. Ratios were obtained from annualized costs derived in each LGA, intervention and counterfactual, the total as well as the disaggregated number of SP doses distributed, and from the total number of women served.
Results
Findings related to coverage and scale-up of SP use
A combined total of 60,428 compounds which comprised 92,240 households with a combined population of 524,580 residents were mapped and enumerated in the three intervention LGAs and registered into the database. According to Sokoto State official figures, there was an estimated population of 167,971 in the counterfactual LGA (Table 4). A total of 114,842 women of reproductive age were registered in the intervention LGAs. Throughout the intervention period, April-November, a total of 25,572 pregnant women in the intervention LGAs and an estimated 5921 in the counterfactual LGA were eligible to receive SP1 (Table 5).
Table 5.
Number and percentage of eligible women who received SP1, SP2 and SP3 by LGA
| Dange Shuni | Goronyo | Silame | Intervention combined | Yabo | Total | |
|---|---|---|---|---|---|---|
| Pregnant women eligible for SP between April and November 2015 | 9136 | 10,850 | 5586 | 25,572 | 5921 | 31,493 |
| Number of SPI doses consumed | 7408 | 10,893 | 6013 | 24,314 | 1527 | 25,841 |
| % Given SP1 | 81 | 100 | 108 | 95 | 26 | 82 |
| Number of SP2 doses consumed | 5334 | 7304 | 4656 | 17,294 | 767 | 18,061 |
| % Given SP2 | 58 | 67 | 83 | 68 | 13 | 57 |
| Number of SP3+ doses consumed | 2922 | 5381 | 3128 | 11,431 | 0 | 11,431 |
| % Given SP3+ | 32 | 50 | 56 | 45 | 0 | 36 |
| Total number of doses SP doses given | 15,664 | 23,578 | 13,797 | 53,039 | 2294 | 55,333 |
| Mean doses of SP delivered to eligible women | 1.71 | 2.17 | 2.47 | 2.07 | 0.39 | 1.76 |
Table 5 shows that between April and November 2015, the percent of eligible women that got SP1 averaged 95% in the intervention LGAs—ranging from 81% in Dange Shuni to 100 and 108% respectively in Goronyo and Silame—compared to 26% in the counterfactual (Figure 5). The high SP1 coverage in Goronyo LGA was also influenced by a test conditional cash transfer programme to encourage women to attend ANC clinics—including those from neighboring LGAs—that was also in effect during the study. In Silame LGA, there were a number of households whose pregnant women were served with SP and not captured in the HRB due to delays in enumeration. Consequently, these were not included in the denominator, and quite likely yielded an overestimated SP1 coverage.
Fig. 5.
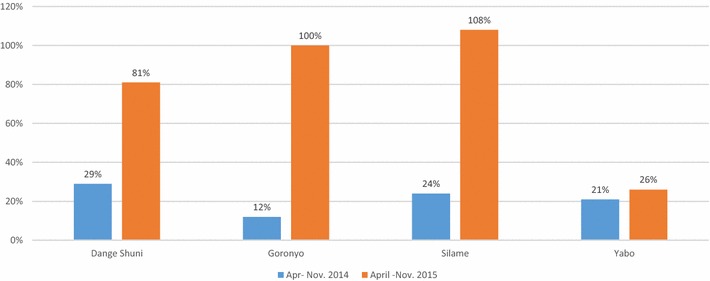
Percentage coverage of pregnant women who took SP1 in three intervention LGAs and one counterfactual LGA, Sokoto State, Apr–Nov 2014 and Apr–Nov 2015
Table 5 shows that the SP2 coverage in the intervention LGAs was 68%—and it ranged from 58% in Dange Shuni to 83% in Silame LGAs–compared to 13% in the counterfactual LGA. The SP3 coverage in the intervention group was 45%–it ranged from 32% in Dange Shuni to 56% in Silame—compared to zero percent in the counterfactual. Data on SP3 was unavailable for the counterfactual as the DHIS2 does not collect data on SP3 doses and higher.
Figure 5 compares SP1 coverage in intervention and counterfactual LGAs in 2014 and 2015. The percent of women that received SP1 in the intervention did not differ from those that did in the counterfactual in the 12 months that preceded the 8 month (April–November) study period, and it averaged 22%.
Figure 6 shows that across the three intervention LGAs, between 55 and 71% of all SP1 through SP6 doses consumed were obtained from community channels. A 26 percentile line drawn through the bar graphs of the intervention LGAs indicate that relative to the 26% coverage in the counterfactual LGA which was an all-facility distribution scheme, there was a proportional increase in health facilities as a source of SP consumed in intervention LGAs. The introduction of community SP distribution may have helped increase SP uptake in facilities in at least two intervention LGAs.
Fig. 6.
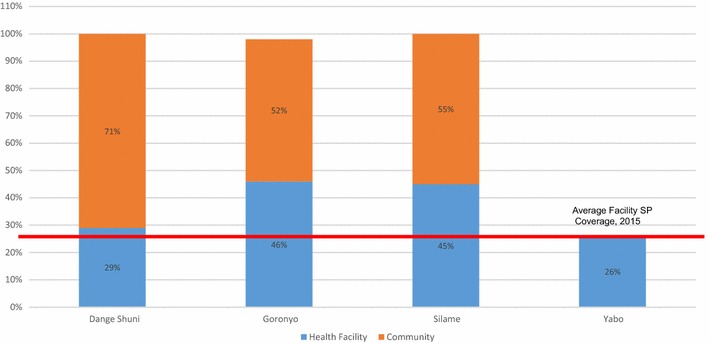
Percentage coverage of pregnant women who took SP1 in three intervention LGAs and one counterfactual LGA by source April–November 2015
The monthly number of women that consumed SP1, SP2 and SP3 in the intervention LGAs, and women that consumed SP1 and SP2 in the counterfactual LGA were plotted (see Fig. 7). There was an early sharp increase in SP1 users suggestive of rapid community adoption aided an already well-established community distribution network. There was a subsequent marked increase in the uptake of SP2 and SP3 suggestive of sustained continuity of use. From June onwards, there was a pattern of convergence between SP1 and SP2 consumption, and by November, the number of women that got SP2 exceeded those that got SP1—another likely indication of successful follow up of pregnant women that held over time. It also reflects a persisting capability of women to demand SP3, and the supply system to meet such demand. In Yabo LGA, the counterfactual, the trend lines of the numbers of women that received SP1 and SP2 per month were much lower and changed little throughout the study period.
Fig. 7.
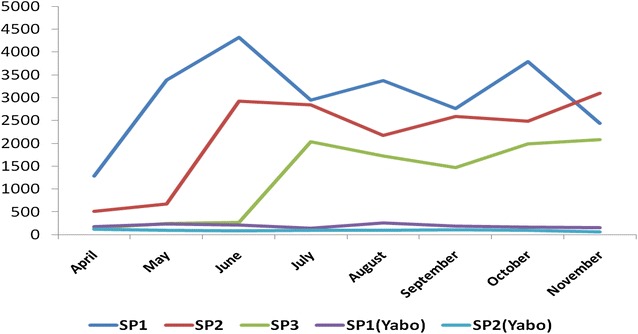
Monthly number of pregnant women who took SP1, SP2, SP3 in three intervention LGAs and one counterfactual LGA, Sokoto State, April–November 2015
Findings related to newborn head circumference
Data on head circumference were significantly more likely to be missing in the intervention (missing n = 2512) versus the counterfactual LGAs (missing n = 9) (Chi-sq p < 0.0001). These were babies who were past 7 days postpartum whose head circumferences were not measured, and therefore excluded. There was a strong correlation between later birth months and increasing number of IPTp-SP doses (r2 = 0.88) in the intervention LGAs. This indicates that more women proactively enrolled earlier in their pregnancies when the community-based distribution of IPTp-SP commenced on April 28, 2015. They were also more likely to have received all three recommended doses of IPTp-SP, than those who enrolled when their pregnancy was in far advanced stages of gestation. This correlation was weak in the counterfactual LGA where there no community mobilization effort (r2 = 0.17) (Fig. 8). The mean head circumference among all live newborns was 355 mm (see Table 6). Table 6 shows that babies born later in the year had smaller head circumferences than those born in April. A month on month decline in mean head circumference was observed in both the intervention and counterfactual LGAs between April and November. In all but 1 month (October) head circumferences were consistently larger in the intervention LGAs (Table 7) (Fig. 9).
Fig. 8.
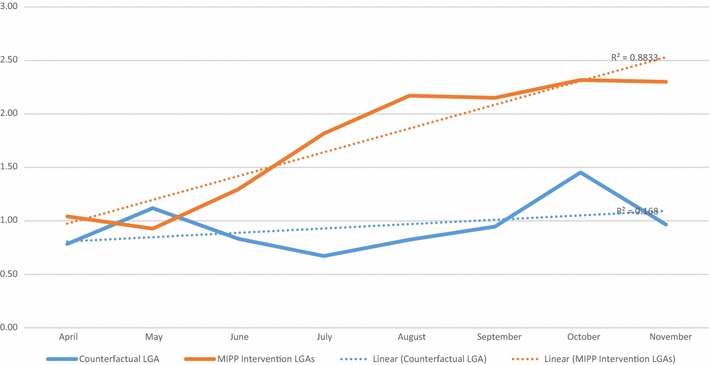
Mean doses of SP between April and November 2015 in the MiPP intervention and counterfactual LGAs
Table 6.
Mean head circumference by month of birth, between April and November 2015
| Month of birth | n | Mean | SD |
|---|---|---|---|
| April | 755 | 360.50 | 22.89 |
| May | 887 | 355.49 | 20.92 |
| June | 838 | 353.06 | 20.86 |
| July | 766 | 354.53 | 23.78 |
| August | 809 | 354.31 | 22.61 |
| September | 860 | 352.98 | 26.17 |
| October | 959 | 355.63 | 20.88 |
| November | 846 | 354.83 | 21.14 |
| Total | 6720 | 355.11 | 22.52 |
Table 7.
Mean head circumference by month of birth between April and November 2015 in the Intervention and counterfactual LGAs
| Month of birth | Intervention LGAs | Counterfactual LGA | Pr > |t| | ||||
|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | ||
| April | 567 | 362.86 | 23.94 | 188 | 353.35 | 17.61 | <0.0001 |
| May | 631 | 356.79 | 22.20 | 256 | 352.30 | 16.99 | 0.00 |
| June | 485 | 354.75 | 22.85 | 353 | 350.75 | 17.52 | 0.01 |
| July | 515 | 355.28 | 25.63 | 251 | 352.99 | 19.41 | 0.21 |
| August | 542 | 356.67 | 23.42 | 267 | 349.53 | 20.06 | <0.0001 |
| September | 626 | 353.73 | 27.89 | 234 | 350.97 | 20.81 | 0.17 |
| October | 643 | 354.13 | 22.04 | 316 | 358.69 | 17.95 | 0.00 |
| November | 574 | 355.80 | 21.24 | 272 | 352.78 | 20.80 | 0.05 |
Fig. 9.
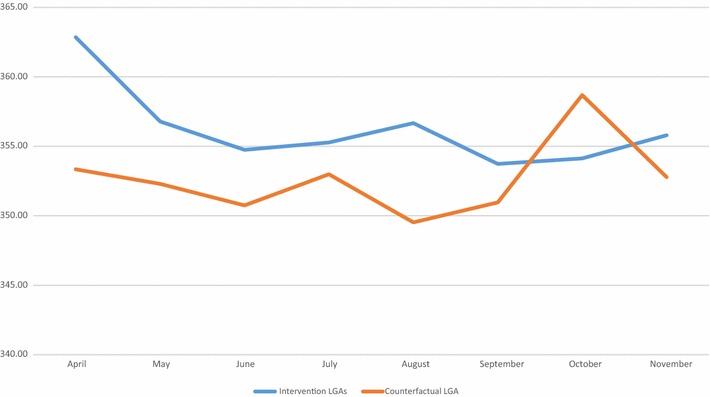
Mean head circumference in the intervention and counterfactual LGAs by birth month (April–November 2015)
Table 8 presents findings from the univariate analyses. As expected, female newborns had significantly smaller head circumferences than male newborns. There was a linear increase in mean head circumference with each additional SP dosage (r-sq. = 0.79) (Fig. 10). Larger and statistically significant mean head circumferences were observed among newborns whose mothers had at least one ANC visit, relative to those whose mothers’ had none. There was no significant association between being primigravid and a smaller head circumference. Newborns in the intervention LGAs had significantly larger head circumferences compared to those in the counterfactual LGA.
Table 8.
Unadjusted mean head circumference (HC) in millimeters (mm) among live newborns born between April and November 2015, and differences in mean HC by available variables
| n | Mean HC in mm | SD | Pr > |t| | |
|---|---|---|---|---|
| Sex of infant | ||||
| Female | 3235 | 353.90 | 22.17 | <0.001 |
| Male (ref) | 3477 | 356.30 | 22.76 | |
| Intervention | ||||
| Yes | 4583 | 356.20 | 23.88 | <0.001 |
| No (ref) | 2137 | 352.70 | 19.07 | |
| SP dosage | ||||
| 0 | 2258 | 353.63 | 23.51 | <0.0001 |
| 1 | 871 | 355.39 | 23.14 | 0.10 |
| 2 | 1682 | 354.90 | 21.37 | 0.01 |
| 3+ (ref) | 1909 | 356.93 | 21.89 | |
| Primigravida | ||||
| Yes | 1225 | 354.30 | 22.57 | 0.18 |
| No (ref) | 5486 | 355.30 | 22.51 | |
| At least 1 ANC visit | ||||
| Yes | 3805 | 356.00 | 20.19 | 0.00 |
| No (ref) | 2915 | 354.00 | 25.20 | |
| Month of birth | ||||
| April | 755 | 360.50 | 22.89 | <0.0001 |
| May | 887 | 355.49 | 20.92 | 0.54 |
| June | 838 | 353.06 | 20.86 | 0.11 |
| July | 766 | 354.53 | 23.78 | 0.79 |
| August | 809 | 354.31 | 22.61 | 0.64 |
| September | 860 | 352.98 | 26.17 | 0.09 |
| October | 959 | 355.63 | 20.88 | 0.45 |
| November (ref) | 846 | 354.83 | 21.14 | |
| Gestational age at delivery (months) | ||||
| 8 | 18 | 342.50 | 26.44 | 0.00 |
| 9 | 6588 | 355.06 | 22.57 | 0.01 |
| 10 (ref) | 113 | 360.26 | 17.29 | |
Fig. 10.
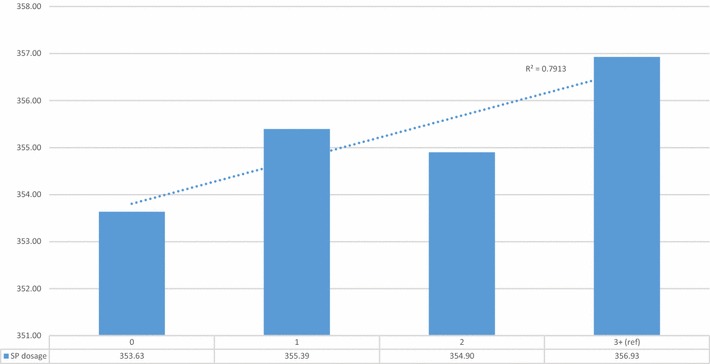
Mean newborn head circumference in mm by number of SP doses during pregnancy, for births between April and November 2015
Table 9 presents the adjusted associations between each of the predictor variables and live newborn head circumference. On average, female newborns had head circumferences that were 2.33 mm smaller than male newborns. Newborns whose mothers had three or more doses of IPTp-SP had a significantly larger head circumference than newborns whose mothers ingested 0, 1 or 2 doses. On average, the newborns of mothers who had attended one or more ANC visits had a significantly larger head circumference, of 2 mm, than babies whose mothers had no ANC visits. Women who resided in the intervention LGAs had newborns with significantly larger mean head circumference at 2.88 mm than those living in the counterfactual LGA. There was a significant, negative, linear trend in head circumference as birth months progressed by 0.64 mm/month from April to November. There was no significant association between mothers being primigravida and a lower newborn head circumference. Newborns that were delivered at 8 months of gestation had on average a head circumference 17.83 mm smaller than those who delivered at 10 months of gestation.
Table 9.
Adjusted associations between selected variables and the mean head circumference (HC) of newborns, between April and November 2015
| Estimate | Standard error | Pr > |t| | |
|---|---|---|---|
| Intercept | 363.78 | 2.77 | <0.0001 |
| Sex of infant | |||
| Female | −2.33 | 0.55 | <0.0001 |
| Male (ref) | |||
| Intervention | |||
| Yes | 2.88 | 0.66 | <0.0001 |
| No (ref) | |||
| SP dosage | |||
| 0 | −2.87 | 0.87 | 0.00 |
| 1 | −1.88 | 0.93 | 0.04 |
| 2 | −2.55 | 0.78 | 0.00 |
| 3+ (ref) | |||
| Primigravida | |||
| Yes | −1.16 | 0.71 | 0.10 |
| No (ref) | |||
| At least 1 ANC visit | |||
| Yes | 2.01 | 0.62 | 0.00 |
| No (ref) | |||
| Month of birth | −0.64 | 0.13 | <0.0001 |
| Gestational age at delivery (months) | |||
| 8 | −17.83 | 5.68 | 0.00 |
| 9 | −3.73 | 2.14 | 0.08 |
| 10 (ref) | |||
n = 6702; F test p < 0.0001; Adj. r-square 0.017; RMSE: 22.6
To test for collinearity between ANC attendance, gestational age and SP dosages two interaction terms were constructed. These interaction terms were used to test for any distinct impact of ANC and gestational age at SP0, SP1, SP2 (relative to SP3) on newborn head circumference. None were significant or of any added explanatory value. This suggests that the effects of SP dosage, gestational age, and ANC attendance on changes in newborn head circumference appeared to operate independently of each other.
The mean head circumference by month of birth in the counterfactual LGA, showed a surge in October in both the number of SP doses and head circumferences. This coincided with data quality audit visits, that likely increased community awareness of SP. October data were excluded and the unadjusted and adjusted analyses were re-run. All estimates increased in magnitude, and with stronger statistical significance. There was also a stronger SP linear association between higher SP doses and increased head circumference, as well as a stronger intervention effect. Therefore, there may have been a “community mobilization” effect of data quality audits carried out in the counterfactual group. See Additional files 4 and 5.
Findings relating to stillbirths
There were 213 stillbirths out of 9453 births that occurred after 7 months of gestation, equivalent to an overall stillbirth rate (SBR) of 23 per 1000 births. The SBR by dose of SP was calculated, and unexpectedly showed that women who ingested zero doses (SBR = 21 per 1000 term births) had a lower SBR than those who had ingested SP1 (SBR = 37 per 1000 term births) or SP2 (SBR = 22 per 1000 term births). (Table 10) Among women who ingested at least one dose of SP, a strong correlation between increasing SP doses and declines in SBR was observed. (r-sq = 0.90) (Fig. 11).
Table 10.
Frequency of Stillbirths by number of SP doses, and Stillbirth rate (SBR) between April and November 2015
| Status at birth | Number of SP doses | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3+ | Total | |
| Livebirth | 2644 | 1142 | 2233 | 3221 | 9240 |
| Stillbirth | 57 | 44 | 51 | 61 | 213 |
| Total births | 2701 | 1186 | 2284 | 3282 | 9453 |
| SBR | 21 | 37 | 22 | 19 | 23 |
A stillbirth was defined as an infant born at 8 months or more gestational age, who showed no signs of life at birth
SBR the number of stillbirths per thousand births
Fig. 11.
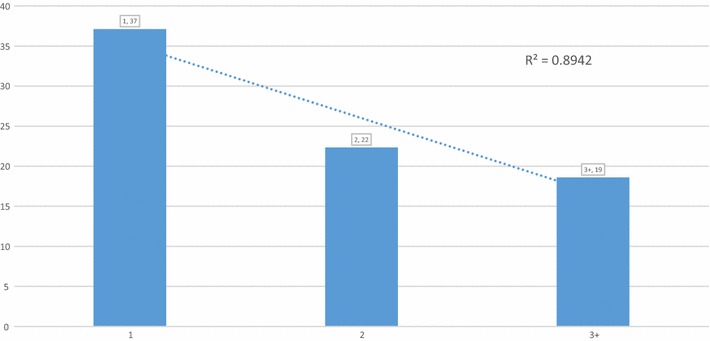
Stillbirth rate by 1, 2 and 3+ doses of SP, for births between April and November 2015
Table 11 presents the unadjusted odds of women having a stillbirth between April and November. The odds of having a stillbirth were higher among women who ingested less than three doses of SP, but was only statistically significant when contrasted to SP1 (OR 2.04). Being resident in the intervention LGA versus the counterfactual LGA was not a significant predictor of a woman having a stillbirth. Newborns that were delivered later in the study were no different from those born earlier in the odds of being a stillbirth. Any ANC attendance significantly reduced the odds of a woman having a stillbirth (OR 0.57). Primigravid women (OR 1.87) and those mothers who gave birth in a facility (OR 1.86) had significantly higher odds of a stillbirth compared to multigravida women and those who gave birth at home.
Table 11.
Unadjusted odds of having a stillbirth (vs. a live birth) between April and November 2015 in four LGA’s in Sokoto State
| Parameter | OR | 95% CIb | |
|---|---|---|---|
| SP dosage | |||
| 0 | 1.14 | 0.79 | 1.64 |
| 1 | 2.04* | 1.37 | 3.02 |
| 2 | 1.21 | 0.83 | 1.76 |
| 3+ (ref) | |||
| Intervention | |||
| Yes | 1.10 | 0.79 | 1.53 |
| No (ref) | |||
| At least 1 ANC visit | |||
| Yes | 0.57* | 0.43 | 0.75 |
| No (ref) | |||
| Primigravidaa | |||
| Yes | 1.87* | 1.38 | 2.53 |
| No (ref) | |||
| Place of birth | |||
| Facility | 1.86* | 1.23 | 2.80 |
| Home (ref) | |||
| Month of birth | |||
| July–November | 0.85 | 0.64 | 1.13 |
| April–June (ref) | |||
9453 births that occurred >28 weeks gestation
an = 9438
b,* Significant at p < 0.05
Table 12 shows the adjusted odds of a woman having a stillbirth. Fewer doses of SP taken was associated with higher odds of stillbirth; only the difference between the first dose and three doses was significant at p < 0.05. (adj. OR 1.77). As in the univariate results, there was no significant association between residing in the intervention (vs. counterfactual) sites and the odds of stillbirth, nor with giving birth later on in the course of the study. Adjusting for all of the other variables in the model, any ANC attendance was associated with a statistically significant 66% decline in the odds of a woman having a stillbirth (adj. OR 0.44). Definitively, primigravida women (adj. OR 1.81) had higher odds of a stillbirth, relative to multigravida women. Women that gave birth in a facility (adj. OR 1.99) were significantly more likely to have had a stillbirth, relative to those who did not give birth in a facility.
Table 12.
Adjusted odds of having a stillbirth (vs. a live birth) between April and November 2015 in four LGA’s in Sokoto State
| Parameter | OR | 95% CI | |
|---|---|---|---|
| SP dosage | |||
| 0 | 0.76 | 0.49 | 1.18 |
| 1 | 1.77 | 1.18 | 2.66 |
| 2 | 1.18 | 0.80 | 1.74 |
| 3+ (ref) | |||
| Intervention | |||
| Yes | 0.90 | 0.62 | 1.30 |
| No (ref) | |||
| At least 1 ANC visit | |||
| Yes | 0.44 | 0.32 | 0.61 |
| No (ref) | |||
| Primigravida | |||
| Yes | 1.81 | 1.33 | 2.47 |
| No (ref) | |||
| Place of birth | |||
| Facility | 1.99 | 1.28 | 3.10 |
| Home (ref) | |||
| Month of birth | |||
| July–November | 0.81 | 0.60 | 1.11 |
| April–June (ref) | |||
*n = 9438; L-R p value < 0.001; c-statistic = 0.65; Hosmer and Lemeshow Goodness-of-Fit pr Chi-sq >0.3753 test
As with head circumference, ANC attendance and higher SP dosages were significantly associated with the odds of stillbirth. None of the tests for any interaction between ANC attendance and SP dosages were either significant or added importance to the model fit. As with head circumference findings, the impact of higher SP dosages and ANC attendance with the odds of stillbirth appeared to operate independently.
Findings relating to costing
Cost per woman
Given the total number of women reached in this study with SP, the average cost per woman who received SP1-3 ranged from N1,132 ($5.7) to N1,518 ($7.6) in the three intervention LGA to N5,326 ($26.7) in the counterfactual LGA (see Table 13). Given that majority of the costs associated with the delivery of SP doses per woman were fixed costs and are, therefore, comparable across LGAs in the intervention and counterfactual alike, the spread in cost per woman reached with SP1-3 in intervention LGAs is explained primarily by differences in the actual number of women that got all three doses of SP. A higher number of women who got all three doses is a function of reaching the most women with SP1 in the first instance and subsequently ensuring that there were few dropouts in women who received SP2 and SP3. The higher cost per woman in the counterfactual LGA reflects the overall low number of women on SPI, the higher dropout by SP2 and the absence of women reached with SP3. The 3.5- to 4.6-fold lower average costs per woman in the intervention LGA suggests that strategies that substantially increase coverage and significantly reduce drop out in-between doses, will lower the average cost per woman.
Table 13.
Cost per woman who received SP 1-6 contrasted by intervention and counterfactual LGAs
| LGAs | ||||
|---|---|---|---|---|
| Goronyo [Cost per woman in 2015 Nigerian Naira (NGN)] | Silame [Cost per woman in 2015 Nigerian Naira (NGN)] | Dange Shuni [Cost per woman in 2015 Nigerian Naira (NGN)] | Yabo [Cost per woman in 2015 Nigerian Naira (NGN)] | |
| SP dose level | ||||
| Women who received SP1 | 723 | 1228 | 947 | 4155 |
| Women who received SP2 | 963 | 1575 | 1235 | 6498 |
| Women who received SP3 | 1710 | 2857 | 2371 | |
| Women who received SP4+ (SP5, SP6) | 4889 | 9269 | 12,169 | |
| Average cost per woman SP1–SP3 | 1132 | 1887 | 1518 | 5326 |
| Average cost per woman SP1–SP4+ | 2071 | 3732 | 4180 | 5326 |
Cost per SP dose
The estimated cost per course per dose of SP1, SP2 and SP3 ranged from NGN 185 ($0.9) to NGN288 ($1.45) in the intervention LGAs compared to a much higher cost of NGN 1382 ($6.98) in the counterfactual LGA. The estimated cost per course per dose of SP1, SP2, SP3 and SP4+ ranged from NGN 239 ($1.21) to NGN373 ($1.89) in the intervention LGAs, compared to NGN 1843 ($9.21) in the counterfactual LGA (see Table 14). The observed substantial difference in the cost of a SP1-3 regimen in the counterfactual LGA is attributable to no reportable distribution activity with SP3 and higher. The incremental cost per dose of SP1, SP2, SP3 and SP4+ on SP1, SP2, SP3 and ranged from NGN 53.5 ($0.27) to NGN85 ($0.43) in the intervention LGAs compared to a much higher NGN 460.87 ($2.33) in the counterfactual. The observed five- to eightfold higher cost per SP dose in the counterfactual LGA is the cost of underperformance of not being at programme scale.
Table 14.
Estimated cost per dose of SP
| LGA | ||||
|---|---|---|---|---|
| Intervention | Counterfactual | |||
| Goronyo [Costs and cost per SP dose in 2015 Nigerian Naira (NGN)] | Silame [Costs and cost per SP dose in 2015 Nigerian Naira (NGN)] | Dange Shuni [Costs and cost per SP dose in 2015 Nigerian Naira (NGN)] | Yabo [Costs and cost per SP dose in 2015 Nigerian Naira (NGN)] | |
| SP dose | ||||
| Women who received SP1 | 1,637,746 | 1,515,582 | 1,453,361 | 1,286,860 |
| Women who received SP2 | 1,637,926 | 1,515,865 | 1,453,586 | 1,288,425 |
| Women who received SP3 | 1,639,192 | 1,517,808 | 1,455,498 | 1,288,425 |
| Women who received SP4+ (SP5, SP6) | 1,646,255 | 1,530,833 | 1,476,856 | 1,288,425 |
| Total cost of SP1–SP3 | 4,914,866 | 4,549,256 | 4,362,447 | 3,863,712 |
| Total cost of SP1–SP4+ | 6,561,121 | 6,080,088 | 5,839,303 | 5,152,137 |
| Total number of women who received any SP1, SP2 and SP3 doses | 22,760 | 13,391 | 15,424 | 2294 |
| Total number of women who received any SP1, SP2, SP3 and SP4+ doses | 23,578 | 13,797 | 15,664 | 2294 |
| Cost per SP1,SP2 and SP3 doses | 216 | 340 | 283 | 1684 |
| Cost per SP1, SP2, SP3 and SP4+ doses | 278 | 441 | 373 | 2246 |
Discussion
To the best of our knowledge, this is the first published implementation research study that has prospectively examined the association between SP use and newborn outcomes, at scale, in community settings. This study is the largest cohort of pregnant women included in a study designed and powered to examine the effects of IPTp-SP delivered at scale.
Study data show that IPTp-SP can be delivered safely to pregnant mothers, in accordance with Nigeria’s Federal Ministry of Health and applicable WHO guidelines. The results have also shown that community-based distribution of SP, twinned with facility-based distribution, produced a superior and sustained coverage in the use of SP by pregnant women, at scale. In this context, community-based distribution of SP substantially increased facility-based distribution of SP. Critical attention given to realizing authentic community ownership, through upfront education and an early community discernment of SP’s beneficial results, contributed to a dramatic increase in the consumption of SP.
The MiPP project did not directly or indirectly intervene on the availability of water to ingest medicines in facilities, although this is a factor that has been cited as a contributor to low IPTp-SP coverage [36–38]. The surge of IPTp-SP use in facilities in the intervention LGAs suggests that increasing demand for IPTP-SP would intrinsically motivate to overcome barriers to accessing water to ingest her medicine. Additionally, in the intervention LGA’s, IPTp-SP was recorded only after it had been observed ingested, by the CBV.
Any antenatal care attendance was strongly predictive of larger newborn head circumference and a reduced odds of stillbirths. The authors call for urgent action by policy makers and programmes to increase communities’ access to and utilization of quality ANC services in Sokoto State and Nigeria. This finding affirms the Global Malaria in Pregnancy Working Group’s call that any efforts intended to increase IPTp-SP delivery at scale, need to also increase ANC usage. The study reinforced, but was not conclusive about, the increased susceptibility of primigravid mothers to having stillbirths. There is already well-established local, tacit knowledge in Sokoto State communities that primigravid mothers suffer greater risks in pregnancy, and therefore need more care. It is recommended that advocacy and community education efforts capitalize on this notion to increase greater use of ANC and health facility delivery services by primigravid women. The study also confirmed a well-established finding that male newborns had larger head circumferences than female ones, and lends biological validity the study findings as a whole.
The finding that women who gave birth in a health facility compared to home deliveries had higher odds of having stillborn babies was puzzling at first glance. However, in a setting like Sokoto State where home delivery is regretfully almost universal, women with complications are more likely to be referred to primary health facilities, and consequently with their newborns suffer higher case fatalities than home births. The implication is that primary health facilities operate as de facto secondary level care facilities, without requisite support. More needs to be done to increase health centres’ preparedness to deliver basic emergency obstetric care. Policy actions that remove barriers to women’s access health facilities for deliveries in Sokoto State and Nigeria are urgently warranted.
Given these findings on the benefits of SP3 doses and higher on newborn survival, the pathway to realize population level benefits rests with getting IPTp-SP interventions implemented at scale. The evidence suggests that a community distribution stratagem, that is well designed to increase coverage, and also amplifies the use of facility-based services, is both feasible and indispensable in Sokoto State and Nigeria. This programme used a health systems approach combined with human-centered design to build on the Nigeria National Primary Health Care Development Agency’s “minimum health care package guidelines”. Lessons from this programme can be used by state governments who would like to accelerate at scale implementation of primary health care service.
SP was readily integrated to other ongoing MNCH activities including the provision of chlorhexidine gel for care of the newborn cord and misoprostol to prevent postpartum haemorrhage. It is also instructive that there were effectively no incidents of stock out of SP of note during the study; this was instrumental to the results. It was helpful that a community health information system, derived from household databases aided the proactive identification of women who received reminders. It enabled timeliness and greater precision in reaching women with SP services. Presently, the Federal Ministry of Health’s web-based health information system, DHIS2 does not collect SP3 or higher doses at all. The DHIS2 should be revised to capture at least three doses of SP, and higher, in line with current national and WHO SP policy guidelines.
Another factor that may have facilitated the scale up of IPTp-SP delivery, was the power of social diffusion. Women who had received SP gave public testimony that they felt their babies at birth, were larger than in previous births; they made uncharacteristic, prideful public displays of their babies. Mothers also gave personal testimonies to their peers that SP made them feel healthier while being pregnant. This phenomenon may have helped to increase the proportion of women who declared their pregnancy earlier, started SP use earlier, and received higher mean doses of SP. It should be emphasized that mothers and communities want to experience tangible results, as an incentive to lend critical support to scaled-up health interventions. With acceptance of the promise of results, communities, as a measure of their trust, used their own funds to recruit additional volunteers to cover hard-to-reach areas. They were also very proactive in the use of information from monthly review meetings to sustain programme momentum.
Inclusive of the cost of SP, it cost between US$0.93 and $1.20 per three doses in the intervention LGAs. These lower cost ratios were the result of increased demand for SP, enabled by community-based distribution plus and facility distribution, as well as the reduced number missed opportunities in clinics. The higher equivalent cost of $6.9 per three doses in the counterfactual LGA, reflects the higher cost associated with a facility-only stratagem of distribution and the ensuing low demand for SP. A scale-up of IPTp-SP that utilizes both community and facility based service delivery, will reduce the cost per woman served and cost per dose given; it is, therefore, recommended in Sokoto State and in Nigeria.
The finding of a decline in the mean newborn head circumference in births month on month from April to November in both intervention and counterfactual groups, suggest factors other than placental malaria at play in worsening newborn outcomes. One likely factor, beyond the scope of this study, is the role of maternal nutrition on newborn outcomes. Mothers whose babies were born in April had more access to post harvest foods for at least 5 months. A combination of better maternal nutrition and better protection from placental malaria is likely to increase the odds of newborn survival. The interplay of maternal nutrition and placental malaria is an area of further study.
Limitations
The data collection instrument in this study was designed to collect a handful of simple metrics that could evaluate the impact of community based IPTp-SP in a context of low literacy. Data collected by CBHV supervisors did not capture many of the socioeconomic, nutritional and behavioral issues that affect newborn outcomes.
The WHO defines a stillbirth as a pregnancy as “a baby born with no signs of life at or after 28 weeks’ gestation” [39]. The definition of stillbirth used in this study was a crude, yet pragmatic, measure in the community programme context of Sokoto State. While this does present a potential for misclassification of stillbirths, the finding of biologically plausible associations between SP doses and a reduction in the odds of stillbirths, enhance the internal validity of the study.
Although every effort was undertaken to standardize head circumference measurements, it is probable that there were variations in where the tape measure was placed. Given the large sample size of newborns, errors in head circumference measurements would be normally distributed. Every effort was made to ensure head circumference measurement as close to birth and no later than 7 days postpartum. On this basis, as mentioned earlier, 2521 newborns principally in the intervention group, that were 8 days or older before they could be measured were excluded. The overall validity of the study is strengthened by findings that confirm an already established, biologically plausible, association between gestational age and the sex of the newborn on newborn head circumference.
The intervention deployed, although primarily the introduction of SP at the community and the training of facility-based health workers to reduce missed opportunities, it also included the restoration of linkages between communities and facilities, to promote referrals. The system for finding and enumerating women, which doubled as community mobilization, and was confined to intervention LGAs, and not introduced into the counterfactual LGA. To have introduced the house-to-house enumeration visits in the counterfactual LGA, would have increased community awareness and increased demand for SP, as was evident in the spike in SP use that occurred with the data collection on birth outcomes. In effect, the house-to-house enumeration served as a co-intervention. As seen in Table 4, the house-to-house enumeration, in two intervention areas, showed a higher number of eligible women than were estimated from official population estimates. This suggests that, if anything, there is most probably have an under estimate of the number of eligible women in the counterfactual area. If this was the case, coverage rates of IPTp-SP in the counterfactual LGA would be even lower than estimated.
Conclusion
The study findings underscore the untapped potential of results-driven primary health care programming inclusive of trained community health workers, to accelerate and safely implement IPTp-SP delivery at scale. The scale up of IPTp-SP programmes, that include a vital community distribution component, is an important stratagem for inclusion in national policy strategies. An optimally scaled-up IPTp-SP programme is more likely to reach the underserved, also the most likely to suffer inequities. Consideration should be given to systems thinking enabled human-centered design principles to help match scale up objectives with strategies most likely to yield local trust, ownership and predictable availability of quality services.
Authors’ contributions
NO, AA, DA, MI, ZM and JA conceived and designed the programme implementation and evaluation plans for this manuscript. NO, AA, DA, JA, MI, ZM, HG, ZN analyzed the data for this manuscript. NO, AA and DA wrote the first draft of this manuscript. All authors contributed to the writing of this manuscript, and agree with the manuscript’s results and conclusions. All authors read and approved the final manuscript.
Acknowledgements
We extend our gratitude to the WDCs, CBHVs, women, and people of the 42 wards where we worked. Additionally, this work would not have been possible without the support of the Sokoto State government officials who supported our efforts and ensured a successful and enabling environment.
Competing interests
To the best of our knowledge, none of the authors have any competing interest, financial or otherwise. This manuscript has not been previously published and is not under consideration in the same or substantially similar form in any other peer-reviewed media.
Availability of data and material
The data are available with some restrictions. Interested parties should contact Dr. Nosa Orobaton at: nosaorobaton@gmail.com for more information.
Consent for publication
No individual can be identified from the data presented here.
Ethics approval and consent to participate
Sokoto Health Ethics Review Committee gave ethical clearance for this study (SKHREC/015/014). Sokoto State Government also gave permission for its personnel to participate in the study. The 42 ward development committees’ chairmen of the 42 wards in the four LGAs in the study area also gave informed consent. Informed verbal consent was obtained from: (i) heads of the households during the compound mapping prior to the collection of detailed household information and (ii) from eligible pregnant women prior to enrollment and after being informed of the benefits and expected side effects of SP. This study was also registered with ClinicalTrials.gov (Protocol Record JSI-MIPP-NG #1) in April of 2016.
Funding
This work was funded by the Bill & Melinda Gates Foundation Grant #OPP1118137.
Abbreviations
- ACT
artemisinin combination therapy
- AFRO
World Health Organization Africa region
- ANC
antenatal care
- CBHV
community based health volunteers
- DHIS2
District Health Information System 2
- DOT
directly observed therapy
- HC
newborn head circumference
- HRB
household register book
- HRS
household registration system
- IPTp
intermittent preventive treatment in pregnancy
- IPTp-SP
intermittent preventive treatment in pregnancy with sulphadoxine-pyrimethamine
- ITN
insecticide treated nets
- LLIN
long lasting insecticide treated nets
- JSI
JSI Research & Training Institute, Inc
- LGA
local government area
- LMP
last menstrual period
- M&E
monitoring and evaluation
- MiP
malaria in pregnancy
- MiPP
malaria in pregnancy project
- MSF
monthly summary form
- RBM
roll back malaria partnership
- RDT
rapid diagnostic testing kits
- SBR
stillbirth rate
- SMOH
Sokoto State Ministry of Health
- TSHIP
Targeted States High Impact Project
- USAID
United States Agency for International Development
- WDC
Ward Development Committee
- WHO
World Health Organization
Additional files
Additional file 1. Data collection form for Outcomes in the Intervention and Counterfactual LGA’s.
Additional file 2. Sample of Pictograms, on the back of color-coded SP administration cards, designed by CBHV supervisors.
Additional file 3. Colour-coded dose cards for SP distribution.
Additional file 4. Unadjusted mean Head Circumference (HC) in millimeters (mm) among live newborns born between April and November 2015 (without October), and differences in mean HC by available variables.
Additional file 5. Adjusted Associations between selected variables and the mean head circumference (HC) of newborns, between April and November 2015 (without October).
Contributor Information
Nosa Orobaton, Email: nosaorobaton@gmail.com.
Anne M. Austin, Email: anne_austin@jsi.com
Dele Abegunde, Email: dele.abegunde@yahoo.com.
Mohammed Ibrahim, Email: ibiauwal@gmail.com.
Zainab Mohammed, Email: drzainabmohammed@gmail.com.
Jumare Abdul-Azeez, Email: jmrbdlzz@gmail.com.
Hakeem Ganiyu, Email: anninfotech@yahoo.com.
Zwalle Nanbol, Email: zwalleyusuf2013@gmail.com.
Bolaji Fapohunda, Email: bolaji.fapohunda8@gmail.com.
Katherine Beal, Email: katherine_beal@jsi.com.
References
- 1.WHO. 10 facts on malaria. Geneva: World Health Organization; 2015. http://www.who.int/features/factfiles/malaria/en/. Accessed 21 Feb 2016.
- 2.WHO. World Malaria Report 2015. Geneva: World Health Organization; 2015. http://apps.who.int/iris/bitstream/10665/200018/1/9789241565158_eng.pdf?ua=1. Accessed 15 Feb 2016.
- 3.Roll Back Malaria Partnership. Progress and impact series: country reports, country reports No. 4. Focus on Nigeria. 2012. http://www.rollbackmalaria.org/files/files/resources/report11-en.pdf. Accessed 10 Feb 2016.
- 4.Nigeria population 2016. Current population of Nigeria. 2016. http://countrymeters.info/en/Nigeria. Accessed 8 Oct 2016.
- 5.WHO. Malaria in pregnant women. Geneva: World Health Organization; 2016. http://www.who.int/malaria/areas/high_risk_groups/pregnancy/en/. Accessed 8 Oct 2016.
- 6.Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, Brabin B, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 7.Kvinnoforum and The Rollback Malaria Forum. A guide to gender and malaria. 2010. https://www.k4health.org/sites/default/files/gm_guide-en%5B1%5D.pdf. Accessed 8 Oct 2016.
- 8.WHO. Guidelines for the treatment of malaria. 3rd ed. Geneva: World Health Organization; 2015. http://www.who.int/malaria/publications/atoz/9789241549127/en/. Accessed 8 Oct 2016.
- 9.WHO. Consensus statement: optimizing the delivery of malaria-in-pregnancy interventions. Geneva: World Health Organization; 2013. http://www.pmi.gov/docs/default-source/default-document-library/tools-curricula/consensusreport_malariapregnancy.pdf?sfvrsn=4. Accessed 8 Oct 2016.
- 10.McClure EM, Goldenberg RL, Dent AE, Meshnick SR. A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. Int J Gynaecol Obstet. 2013;121:103–109. doi: 10.1016/j.ijgo.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. doi: 10.1086/315449. [DOI] [PubMed] [Google Scholar]
- 12.Sreeramareddy CT, Chuni N, Patil R, Singh D, Shakya B. Anthropometric surrogates to identify low birth weight Nepalese newborns: a hospital-based study. BMC Pediatr. 2008;8:16. doi: 10.1186/1471-2431-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rijken MJ, Rijken JA, Papageorghiou AT, Kennedy SH, Visser GHA, Nosten F, et al. Malaria in pregnancy: the difficulties in measuring birthweight. BJOG. 2011;118:671–678. doi: 10.1111/j.1471-0528.2010.02880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elizabeth NL, Christopher OG, Patrick K. Determining an anthropometric surrogate measure for identifying low birth weight babies in Uganda: a hospital-based cross sectional study. BMC Pediatr. 2013;13:54. doi: 10.1186/1471-2431-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villar J, Cheikh Ismail L, Victora CG, Ohuma EO, Bertino E, Altman DG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 16.Blencowe H, Cousens S, Jassir FB, Say L, Chou D, Mathers C, et al. National, regional, and worldwide estimates of stillbirth rates in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2016;4:e98–e108. doi: 10.1016/S2214-109X(15)00275-2. [DOI] [PubMed] [Google Scholar]
- 17.Lawn JE, Blencowe H, Pattinson R, Cousens S, Kumar R, Ibiebele I, et al. Stillbirths: Where? When? Why? How to make the data count? Lancet. 2011;377:1448–1463. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 18.Kerber KJ, Mathai M, Lewis G, Flenady V, Erwich JJHM, Segun T, et al. Counting every stillbirth and neonatal death through mortality audit to improve quality of care for every pregnant woman and her baby. BMC Pregnancy Childbirth. 2015;15(Suppl 2):S9. doi: 10.1186/1471-2393-15-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Geertruyden J-P, Thomas F, Erhart A, D’Alessandro U. The contribution of malaria in pregnancy to perinatal mortality. Am J Trop Med Hyg. 2004;71:35–40. [PubMed] [Google Scholar]
- 20.Radeva-Petrova D, Kayentao K, ter Kuile FO, Sinclair D, Garner P. Drugs for preventing malaria in pregnant women in endemic areas: any drug regimen versus placebo or no treatment. Cochrane Database Syst Rev. 2014;10:CD000169. doi: 10.1002/14651858.CD000169.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Federal Ministry of Health (FMOH). National guidelines and strategies for malaria prevention and control during pregnancy. A publication of the Federal Ministry of Health, Nigeria; Malaria Control Programme. Abuja: FMOH; 2005.
- 22.National Federal Ministry of Health (FMOH), WHO. National drug policy—Nigeria: 5.0. Targets of the National Drug Policy. 2005. http://collections.infocollections.org/whocountry/en/d/Js6865e/8.html. Accessed 8 Oct 2016.
- 23.Amoran OE, Ariba AA, Iyaniwura CA. Determinants of intermittent preventive treatment of malaria during pregnancy (IPTp) utilization in a rural town in Western Nigeria. Reprod Health. 2012;9:12. doi: 10.1186/1742-4755-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Population Commission (NPC), Nigeria, and ICF International. Nigeria demographic ands health survey 2013. Abuja: NPC and ICF International; 2013. http://dhsprogram.com/pubs/pdf/FR293/FR293.pdf. Accessed 8 Oct 2016.
- 25.WHO Global Malaria Programme. WHO Policy brief for the implementation of intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva: World Health Organization; 2013 http://www.who.int/malaria/publications/atoz/Policy_brief_IPTp-SP_implementation_11april2013.pdf.pdf. Accessed 8 Oct 2016.
- 26.WHO. Evidence Review Group: Intermittent preventive treatment of malaria in pregnancy (IPTp) with sulfadoxine-pyrimethamine (SP). Geneva: WHO Headquarters; 2012. http://www.who.int/malaria/mpac/sep2012/iptp_sp_erg_meeting_report_july2012.pdf. Accessed 8 Oct 2016.
- 27.Okeibunor JC, Orji BC, Brieger W, Ishola G, Otolorin E, Rawlins B, et al. Preventing malaria in pregnancy through community-directed interventions: evidence from Akwa Ibom State, Nigeria. Malar J. 2011;10:227. doi: 10.1186/1475-2875-10-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Bank. Improving primary health care delivery in Nigeria: evidence from Four States, 2010, Working Bank Paper. 2010. https://openknowledge.worldbank.org/bitstream/handle/10986/5958/540370PUB0heal101Official0Use0Only1.pdf?sequence=1. Accessed 8 Oct 2016.
- 29.Sokoto State Government, Sokoto State Ministry of Health. Strategic Health Development Plan (2010–2015). 2010. http://www.mamaye.org.ng/sites/default/files/evidence/SOKOTO revised 05.01.2011.pdf. Accessed 8 Oct 2016.
- 30.National Federal Ministry of Health (FMOH). Nigeria Health Management Information System. https://dhis2nigeria.org.ng/dhis/dhis-web-commons/security/login.action;jsessionid=26A55D97A639155E56A068426DAA4674. Accessed 10 Feb 2016.
- 31.Uauy R, Casanello P, Krause B, Kuzanovic JP, Corvalan C. Conceptual basis for prescriptive growth standards from conception to early childhood: present and future. BJOG. 2013;120(Suppl 2):3–8. doi: 10.1111/1471-0528.12057. [DOI] [PubMed] [Google Scholar]
- 32.Meuris S, Piko BB, Eerens P, Vanbellinghen AM, Dramaix M, Hennart P. Gestational malaria: assessment of its consequences on fetal growth. Am J Trop Med Hyg. 1993;48:603–609. doi: 10.4269/ajtmh.1993.48.603. [DOI] [PubMed] [Google Scholar]
- 33.National Population Commission [Nigeria] and ICF Macro. Nigeria Demographic and Health Survey 2008. Abuja: NPC and ICF International; 2009. http://www.dhsprogram.com/pubs/pdf/FR222/FR222.pdf. Accessed 8 Oct 2016.
- 34.Peters PJ, Thigpen MC, Parise ME, Newman RD. Safety and toxicity of sulfadoxine/pyrimethamine: implications for malaria prevention in pregnancy using intermittent preventive treatment. Drug Saf. 2007;30:481–501. doi: 10.2165/00002018-200730060-00003. [DOI] [PubMed] [Google Scholar]
- 35.Brabin BJ, Romagosa C, Abdelgalil S, Menéndez C, Verhoeff FH, McGready R, et al. The sick placenta-the role of malaria. Placenta. 2004;25:359–378. doi: 10.1016/j.placenta.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Hill J, Hoyt J, van Eijk AM, D’Mello-Guyett L, ter Kuile FO, Steketee R, et al. Factors affecting the delivery, access, and use of interventions to prevent malaria in pregnancy in sub-Saharan Africa: a systematic review and meta-analysis. PLoS Med. 2013;10:e1001488. doi: 10.1371/journal.pmed.1001488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kibusi SM, Kimunai E, Hines CS. Predictors for uptake of intermittent preventive treatment of malaria in pregnancy (IPTp) in Tanzania. BMC Public Health. 2015;15:540. doi: 10.1186/s12889-015-1905-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangaré LR, Stergachis A, Brentlinger PE, Richardson BA, Staedke SG, Kiwuwa MS, et al. Determinants of use of intermittent preventive treatment of malaria in pregnancy: Jinja, Uganda. PLoS ONE. 2010;5:e15066. doi: 10.1371/journal.pone.0015066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO. Stillbirths. Geneva: World Health Organization; 2015. http://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/#.V-hhss5X1hw.mendeley. Accessed 8 Oct 2016.


